Editado por: Rosario Amaya Villar - Unidad de Cuidados Intensivos, Hospital Universitario Virgen del Rocio, Sevilla, España
Última actualización: Febrero 2024
Más datosTo evaluate the impact of the novel P/FPE index to classify ARDS severity on mortality of patients with ARDS.
DesignA retrospective cohort study.
SettingTwelve-bed medical and surgical intensive care unit from January 2018 to December 2020.
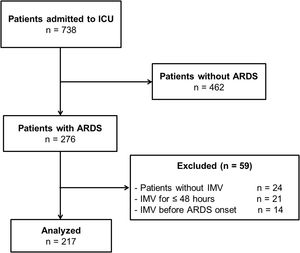
PatientsA total of 217 ARDS patients managed with invasive mechanical ventilation >48h.
InterventionsNone.
VariablesARDS severity on day 1 and day 3 was measured based on PaO2/FiO2 ratio and P/FPE index [PaO2/(FiO2×PEEP)]. Primary outcome was the hospital mortality.
ResultsHospital mortality rate was 59.9%. Relative to PaO2/FiO2 ratio, 31.8% of patients on day 1 and 77.0% on day 3 were reclassified into a different category of ARDS severity by P/FPE index. The level of PEEP was lower by P/FPE index-based ARDS severity classification than by using PaO2/FiO2 ratio. The performance for predicting mortality of P/FPE index was superior to PaO2/FiO2 ratio in term of AROC (day 1: 0.72 vs. 0.62; day 3: 0.87 vs. 0.68) and CORR (day 1: 0.370 vs. 0.213; day 3: 0.634 vs. 0.301). P/FPE index improved prediction of risk of death compared to PaO2/FiO2 ratio as showed by the qNRI (day 1: 72.0%, p<0.0001; day 3: 132.4%, p<0.0001) and IDI (day 1: 0.09, p<0.0001; day 3: 0.31, p<0.0001).
ConclusionsAssessment of ARDS severity based on P/FPE index seems better than PaO2/FiO2 ratio for predicting mortality. The value of P/FPE index for clinical decision-making requires confirmation by randomized controlled trials.
Evaluar el impacto del índice P/FPE para clasificar la severidad del SDRA y su relación con la mortalidad.
DiseñoEstudio de cohorte retrospectivo.
ContextoUnidad de cuidados intensivos polivalentes de 12 camas desde enero de 2018 hasta diciembre de 2020.
PacientesSe estudió a 217 pacientes con SDRA con ventilación invasiva>48 horas.
IntervencionesNinguna.
VariablesLa severidad del SDRA se evaluó el primer y el tercer día, según el índice PaO2/FiO2 y el índice P/FPE (PaO2/[FiO2×PEEP]). El desenlace primario evaluado fue la mortalidad hospitalaria.
ResultadosLa mortalidad hospitalaria fue 59,9%. Con relación al índice PaO2/FiO2, el 31,8% de los pacientes el día 1 y el 77,0% el día 3 fue reclasificado en categorías diferentes de severidad del SDRA mediante el índice P/FPE. El nivel de PEEP fue más bajo con el uso del índice P/FPE que con el PaO2/FiO2. La predicción de la mortalidad fue superior con el índice P/FPE que con PaO2/FiO2, en términos de AROC (día 1: 0,72 vs. 0,62; día 3: 0,87 vs. 0,68) y CORR (día 1: 0,370 vs. 0,213; día 3: 0,634 vs. 0,301). El índice P/FPE mejoró la predicción del riesgo de muerte comparado con el PaO2/FiO2, como demuestra el qNRI (día 1: 72,0%, p<0,0001; día 3: 132,4%, p<0,0001) y el IDI (día 1: 0,09, p<0,0001; día 3: 0,31, p<0,0001).
ConclusionesLa evaluación de severidad del SDRA mediante el índice P/FPE parece ser mejor que la del índice PaO2/FiO2 para predecir la mortalidad. El valor del P/FPE para la toma de decisiones clínicas requiere confirmación mediante ensayos clínicos.
Acute respiratory distress syndrome (ARDS) is a heterogeneous syndrome characterized by an acute inflammation, increased permeability, and edema of the lung tissue, with a typical histological hallmark of diffuse alveolar damage, leading to alveolar collapse and a severe impairment in oxygen diffusion.1 ARDS accounts for 10% of intensive care unit (ICU) admissions and 23% of patients receiving mechanical ventilation, with a mortality rate that ranges from 35% in mild cases to 46% in severe cases.2 According to the Berlin definition, severity of ARDS is classified into 3 categories (mild, moderate, and severe) based on the arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio, with a minimum positive end-expiratory pressure (PEEP) level of 5cm H2O.3 These criteria have been useful for designing clinical trials and observational studies, as well as for improving clinical decision-makings and therapeutic interventions.4
However, the PaO2/FiO2 ratio has some limitations for assessing ARDS severity. First, PaO2/FiO2 ratio is affected by ventilatory setting such as PEEP and inspiratory/expiratory time ratio. Villar et al. improved ARDS risk stratification using standard ventilatory settings (PEEP≥10cmH2O and FiO2≥0.5).5,6 Second, PaO2/FiO2 ratio may change over time. A number of authors found a better mortality prediction by patients’ reclassification ≥24h after ARDS onset.5–7 And third, PaO2/FiO2 ratio is not always linked to mortality in patients with ARDS.8,9
Recently, Sayed and coworkers proposed a novel criterion to address Berlin definition gap by including PEEP in the new index named P/FPE, defined as PaO2/(FiO2×PEEP). The increase the PEEP level with the same FiO2 yields different degree of blood oxygenation. By using machine learning approaches, the authors demonstrated that the P/FPE index after onset and at third day is markedly better than the current PaO2/FiO2 ratio to assess ARDS severity.10 The present study was aimed to evaluate the impact of the P/FPE index on mortality of patients with ARDS.
Patients and methodsDesign and settingThe current study is presented as stated by The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement (Supplementary material 1).11 This is a retrospective cohort study of patients collected between January 2018 and December 2020 in the medical and surgical ICU-8 of the Hermanos Ameijeiras Hospital. This is a 640-bed, university-affiliated, tertiary referral hospital in Havana, Cuba. The ICU-8 has 12 beds and provides care for approximately 300–350 critically ill patients per year. Information regarding origin, demography, epidemiology, chronic comorbidities, clinical status, laboratory tests, image results, and outcomes of all patients were recorded in the ICU-8 database by the attending physician day by day from patients’ admission until discharge or death. Quality of data was daily verified by a supervisor physician. A complete list of data used for this study is provided in Supplementary material 2.
The current study was conducted in accordance with the 1964 Helsinki Declaration, and was approved by the Scientific Council and the Ethics Committee for Scientific Research of the Hermanos Ameijeiras Hospital (Approval number 01-10-06-2021). Written informed consent was waived in view of retrospective nature of the study.
SubjectsAll consecutive subjects with ARDS admitted to ICU were included. The following subjects were excluded: 1. Subjects without invasive mechanical ventilation (IMV), because failure in noninvasive respiratory support therapies may have a negative impact on outcomes12,13; 2. Subjects with duration of IMV ≤48h to improve ARDS classification,7,9,10,14 and avoid confounders related to systemic pathophysiological disturbances of the acutely ill patients; and 3. Subjects on IMV before ARDS onset, since several clinical, therapeutic, and ventilatory setting confounders may contribute for developing ARDS in subjects previously intubated for other reasons and may have effects on outcomes.15,16 Finally, 217 subjects were analyzed (Fig. 1).
Data collectionThe following data were recorded on ICU admission: age, sex, body mass index, predictive body weight (PBW), history of chronic diseases, length of hospitalization before ICU admission, reason for ICU admission, type of patient, Simplified Acute Physiology Score (SAPS) 3, Sequential Organ Failure Assessment (SOFA) score, and use and dose of vasoactive drugs. Within 2h after starting IMV the following variables were collected: ventilatory setting (ventilatory mode, peak inspiratory pressure, plateau pressure, mean pressure, PEEP, driving pressure, tidal volume, tidal volume/PBW, respiratory rate, minute ventilation, standardized minute ventilation, static compliance, and FiO2), recruitment maneuvers, prone positioning, infusion of neuromuscular blocking agents, pH and blood gases parameters (hemoglobin oxygen saturation, arterial partial pressure of oxygen, arterial partial pressure of carbon dioxide). Risk factors for ARDS were collected on ARDS diagnosis. ICU-acquired ARDS was defined as ARDS onset >48h after ICU admission.17
ARDS severity evaluationSeverity of ARDS was assessed on day 1 (within 2h after starting IMV) and day 3 (within 48–72h after starting IMV) with the patient in supine position. All subjects were stratified into mild (200mm Hg<PaO2/FiO2 ratio≤300mm Hg), moderate (100mm Hg<PaO2/FiO2 ratio≤200mm Hg) or severe ARDS (PaO2/FiO2 ratio ≤100mm Hg) in line with the Berlin definition.3 According to P/FPE index, patients were classified as mild (40<P/FPE index≤60), moderate (20<P/FPE index≤40) or severe ARDS (P/FPE index≤20).10 All cases had PEEP≥5cm H2O; minimal PaO2/FiO2 ratio and P/FPE index values were used because minimal values during the day might better predict mortality. Diagnosis and severity of ARDS along with decision-making for therapeutic interventions were collectively taken by the physician team of the ICU (all of them blinded to the study objective).
Ventilatory managementVentilatory adjustments of patients were left to the attending physician, but by using a protective approach. For patients with PaO2/FiO2 ratio≤150mm Hg, prone positioning, lung recruitment maneuvers, higher PEEP, and neuromuscular blocking agents were considered.18 Sedative and analgesic drugs were used as needed. Ventilatory settings and arterial blood gases on day 1 and day 3 are depicted in Supplementary Table 1 (Supplementary material 3).
OutcomesPrimary outcome of interest was the hospital mortality. Secondary outcomes were ICU mortality, duration of IMV, length of ICU stay, and length of hospitalization.
Statistical analysisStudy objective was unknown for attending physicians, nurses and patients, which allowed to minimize the following sources of biases: 1. Over or underreport of ARDS; 2. Selective ICU admission of patients with ARDS; 3. Selective detection of ICU-acquired ARDS; and 4. Selective use of any therapeutic intervention with impact on clinical outcomes.
Assuming hospital mortality rates of 20% in mild ARDS patients and 40% in severe ARDS patients on 3 day after starting IMV, as previously reported by Sayed and coworkers using large datasets,10 with a statistical power of 80% and two-side confidence level of 95%, we calculated a sample size of 166 patients. Finally, we enrolled 217 patients.
Multiple imputation method was used for treating missing values. Categorical variables are shown as absolute numbers with percentage, whereas numerical variables are represented as median with 25–75 interquartile rank (IQR). Differences between groups were assessed using chi-square test for categorical variables and Mann–Whitney U test for continuous variables.
Multivariate logistic regression analysis was used to explore the impact of ventilatory settings and respiratory indexes on primary outcome. Model assumptions were verified. To avoid collinearity, after checking the correlation matrix, only weakly correlated and clinically significant covariates were included. SOFA score was included as a covariate in order to interpret results in a valid clinical scenario. Two models were explored, the first used the PaO2/FiO2 ratio as a covariate, and the second used the P/FPE index.
The area under receiver operating characteristic curve (AROC) and the correlation between the predicted and actual value (CORR) were used to assess the performance of the PaO2/FiO2 ratio and P/FPE index in predicting hospital mortality. For assessment the incremental value of the P/FPE index compared to the PaO2/FiO2 ratio, the quantitative net reclassification improvement (qNRI, for quantifying the amount of correct change in predicting hospital mortality by using the P/FPE index relative to the PaO2/FiO2 ratio) and the integrated discrimination improvement (IDI, for quantifying the increase in separation of events and nonevents of death by using the P/FPE index relative to the PaO2/FiO2 ratio) with its 95% confidence interval (CI) were estimated.
Statistical tests with a two-tailed p-value <0.05 were considered as significant. Data were analyzed using IBM®SPSS® Statistics 23.0 (IBM, Chicago, IL, USA).
ResultsCharacteristics of patientsIn the 217 studied patients, the most common chronic comorbidities were hypertension, cancer, and immunoincompetence. The main reasons for ICU admission were acute respiratory failure, shock, and disturbed consciousness. Nonsurgical and surgical patients accounted for 61.8% and 38.2% of participants, respectively. The median SOFA score was 8.0 points, and the median SAPS 3 score was 55.0 points. During the ICU stay, vasoactive drugs were used in 36.4% of patients. ICU and hospital mortality rates were 41.0% and 59.9%, respectively. History of cardiovascular diseases, type of patient, and SOFA score were associated with hospital mortality in univariate analysis (Table 1).
General characteristics of subjects.
| Characteristics | Total (n=217) | Survivors (n=87) | Nonsurvivors (n=130) | p |
|---|---|---|---|---|
| Age, years | 63.0 (53.0–73.0) | 62.0 (51.5–72.0) | 64.5 (54.0–73.0) | 0.261 |
| Sex, male | 103 (47.5) | 45 (51.7) | 58 (44.6) | 0.304 |
| Body mass index, kg/m2 | 25.0 (24.1–27.5) | 25.4 (24.3–27.4) | 24.8 (23.5–27.5) | 0.444 |
| Chronic diseasesa | ||||
| Chronic respiratory disease | 35 (16.1) | 10 (11.5) | 25 (19.2) | 0.129 |
| Diabetes mellitus | 48 (22.1) | 16 (18.4) | 32 (24.6) | 0.279 |
| Immunoincompetence | 64 (29.5) | 21 (24.1) | 43 (3.1) | 0.157 |
| High blood pressure | 128 (59.0) | 42 (48.3) | 86 (66.2) | 0.009 |
| Coronary artery disease | 38 (17.5) | 9 (10.3) | 29 (22.3) | 0.023 |
| Other cardiovascular disease | 33 (15.2) | 8 (9.2) | 25 (19.2) | 0.044 |
| Cancer | 82 (37.8) | 30 (34.5) | 52 (40.0) | 0.411 |
| Chronic kidney disease | 25 (11.5) | 8 (9.2) | 17 (13.1) | 0.380 |
| Chronic liver disease | 12 (5.5) | 5 (5.7) | 7 (5.4) | 1.000 |
| Length of stay before ICU admission, days | 6.0 (3.0–16.0) | 7.0 (2.0–14.0) | 6.0 (3.0–16.0) | 0.461 |
| Reason for ICU admissionb | ||||
| Acute respiratory failure | 180 (82.9) | 77 (88.5) | 103 (79.2) | 0.075 |
| Shock | 64 (29.5) | 23 (26.4) | 41 (31.5) | 0.419 |
| Rhythm disturbances | 9 (4.1) | 2 (2.3) | 7 (5.4) | 0.264 |
| Acute abdomen | 20 (9.2) | 7 (8.0) | 13 (10.0) | 0.626 |
| Severe acute pancreatitis | 5 (2.3) | 2 (2.3) | 3 (2.3) | 0.997 |
| Disturbed consciousness | 49 (22.6) | 20 (23.0) | 29 (22.3) | 0.906 |
| Intracranial mass effect | 24 (11.1) | 14 (16.1) | 10 (7.7) | 0.053 |
| Liver failure | 7 (3.2) | 1 (1.1) | 6 (4.6) | 0.247 |
| Type of patient | 0.007 | |||
| Nonsurgery | 134 (61.8) | 44 (50.6) | 90 (69.2) | |
| Elective surgery | 40 (18.4) | 24 (27.6) | 16 (12.3) | |
| Emergency surgery | 43 (19.8) | 19 (21.8) | 24 (18.5) | |
| SAPS 3 score, points | 55.0 (44.0–66.0) | 55.0 (42.5–65.5) | 55.0 (44.0–66.0) | 0.957 |
| SOFA score, points | 8.0 (6.0–11.0) | 4.0 (3.0–7.0) | 8.0 (8.0–12.0) | <0.0001 |
| Risk factor for ARDSc | ||||
| Pneumonia | 88 (40.6) | 40 (46.0) | 48 (36.9) | 0.183 |
| Extrapulmonary sepsis | 67 (30.9) | 24 (27.6) | 43 (33.1) | 0.391 |
| Aspiration | 15 (6.9) | 8 (9.2) | 7 (5.4) | 0.278 |
| Noncardiogenic shock | 75 (34.6) | 31 (35.6) | 44 (33.8) | 0.786 |
| Blood transfusion | 9 (4.1) | 2 (2.3) | 7 (5.4) | 0.320 |
| Drug overdose | 6 (2.8) | 0 (0.0) | 6 (4.6) | 0.083 |
| Other risk factor | 10 (4.6) | 4 (4.6) | 6 (4.6) | 1.000 |
| No risk factor | 15 (6.9) | 4 (4.6) | 11 (8.5) | 0.271 |
| ICU-acquired ARDS | 49 (22.6) | 12 (13.8) | 37 (28.5) | 0.011 |
| Time from ICU admission to ARDS onset, days | 4.0 (3.5–5.0) | 4.0 (3.3–4.) | 4.0 (3.5–5.0) | 0.556 |
| Duration of MV, days | 7.0 (4.0–10.0) | 6.0 (4.0–10.0) | 7.0 (4.0–10.0) | 0.820 |
| Length of ICU stay, days | 9.0 (6.0–13.0) | 10.0 (8.0–14.0) | 8.0 (5.0–12.0) | <0.0001 |
| Length of hospitalization, days | 12.0 (7.0–16.5) | 16.0 (12.0–19.0) | 8.5 (5.0–13.3) | <0.0001 |
Data are presented as the number (%) or the median (interquartile range). The p-values are calculated using the Mann–Whitney U test for continuous variables and chi-square test for categorical variables.
ARDS=acute respiratory distress syndrome; ICU=intensive care unit; MV=invasive mechanical ventilation; SAPS=Simplified Acute Physiology Score; SOFA=Sequential Organ Failure Assessment.
Pneumonia, noncardiogenic shock, and extrapulmonary sepsis were the most common risk factors for ARDS. ICU-acquired ARDS accounted for 22.9% of subjects and was related to increased mortality (13.8% vs. 28.5%; p=0.011). General characteristics of patients are depicted in Table 1. Relationship of ventilatory settings and arterial blood gases with hospital mortality is illustrated in Supplementary Tables 2 and 3 (see Supplementary material 3).
ARDS severity classificationThe median PaO2/FiO2 ratio and P/FPE index on first day after starting IMV was 187.0mm Hg (IQR 117.3–221.8mm Hg) and 21.6 (IQR 10.2–33.2), respectively. On third day, the median value of PaO2/FiO2 ratio was 222.5mm Hg (IQR 176.7–297.8mm Hg) and the median value of P/FPE index was 23.3 (IQR 15.8–37.5).
According to the PaO2/FiO2 ratio on day 1, 30.9%, 46.5%, and 22.6% of patients had a mild, moderate, and severe ARDS, respectively; on day 3, 23.0% of patients were free of ARDS while patients with mild, moderate, and severe ARDS accounted for 42.4%, 31.3%, and 3.2%, respectively. Using the P/FPE index on day 1, patients with mild, moderate, and severe ARDS accounted for 18.0%, 39.6%, and 42.4%, respectively; on day 3, 6.5% of patients were free of ARDS, whereas 14.3%, 40.6%, and 38.7% had a mild, moderate, and severe ARDS, respectively.
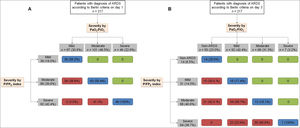
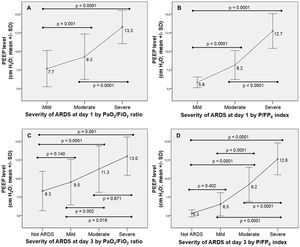
A number of patients were reclassified into a different category of ARDS severity by using the P/FPE index (31.8% on day 1 and 77.0% on day 3) (Fig. 2). Of note, 72% of patients without ARDS by the PaO2/FiO2 ratio on day 3 remained with ARDS by using the P/FPE index (Fig. 2). Severity of ARDS was linked to a higher PEEP level; however, the level of PEEP was lower by the P/FPE index-based ARDS severity than using the PaO2/FiO2 ratio-based ARDS severity (Fig. 3).
Agreement in classification of ARDS severity using PaO2/FiO2 ratio and P/FPE index on day 1 (A) and day 3 (B). Blue boxes represent patients whose categories remained unchanged. Red boxes represent patients who were reclassified to a more severe category. Green boxes represent patients who were reclassified to a milder category. P/F=PaO2/FiO2 ratio; P/FPE index=PaO2/(FiO2×PEEP).
Relationship between PEEP level and ARDS severity according to the PaO2/FiO2 ratio at day 1 (pictures A) and day 3 (picture C), and the P/FPE index at day 1 (pictures B) and day 3 (picture D). ARDS=acute respiratory distress syndrome; PaO2/FiO2=arterial partial pressure of oxygen to fraction of inspired oxygen ratio; PEEP=positive end-expiratory pressure; P/FPE index=PaO2/(FiO2×PEEP).
PaO2/FiO2 ratio and P/FPE index was related to mortality both on day 1 and day 3 (Supplementary figure 1, Supplementary material 3). However, on multivariate analyses only the P/FPE index was consistently associated with hospital mortality (Table 2). Driving pressure, tidal volume/PBW and SOFA score were also independent risk factors related to increased mortality (Table 2).
Ventilatory settings and respiratory indexes related to hospital mortality by multivariate logistic regression analysis.
| Risk factors | OR | 95% CI | p |
|---|---|---|---|
| Model including PaO2/FiO2ratio (day 1) | |||
| Driving pressure | 1.52 | 1.26–1.84 | <0.0001 |
| VT/PBW | 3.51 | 1.55–7.98 | 0.003 |
| Respiratory rate | 1.11 | 1.00–1.23 | 0.047 |
| PaO2/FiO2 ratio | 0.996 | 0.989–1.002 | 0.213 |
| SOFA score | 2.19 | 1.69–2.83 | <0.0001 |
| Model including P/FPEindex (day 1) | |||
| Driving pressure | 1.58 | 1.29–1.95 | <0.0001 |
| VT/PBW | 3.17 | 1.30–7.74 | 0.011 |
| Respiratory rate | 1.11 | 1.00–1.24 | 0.051 |
| P/FPE index | 0.93 | 0.89–0.97 | 0.001 |
| SOFA score | 2.27 | 1.70–3.02 | <0.0001 |
| Model including PaO2/FiO2ratio (day 3) | |||
| Driving pressure | 1.33 | 1.06–1.66 | 0.015 |
| VT/PBW | 3.87 | 1.17–12.84 | 0.027 |
| Respiratory rate | 1.10 | 1.00–1.22 | 0.054 |
| PaO2/FiO2 ratio | 0.994 | 0.989–1.000 | 0.048 |
| SOFA score | 2.59 | 1.92–3.50 | <0.0001 |
| Model including P/FPEindex (day 3) | |||
| Driving pressure | 1.50 | 1.13–1.98 | 0.005 |
| VT/PBW | 5.34 | 1.21–23.51 | 0.027 |
| Respiratory rate | 1.11 | 0.99–1.25 | 0.071 |
| P/FPE index | 0.87 | 0.83–0.92 | <0.0001 |
| SOFA score | 2.50 | 1.78–3.52 | <0.0001 |
Data are presented as the odds ratio (OR) with 95% confidence interval (CI). The p-values were calculated using the multivariate logistic regression analysis.
PaO2/FiO2=arterial partial pressure of oxygen to fraction of inspired oxygen ratio; P/FPE index=PaO2/(FiO2×PEEP); SOFA=Sequential Organ Failure Assessment; VT/PBW=tidal volume/predictive body weight.
The capability of the PaO2/FiO2 ratio and P/FPE index for predicting hospital mortality improved from day 1 to day 3. Nonetheless, the performance of the P/FPE index was superior to the PaO2/FiO2 ratio in term of AROC (day 1 0.72 vs. 0.62; day 3 0.87 vs. 0.68) and CORR (day 1 0.370 vs. 0.213; day 3 0.634 vs. 0.301) (Table 3 and Supplementary figure 2, Supplementary material 3). P/FPE index improved the prediction of risk of death compared to PaO2/FiO2 ratio as showed by the qNRI (day 1 72.0%, p<0.0001; day 3 132.4%, p<0.0001) and IDI (day 1 0.09, 95% CI 0.06–0.12, p<0.0001; day 3 0.31, 95% CI 0.26–0.35, p<0.0001) (Supplementary figures 3 and 4, Supplementary material 3).
Performance of PaO2/FiO2 Ratio and P/FPE Index in Predicting Hospital Mortality.
| Index | AROC | CORR | |||
|---|---|---|---|---|---|
| Estimate | 95% CI | p | Estimate | p | |
| Assessment on day 1 | |||||
| PaO2/FiO2 ratio | 0.62 | 0.54–0.69 | 0.004 | 0.213 | 0.002 |
| P/FPE index | 0.72 | 0.65–0.78 | <0.0001 | 0.370 | <0.0001 |
| Assessment on day 3 | |||||
| PaO2/FiO2 ratio | 0.68 | 0.60–0.75 | <0.0001 | 0.301 | <0.0001 |
| P/FPE index | 0.87 | 0.82–0.92 | <0.0001 | 0.634 | <0.0001 |
Data are presented as the area under receiver operating characteristic curve (AROC) with 95% confidence interval (CI), and the correlation between the predicted and actual value (CORR). The p-values were calculated using the Pearson's correlation test for CORR.
ICU=intensive care unit; PaO2/FiO2=arterial partial pressure of oxygen to fraction of inspired oxygen ratio; P/FPE index=PaO2/(FiO2×PEEP).
The present study found that 31.8% of patients with ARDS on day 1 and 77.0% on day 3 were reclassified into a different category of ARDS severity using the P/FPE index. The performance for predicting hospital mortality increased with the P/FPE index, compared to the PaO2/FiO2 ratio. The P/FPE index and ventilatory settings, such as tidal volume and driving pressure, were independently related to increased mortality in multivariate analysis. ARDS severity stratification improved on third day after ARDS diagnosis. Mean PEEP level was lower when ARDS severity was categorized according to the P/FPE index rather than the PaO2/FiO2 ratio.
In order to overcome the gap of the Berlin criteria, Sayed et al. recently proposed the PaO2 to (FiO2×PEEP) ratio, named P/FPE index, as a novel criterion to reclassify ARDS patients in terms of severity.10 Improvement of ARDS severity classification is essential for current critical care medicine since misclassification may lead to errors in clinical judgment and decision-making. For instance, we found that a number of patients were reclassified in a higher category of ARDS severity using the P/FPE index, whereas 72.0% of patients without ARDS on day 3 according to the PaO2/FiO2 ratio truly had ARDS by the P/FPE index. Recently, Palanidurai et al. observed that more than half of the patients were reclassified into a different severity category of ARDS by the P/FPE index, compared to the PaO2/FiO2 ratio. These similar results indicate that changes in severity classification with the P/FPE index reflect the true severity of ARDS and the applied PEEP strategy.19
Under-recognition of ARDS is a common and serious problem with important clinical consequences, particularly in terms of therapeutic options not considered.20 The LUNG SAFE study demonstrated that the diagnosis of ARDS is delayed or missed in 40% of patients.2 P/FPE index is able to identify patients with ARDS who would not be classified with ARDS according to the PaO2/FiO2 ratio, and is also a better predictor of mortality.19 Therefore, by using the P/FPE index clinicians may implement strategies to improve mortality such as optimal PEEP, lower FiO2, prone positioning and the use of neuromuscular blocking agents.
PEEP is a confounder in clinical practice since optimal PEEP is difficult to obtain through several methods,21 and clinicians commonly prescribes high PEEP in patients with adequate oxygenation goals.6,22 Clinical interpretation of the PaO2/FiO2 ratio may be biased by nonpulmonary factors such as hemoglobin concentration and arterial-venous oxygen content difference.23 Consequently, oxygen toxicity with adverse impact on outcomes may be developed due to excess FiO2.24 P/FPE index is attractive because physicians can achieve the best combination of FiO2 and PEEP to reach adequate oxygenation. In our cohort, the frequency of prone positioning was similar to that reported in epidemiologic studies while infusion of neuromuscular blocking agents was lower.2 However, the use of these drugs is controversial because of its side effects and unclear benefits25; currently they are indicated only for the treatment of ventilatory asynchrony, supporting pronation, or assuring protective ventilation goals.21
Our findings demonstrated the clinical validity of P/FPE index. The better performance, compared to PaO2/FiO2 ratio, shows the usefulness of P/FPE index in predicting mortality. Palanidurai et al. also found that P/FPE index has a greater predictive validity for predicting hospital mortality in ARDS patients than the PaO2/FiO2 ratio.19 In fact, the AROC for P/FPE index (0.71 vs. 0.72) and PaO2/FiO2 ratio (0.66 vs. 0.62) was similar to the present study, which supports external validity of our results.
ARDS is one of the major reasons of ICU admission, and continues to have high mortality rates despite advances in supportive care.2,26 Ventilatory support is the keystone in the management of patients with ARDS, but ventilatory setting may have an impact on outcomes.27 Since tidal volume and driving pressure, along with the P/FPE index, were related to increased mortality in multivariate analysis, our findings suggest that the negative effect of ventilatory variables remains unchanged through the course of the disease, which is in line with recent evidences.28,29 Additionally, the relationship between the P/FPE index with mortality explains ARDS severity, but also the potentially harmful effects of PEEP.
The present study confirmed that ARDS severity stratification is improved on third day after ARDS diagnosis.7,10 Lai et al. demonstrated that the PaO2/FiO2 ratio after a period of stabilization may be a more appropriate predictor of mortality than the initial PaO2/FiO2 ratio at the onset of ARDS.30 Chiu et al. found that patients with resolved or improving ARDS severity on day 3 had lower mortality, whereas patients with the same or worsening ARDS severity on day 3 had higher mortality.7 Apparently, patients need to be exposed to a sufficient period of time for response to medical therapies and adjusted IMV settings before being classified.
We found that a lower mean PEEP was used in all class of ARDS severity when patients were stratified by P/FPE index, which might reduce the risk of excess PEEP. Palanidurai et al. observed that the predictive validity of P/FPE index improved with progressively higher levels of PEEP, indicative of the negative effects of higher PEEP.19 PEEP is not considered for ARDS severity evaluation by the Berlin definition. Adding PEEP to the PaO2/FiO2 ratio takes into consideration the respiratory system compliance and lung recruitment. Furthermore, by using PEEP as a quantitative variable, P/FPE index conserves information and improves accuracy of estimations.31 However, although PEEP increases functional residual capacity and improves blood oxygenation, tissue oxygen delivery decreases because of reduced cardiac output.32 PEEP also increases the risk of volutrauma and ventilator-induced lung injury,33 causing increased mortality when a high-PEEP strategy is used.34
This study has a number of strengths. First, the study was conducted in a center with high standard of health care and in an ICU with qualified intensivists 24h a day, seven days a week. Second, potential sources of bias were reduced, which lend additional strength to our analysis. Third, this is a well-powered study with a representative sample size so estimation errors were minimized. Fourth, by multivariate analysis we were able to control for potential confounders including ventilatory setting, and severity of illness. Fifth, the LUNG SAFE study showed that most ARDS patients are not ventilated using a protective ventilation approach.2 We reached ventilatory goals on day 3, which indicates an improvement in quality of ventilatory management according to current recommendations.18,29
There are several limitations of our study. First, this is an observational study from a single center, thus results may not be representative of other institutions or regions. Second, a mixed cohort of surgical and nonsurgical patients with several clinical and pathophysiological disorders were analyzed, which could have effects on outcomes. Indeed, hospital mortality rate was higher than reported in recent epidemiologic studies.2 Since our hospital is a national referral center, case-mix of patients with more severe diseases were more likely included compared with community-based hospitals. Compared with patients enrolled in the LUNG SAFE study,2 our analyzed patients had more chronic diseases including immunoincompetence, cardiovascular disease, and cancer, and had a higher rate of extrapulmonary sepsis and noncardiogenic shock, all which may explain the higher mortality rate observed in the study. Third, trauma patients were not included in the study so results cannot be applied to this type of patients. Fourth, several phenotypes and subphenotypes have been recognized in ARDS patients with impact on outcomes.35,36 In the present study, analyses were not stratified according to ARDS phenotypes; consequently, further studies are required to define the effects of the interaction or association of P/FPE index-based ARDS severity and ARDS phenotypes, as well as its clinical implications, which opens a new agenda of work for future researches. Finally, we explored ARDS severity within the first 72h after starting IMV, and the clinical course of patients beyond this period of time may affect outcomes.
In conclusion, assessment of ARDS severity based on P/FPE index is better than current PaO2/FiO2 criteria for predicting mortality, especially on third day after starting IMV. P/FPE index is easy to use at the bedside by involving information of the two therapeutic strategies used for managing hypoxemia such as FiO2 and PEEP. We recommend further clinical trials to clarify the advantages of ARDS severity classification based on P/FPE index for clinical decision-making.
Author's contributionsFDMB contributed in the concepts, design, definition of intellectual content, literature search, clinical studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review.
REM and VOR contributed in the design, definition of intellectual content, clinical studies, data acquisition, manuscript editing, and manuscript review.
TTS contributed in the literature search, manuscript preparation, manuscript editing, and manuscript review.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that they have no competing interest.