To assess the psychometric properties of the behavioral indicators of pain scale (ESCID) when applied to a wide range of medical and surgical critical patients.
DesignA multicentre, prospective observational study was designed to validate a scale measuring instrument.
SettingTwenty Intensive Care Units of 14 hospitals belonging to the Spanish National Health System.
ParticipantsA total of 286 mechanically ventilated, unable to self-report critically ill medical and surgical adult patients.
ProcedurePain levels were measured by two independent evaluators simultaneously, using two scales: ESCID and the behavioral pain scale (BPS). Pain was observed before, during, and after two painful procedures (turning, tracheal suctioning) and one non-painful procedure.
Main variablesESCID reliability was measured on the basis of internal consistency using the Cronbach-α coefficient. Inter-rater and intra-rater agreement were measured. The Spearman correlation coefficient was used to assess the correlation between ESCID and BPS.
ResultsA total of 4386 observations were made in 286 patients (62% medical and 38% surgical). High correlation was found between ESCID and BPS (r=0.94–0.99; p<0.001), together with high intra-rater and inter-rater concordance. ESCID was internally reliable, with a Cronbach-α value of 0.85 (95%CI 0.81–0.88). Cronbach-α coefficients for ESCID domains were high: facial expression 0.87 (95%CI 0.84–0.89), calmness 0.84 (95%CI 0.81–0.87), muscle tone 0.80 (95%CI 0.75–0.84), compliance with mechanical ventilation 0.70 (95%CI 0.63–0.75) and consolability 0.85 (95%CI 0.81–0.88).
ConclusionESCID is valid and reliable for measuring pain in mechanically ventilated unable to self-report medical and surgical critical care patients.
Clinicaltrials.govEvaluar las propiedades psicométricas de la Escala de Conductas Indicadoras de Dolor (ESCID), aplicada a una muestra amplia de pacientes críticos de patología médica y posquirúrgica.
DiseñoEstudio multicéntrico, observacional, prospectivo de validación de una escala como instrumento de medida.
ÁmbitoVeinte Unidades de Cuidados Intensivos de 14 hospitales del Sistema Nacional de Salud español.
ParticipantesDoscientos ochenta y seis pacientes críticos adultos, sometidos a ventilación mecánica, sin capacidad de comunicación, de patología médica y posquirúrgica.
IntervenciónSe midió el nivel de dolor de los pacientes por 2 observadores de manera simultánea y utilizando dos escalas: ESCID y la Behavoiral Pain Scale. El dolor fue medido antes, durante y después de la aplicación de dos procedimientos dolorosos (movilización y aspiración endotraqueal) y un procedimiento no doloroso.
Variables de interésLa fiabilidad de ESCID se midió mediante la consistencia interna determinada con el coeficiente alfa de Cronbach. Se midió la concordancia inter- e intraobservadores. Se determinó la correlación entre las escalas ESCID y Behavoiral Pain Scale mediante el coeficiente de Spearman.
ResultadosSe realizaron 4.386 observaciones de dolor en 286 pacientes (62% patología médica y 38% posquirúrgica). Se evidencia una alta correlación entre ESCID y Behavoiral Pain Scale (r=0,94-0,99; p<0,001) así como una alta concordancia inter- e intraobservador. La escala ESCID presenta buena consistencia interna, con un valor de α-Cronbach de 0,85 (IC 95% 0,81-0,88). Los 5 dominios de ESCID presentan alta consistencia interna con α-Cronbach: musculatura facial 0,87 (IC 95% 0,84-0,89), tranquilidad 0,84 (IC 95% 0,81-0,87), tono muscular 0,80 (IC 95% 0,75–0,84), adaptación a ventilación mecánica 0,70 (IC 95% 0,63–0,75) y confortabilidad 0,85 (IC 95% 0,81–0,88).
ConclusiónESCID es válida y fiable para medir el dolor en pacientes críticos médicos y posquirúrgicos, no comunicativos y sometidos a ventilación mecánica.
Clinicaltrials govThe incidence of pain in adult critical care patients is higher than 50% and it can be experienced at rest or during routine clinical care procedures1–5. Inadequate management of pain is associated with a stress response that includes changes in physiological parameters, neuro-endocrine secretion and immunomodulation6,7. It is also associated with agitation8 and sleep pattern alterations9, and it can be a contributing risk factor for delirium10 and post-traumatic stress disorder in critical care patients11.
The systematic evaluation of pain integrated in the pain-agitation-delirium (PAD) management protocols has been associated with reductions in the incidence of pain, use of analgesics, duration of mechanical ventilation (MV) and length of stay (LOS) in the Intensive Care Unit (ICU) 1,12–15. Adequate pain treatment necessarily requires the use of reliable tools that aid in its detection and measurement16,17.
Recent international guidelines recommend the use of scales based on behavioral indicators of pain for patients who are unable to self-report, provided that their motor function is preserved and behaviors are observable18,19. For these patients, the behavioral pain scale (BPS)20 and the critical care pain observation tool (CPOT)21,22 are recommended due to their psychometric properties17,23.
In 2011, Latorre et al.24 validated a new tool, the behavioral indicators of pain scale or ESCID from its acronym in Spanish, as a useful tool for assessing pain in mechanically ventilated and unable to self-report critically ill adult patients. The ESCID scale was developed after the original Campbell scale, proposed by The Analgesia and Sedation Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units, taking in to account that the Campbell scale could be an appropriate instrument to assess pain and quantify its intensity in non-communicative, critically ill patients25. However, the Campbell scale has two limitations: it is not been subjected to validation studies in critically ill patients and it is not applicable to patients with mechanical ventilation because it does not include it as an indicator. In the validated version of the ESCID scale, the indicator ‘vocalization’ from the original Campbell scale was replaced by ‘compliance with mechanical ventilation’. Additionally, each indicator of the scale was more precisely defined and quantified to minimize subjectivity of the observer. The ESCID scale provides a greater number of behavioral indicators, which can help decrease the artifacts produced by causes unrelated to pain, considering the positive correlation between the number of behavioral indicators exhibited by the patient and pain shown26.
The ESCID scale has shown good psychometric properties: the internal consistency measured with the Cronbach's Alpha Coefficient was 0.70–0.80; a high correlation between ESCID and BPS scales; and good interrater and intrarater reliability. However, the ESCID scale validation study has some limitations that affect its external validity, as is the fact that it was conducted in a single ICU with a sample of 42 critical care patients with exclusively medical pathologies24.
The interest of the current study is to determine the psychometric properties of the ESCID scale when applied to a wide range of medical and surgical critical care patients in various ICUs of different hospitals27. Our primary hypothesis is that the ESCID scale is a valid and reliable tool for measuring pain in mechanically ventilated and unable to self-report critical care patients.
This study pretends to validate the ESCID scale by addressing the following research objectives: (a) to assess interrater agreement when using pain scales; (b) to assess intrarater agreement when using pain scales in repeated measures for the same patient; (c) to examine the correlation between the ESCID scale and the BPS while using them simultaneously in the same patient and at the same time, when patients are exposed to a non-nociceptive procedure and two nociceptive procedures; (d) to examine the relationship between applying a painful routine care procedure and the changes in physiological indicators of pain; (e) to evaluate the possible differences in the application of the scales for measuring pain in patients with medical and surgical pathologies; and (f) to evaluate the possible differences in the application of scales to measure pain in patients with different levels of sedation.
Patients and methodsMulticenter, prospective and observational study to validate a scale-measuring instrument was conducted in 20 Intensive Care Units of 14 Spanish National Health System hospitals with different types of patients admitted for medical and surgical pathologies. The study was initiated in January 2012 and finalized in January 201427. The protocol was approved by the Clinical Investigation Ethics Committee of Puerta de Hierro Majadahonda University Hospital (Spain), the coordinating center (University Hospital Ethics Committee, record n° 273, January 23, 2012), and all corresponding Ethics Committees in each hospital participating in the study.
Written informed consent was obtained from the next of kin or the patient's legal guardian, and was ratified afterwards by the patient itself. To maintain anonymity and guarantee confidentiality, all participants were identified by a code number that was maintained throughout the study.
Study populationInclusion criteria were: age ≥18 years; receiving MV; showing no motor or verbal communication; ability to understand the Spanish language; and the presence of a legal guardian with the authority to give consent to the patient's participation in the study if the patient was unable to do so.
Exclusion criteria were: quadriplegia; severe polyneuropathy; treatment with neuromuscular blocking agents; neurological disease that resulted in a score <4 in the motor section of the Glasgow Coma Scale (GCS); and the suspicion or presence of delirium assessed using the Confusion Assessment Method for the ICU. All these conditions resulted in study exclusion as they had the potential to alter a patient's behavioral responses to pain.
ProtocolA group of nurses from each of the ICU were responsible for collecting data. All the ICU nurses implicated in the study received a 90min debrief where the principal investigator discussed the recruitment of patients, the Informed Consent form, the study procedures, correct use of the ESCID and BPS scales27, and the data collection.
Prior to commencing the study, a pilot trial was carried out in each ICU (5 patients per unit) to detect any possible difficulties that might arise so that they could be remedied beforehand.
Pain was assessed using two scales, BPS20 and ESCID (Table 1), simultaneously applied by two independent observers who were blinded to each other's assessments. Observations coincided with the application of two routine care procedures previously documented as painful26: turning/repositioning and endotracheal suctioning. Furthermore, to achieve greater consistency and establish a comparative element, as proposed in other studies20,28,29, the same pain assessment was carried out by applying a non-painful procedure that consisted on gently rubbing a gauze cloth over a portion of the patient's healthy skin tissue.
The behavioral indicators of pain scale (ESCID).
| Behavioral indicators of pain scale (ESCID) | |
| Number of observation domains | 5 |
| Number of descriptors per domain | 3 |
| Rated per descriptor | 0–2 |
| Total score | 0–10 |
| Facial expression | Score |
| Relaxed | 0 |
| Tense, frowning/grimacing | 1 |
| Regularly frowning/clenched jaw | 2 |
| Calmness | |
| Calm, relaxed, normal movements | 0 |
| Occasional restless movement, shifting position | 1 |
| Frequent movement, including head or limbs | 2 |
| Muscle tone | |
| Normal | 0 |
| Increased. Flexion of fingers and/or toes | 1 |
| Rigid | 2 |
| Compliance with mechanical ventilation (MV) | |
| Tolerates MV | 0 |
| Coughs, however, tolerates MV | 1 |
| Fights with the respirator | 2 |
| Consolability | |
| Comfortable, quiet | 0 |
| Reassured by touch or talk. Distractible | 1 |
| Difficult to comfort by touch or talking | 2 |
To avoid any possible bias, pain measurement was performed once for each patient and procedure. All the patients were free of physical restraints during the pain assessments, in order to avoid bias in the evaluation of the body movements as behaviors of pain.
Pain assessment was carried out at 3 time points as follows:
- •
First measurement (at rest): 5min before starting the procedure.
- •
Second measurement: during the procedure (turning/repositioning and non-painful procedure) or 10–30s after completing the procedure (endotracheal suctioning).
- •
Third measurement: 15min after the procedure was completed.
The following independent variables were obtained from each participant through continuous hemodynamic monitoring, the patient's clinical history and direct observation:
- •
Demographic data: age, gender.
- •
Clinical data: heart rate, invasive blood pressure, and respiratory rate. Sedation and analgesic medication administered during the previous 24h by both continuous infusion and bolus were recorded as well as the scores from the Richmond sedation agitation scale (RASS). For patients with neurological disorders, the GCS was applied before the procedure. In addition, the following clinical data were collected: current pathology, reason for ICU admission, medical history (comorbidities, history of chronic pain, chronic use of analgesics) and severity index determined by simplified acute physiology score (SAPS II).
The ESCID and BPS scales were used to measure the pain level as dependent variables.
Statistical analysisThe sample size was calculated by assuming several criteria: (1) taking into account that multivariable techniques, such as factor analysis, usually require at least 10 participants per variable to achieve replicable findings30; (2) given the heterogeneity of critical care medical and surgical patients, the aim was to ensure sufficient participation to obtain a representative sample; (3) to guarantee the accuracy of the measurements by reducing any possible bias; and (4) more than 50 participants as a quality criterion for testing scale: construct validity17.
Measurement of psychometric propertiesPsychometric properties related to the use of the pain assessment tools were determined using the recommendations made by Gélinas et al.17, which proposed the use of established scales as published in recent guidelines18,19.
Internal consistencyESCID reliability was measured by the internal consistency of each item using Cronbach's Alpha Coefficient. A Cronbach-α value higher than 0.7 reflects a satisfactory internal consistency, which is a high inter-relation between each domain of the tool. For greater consistency, the calculation considered the mean of the assessments of each item at the three time points in which the scale was used (before, during and after the procedure).
Interrater and intrarater reliabilityRepeated measures analysis of variance (ANOVA) was used to analyze interrater and intrarater agreement through comparing the components of the ESCID and BPS scales, to measure the changes between the results obtained according to the time, observer and procedure.
Discriminant validationDiscriminant validation was determined by comparing total scores obtained during different situations and stimuli, i.e., at rest and during a procedure (turning/repositioning, endotracheal suctioning or non-painful procedure). The main effects of the three original variables (procedure, time and observer) were compared as were the three first-order interactions (time×procedure, time×observer and procedure×observer).
Convergent validationConvergent validation is another strategy for verifying reliability and validity of the use of pain assessment tools. Refers to the ability of the assessment tool to correlate with another tool, ideally using a different method, measuring the same construct (i.e., pain). The BPS is considered one of the most valid and reliable scales available to evaluate pain in non-communicative ICU patients (Cronbach-α 0.63–0.72, Kappa coefficients 0.67–0.83)17,18.
Spearman's correlation coefficient was used to evaluate the correlation between the ESCID and BPS scales in terms of the measurements taken before, during and after each procedure.
Presentation of dataQuantitative data are shown as frequencies, percentages, means, medians, standard deviations and 25th and 75th percentiles (p25 and p75). Inference using the t-test and Spearman's correlation were applied to explore the association between the level of pain and the independent variables. Analysis of variance (ANOVA) was used to compare the means of more than two groups. All the multiple comparison tests were adjusted by means of Bonferroni correction. A p value of <0.05 was considered statistically significant.
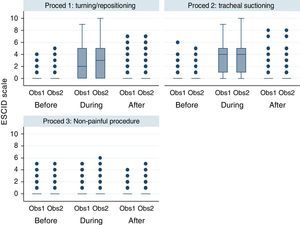
Graphic representation of the pain variable according to the ESCID scale (Fig. 2) is shown using a box-plot approach. Each box shows p25, median and p75. Each whisker covers values 1.5 times the interquartile range and the circles represent the extreme values (outliers).
Data were analyzed using SPSS software (v.19.0. SPSS Inc. Chicago, IL, USA).
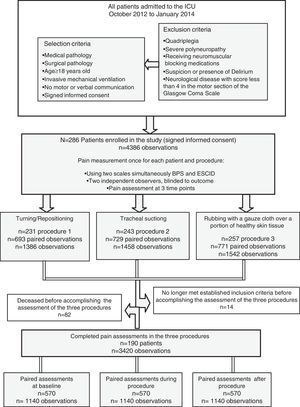
ResultsTwo hundred eighty-six patients from 20 ICUs were included during the recruitment period. During the study period, 4386 observations were recorded by 150 observers. A consort flow chart of patient enrolment is shown in Fig. 1. The patients’ characteristics are shown in Table 2.
Demographic and medical characteristics of the patients included for analysis (n=190).
| Age (years), median [25th to 75th percentiles] | 61 [50–73] |
| Sex (F/M) n (%) | 55 (29)/135 (71) |
| Type of admission to the ICU (medical/surgical) n (%) | 120 (63)/70 (37) |
| Chronic pain syndrome, n (%) | 13 (7) |
| Time of chronic pain (months), median [25th to 75th percentiles] | 12 [9–24] |
| Time of analgesics (months), median [25th to 75th percentiles] | 12 [12–24] |
| Reason for admission to the ICU | |
| Respiratory, n (%) | 44 (23) |
| Cardiac surgery, n (%) | 40 (21) |
| Trauma, n (%) | 25 (13) |
| Gastrointestinal, abdominal surgery, n (%) | 25 (13) |
| Neuro/neurosurgery, n (%) | 17 (9) |
| Infection/sepsis, n (%) | 17 (9) |
| Cardiac, n (%) | 13 (7) |
| Miscellaneousa, n (%) | 9 (5) |
| Time between admission to ICU and enrolment (days), median [25th to 75th percentiles] | 3 [1–7] |
| SAPS II score within 24 h after admission to ICU, median [25th to 75th percentiles] | 44 [34–57] |
| Mechanical ventilation upon enrolment, n (%) | 190 (100) |
| For procedure (n=570) | |
| Sedation (continuous intravenous infusion), n (%) | 485 (85) |
| Sedation (intravenous boluses), n (%) | 27 (5) |
| Analgesia (continuous intravenous infusion), n (%) | 450 (79) |
| Analgesia (intravenous boluses), n (%) | 51 (9) |
| RASS level, median [25th to 75th percentiles] | −4 [−5; −2] |
| RASS level >−4, n (%) | 256 (45) |
| RASS level −4, n (%) | 160 (28) |
| RASS level −5, n (%) | 154 (27) |
Finally, 190 patients completed the pain assessments in all of three procedures proposed in the study, resulting in 3420 observations being recorded and included in the analysis (Fig. 1). These patients had similar characteristics to those defined for the study population (Table 2).
Internal consistencyESCID presented a high reliability with a Cronbach-α coefficient of 0.85 [95% confidence interval (CI), 0.81–0.88]. The facial expression [0.87 (95%CI 0.84–0.89)], calmness [0.84 (95%CI 0.81–0.87)], muscle tone [0.80 (95%CI 0.75–0.84)] and consolability [0.85 (95%CI 0.81–0.88)] domains presented a high internal consistency with a Cronbach-α coefficients ≥0.80. The compliance with mechanical ventilation domain presented good internal consistency [0.70 (95%CI 0.63–0.75)].
On evaluating the reliability of the ESCID scale with respect to the type of ICU admittance, it was observed that the internal consistency of the scale was greater in medical patients: the Cronbach-α coefficient was 0.85 (95%CI 0.81–0.89) for medical patients and 0.80 (95%CI 0.73–0.86) for surgical patients.
When evaluating the reliability of the ESCID scale with respect to the sedation level (measured by RASS), it was observed that the internal consistency of the scale was reduced in patients with the highest level of sedation (RASS score −5, unarousable patients) with a Cronbach-α coefficient of 0.63 (95%CI 0.55–0.71).
Interrater and intrarater reliabilityThere was a high interrater and intrarater conformity when the ESCID scale was applied at the three moments of each procedure (Fig. 2). There were no statistically significant intrarater differences (p=0.241), however differences were observed between procedures (p<0.001) given that the values were similar for painful procedure but differed for non-painful ones. The values show evaluation of increased pain during the application of painful procedures which normalized in the measurement taken 15min after. However, there were no changes in the pain levels at the three moments during non-painful procedures.
Pain measured with ESCID scale in mechanically ventilated, unable to self-report critically ill patients, by two independent observers before, during and after three different procedures. This figure shows the median scores of the ESCID scale evaluated by two observers according to different situations: before, during and after turning/repositioning, tracheal suctioning and non-painful procedure (rub with a gauze cloth over a portion of healthy skin tissue of the patient). The numbers show a significant increasing of the level of pain during the two painful procedures, that normalized in the assessment after 15min finishing this procedure. However, there were no modifications in the pain level in the three assessment times during the non-painful procedure. There is a good inter-observer concordance. The points are outliers or values outside these limits. Note: Proced: procedure; Obs: observer.
The median punctuation of the ESCID and BPS scales (Table 3) demonstrate a difference of 0.0 or 0.1 points between the two observers at the three measuring time points. These results demonstrate a high intrarater and interrater conformity.
Analysis of variance for repeated measures (ANOVA) for BPS and ESCID.
| Turning/repositioning | Tracheal suctioning | Non-painful procedure | |
|---|---|---|---|
| n=190 | n=190 | n=190 | |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| BPS scalea | |||
| Before | |||
| Observer 1 | 3.1 (0.4) | 3.2 (0.5) | 3.2 (0.5) |
| Observer 2 | 3.2 (0.5) | 3.2 (0.5) | 3.2 (0.6) |
| During procedure | |||
| Observer 1 | 4.7 (1.6) | 5.4 (1.8) | 3.2 (0.6) |
| Observer 2 | 4.8 (1.6) | 5.3 (1.8) | 3.3 (0.7) |
| After | |||
| Observer 1 | 3.2 (0.7) | 3.2 (0.5) | 3.1 (0.4) |
| Observer 2 | 3.3 (0.7) | 3.2 (0.5) | 3.2 (0.5) |
| ESCID scalea | |||
| Before | |||
| Observer 1 | 0.2 (0.7) | 0.3 (0.8) | 0.2 (0.7) |
| Observer 2 | 0.3 (0.8) | 0.3 (0.8) | 0.3 (0.9) |
| During procedure | |||
| Observer 1 | 2.6 (2.5) | 3.4 (2.5) | 0.3 (0.9) |
| Observer 2 | 2.7 (2.4) | 3.3 (2.4) | 0.4 (1.0) |
| After | |||
| Observer 1 | 0.3 (1.0) | 0.3 (0.8) | 0.2 (0.6) |
| Observer 2 | 0.4 (1.1) | 0.3 (0.8) | 0.3 (0.8) |
The response to pain in BPS and ESCID changes over time, increasing during the procedure (p<0.001). There are differences between painful and non-painful procedures (p<0.001). There are not inter-observer differences. There is no significative effect of the interaction neither among time and observer (p=0.720) nor procedure and observer (p=0.083). SD: standard deviation.
The response to pain as measured by the ESCID and BPS scales differs over time (p<0.001). When performing Bonferroni's multiple comparison test, it can be seen that this difference is due to increased mean pain scores during the painful procedures with respect to the measurements taken before and after, with no statistically significantly differences between the latter two. On the other hand, there were no variations during the non-painful procedure (Table 3, Fig. 2) which establishes that there were differences in the procedure factor (p<0.001), but no differences in the observer factor (p>0.5).
Convergent validationThere was a good correlation between the ESCID and BPS scales when applied at the three moments during each procedure. Spearman's correlation between the ESCID and BPS scales was r=0.94–0.99 (p<0.001). Taking time changes into consideration, Spearman's correlation between the two scales prior to the procedure was r=0.96, during r=0.95, and after r=0.96.
Other relevant resultsThere were no statistically significant differences between pain measured with the ESCID and BPS scales and the type of patient (medical or surgical) during the three procedures at the three moments.
There were no statistically significant differences between the level of sedation (RASS >−4, −4 and −5) and patient type (medical or surgical).
With respect to physiological indicators (Table 4), it was observed that the parameters remained stable with a slight variation during the application of painful procedures, which normalized with respect to measurements taken at rest. These changes were not observed during non-painful procedures.
Description of the changes in physiological indicators before, during and after the procedures.
| Turning/repositioning | Tracheal suctioning | Non-painful procedure | |
|---|---|---|---|
| n=190 | n=190 | n=190 | |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Before | |||
| SAP (mmHg) | 125 (21) | 126 (20) | 120 (21) |
| DAP (mmHg) | 60 (12) | 61 (12) | 58 (11) |
| HR (beats per minute) | 84 (18) | 86 (20) | 84 (19) |
| RR (breaths per minute) | 19 (5) | 19 (5) | 18 (5) |
| During | |||
| SAP (mmHg) | 132 (25) | 133 (25) | 123 (19) |
| DAP (mmHg) | 65 (15) | 65 (14) | 58 (11) |
| HR (beats per minute) | 88 (19) | 90 (20) | 85 (19) |
| RR (breaths per minute) | 21 (7) | 23 (8) | 18 (5) |
| After | |||
| SAP (mmHg) | 125 (21) | 125 (21) | 122 (18) |
| DAP (mmHg) | 60 (12) | 60 (11) | 58 (11) |
| HR (beats per minute) | 85 (18) | 86 (19) | 84 (19) |
| RR (breaths per minute) | 18 (5) | 19 (5) | 19 (7) |
SAP: systolic arterial pressure; DAP: diastolic arterial pressure; HR: heart rate; RR: respiratory rate; SD: standard deviation.
The main finding of this study is that the ESCID scale has good psychometric properties. Considering the results obtained in relation to the internal consistency, interrater and intrarater reliability as well as the discriminant validation of the ESCID scale, it can be concluded that this tool is a valid and reliable tool for measuring pain in unable to self-report mechanically ventilated critically ill patients. These results are consistent with those reported in the first validation of the ESCID scale performed with a small sample of patients in a single medical ICU24.
The psychometric properties of the ESCID scale are similar to those shown by the BPS20,23,28,29 and the CPOT21–23 scales, which is why the use of such behavioral scales has been recommended in recent guidelines18,19. The internal consistency of the ESCID scale had a Cronbach-α coefficient of 0.85, similar to other studies such as that of Chanques et al.23, which reported a Cronbach-α coefficient of 0.80 for the BPS and 0.81 for the CPOT. On the other hand, the ESCID scale demonstrated a high internal consistency in its five domains with Cronbach-α coefficients of 0.70–0.87.
In both painful and painless procedures, ESCID shows a high degree of correlation with the BPS, the tool of reference which has been used in different studies1,20,23,28,29, in addition to demonstrating a high degree of interrater and intrarater conformity in the measurements performed.
The ESCID scale has two additional domains with respect to the BPS and is also innovative with respect to other scales, such as the BPS and the CPOT16,17,23, with the introduction of the “consolability” domain which reflects the patient's reaction to interaction with the observer through verbal and/or tactile stimuli. The “Consolability” domain has proven useful and reliable in other pain behavioral scales, such as FLACC in pediatric patients31 and PAINAD in patients with advanced dementia32, but has not previously been tested in scales to measure pain in critically ill patients.
The fact that the “consolability” domain had a high Cronbach-α coefficient reinforces the internal consistency of the ESCID scale, especially knowing that 150 different observers participated in the study with high interrater and intrarater concordance.
To avoid bias, as in several previous studies28,29, a single measurement was performed per patient and procedure. In addition, all observations were made by 2 observers who were blinded to each other's evaluation.
The reliability of the ESCID scale according to the type of ICU admission was high in both cases, although Cronbach-α coefficient was better in patients with medical compared to surgical pathologies.
The internal consistency of the ESCID scale displayed a significant decrease in very profoundly sedated patients (RASS score -5) with a Cronbach-α coefficient of 0.63, due to bias or abolition of behavioral indicators, therefore its use may be limited in these patients. This finding was consistent with the literature where other studies have established the existence of some limitations in the use of the BPS and the CPOT, which make them inapplicable in cases of deep sedation, neuromuscular block, tetraplegia or polyneuropathy. This concurs with the guidelines published by Barr et al. 18, which established that behavioral scales should be used in patients whose motor functions are intact and in whom behavioral indicators are observable.
The ESCID scale is the only scale which has been subjected to a psychometric validation process in the Spanish language. This is relevant because it increases the possibility of integrating it into PAD management protocols when implementing these in Spanish-speaking countries. The use of a tool which is validated and adapted to the language is crucial18 because this can produce improvements in the outcomes of critical care patients1,3,12–15.
The nurses responsible for data collection were previously trained in the use of the ESCID and BPS scales. While some reliability studies have shown lower interrater conformity values in the use of the BPS, in which the subjective interpretation of the included items has been established as its main limitation17,33, prior knowledge of behavioral scale use could promote correct application23, as demonstrated in the good interrater conformity obtained using the ESCID and BPS scales in the present study. The ESCID scale also features a user guide that furthers the knowledge and application of this scale aiming to minimize observer bias27.
As in previous studies, the presence of procedural pain is evident. Major studies, such as the Thunder Project II26 and the Europain5, demonstrated the presence of pain during turning/repositioning and tracheal suctioning procedures. In this study, the patients presented increased pain scores when the procedures were carried out: 1.6 (BPS) and 2.4 (ESCID) during turning/repositioning, and 2.2 (BPS) and 3.1 (ESCID) during tracheal suctioning.
This result could justify the conclusion that the analgesia used prior to performing such procedures was insufficient. In fact, in our study, only 9% of patients received a bolus of analgesia prior to painful procedures. It is important to establish pre-emptive analgesia34–36 for improved pain control and, consequently, decrease adverse events related to pain and increase the adequate administration of analgesics and sedatives37.
An effort is required to implement validated tools for the detection of pain, which are currently underused38–40, to avoid pain going undetected and untreated as has been demonstrated in some studies3,41,42.
In our sample, no significant differences were observed between the level of pain and the type of admission (medical or surgical). Similarly, no significant differences were found between the level of sedation and the type of admission. This confirms the findings of studies into procedural pain in both medical and surgical patients, regardless of the reason for admittance2,3,5,26.
This study has several limitations, such as the exclusion of patients with suspicion or diagnosis of delirium. Moreover, the diagnosis of delirium is not possible in deeply sedated patients (RASS score −4 or −5), in whom the CAM-ICU tool is not applicable, so it is impossible to determine whether these patients had delirium during the measurements of pain. Although the presence of delirium was considerer an exclusion criteria, delirium screening was not routinely done in all of the patients so the possibility exists that delirious patients might have been included. Consequently, subsequent studies should be performed to determine the influence of delirium on the ESCID scale domains.
The ESCID scale has been validated in the Spanish language in non-communicative medical and surgical patients. Using the ESCID scale in other ICU patient populations and translating the same into foreign languages will require further validation testing.
ConclusionsThe ESCID scale offers good psychometric characteristics for pain assessment in non-communicative, mechanically ventilated critical care patients. As shown in our study, which included a large sample of patients with different types of admission, the ESCID scale is a valid, reliable and reproducible tool, and its integration into PAD management protocols could help to prevent significant adverse events resulting from poor pain management.
Pain monitoring should be performed routinely, especially in potentially painful procedures that are performed on critical care patients in routine clinical practice.
Funding disclosuresThis study was financed by the Spanish Health Research Fund (PI11/00766, Health Ministry. Spanish National Research, Development and Innovation Plan 2008–2001. Spanish title: “Estudio multicéntrico de validez y fiabilidad de la Escala de Conductas Indicadoras de Dolor ESCID para medir el dolor en pacientes críticos, no comunicativos y sometidos a ventilación mecánica”) after a peer-reviewed funding process. Also co-financed by FEDER Fund and Economy and Competitiveness Ministry.
Author's contributionsI.L., M.S., M.A., L.H., study concept and design. I.L., M.S., data analysis and interpretation. I.L., M.S., M.A., L.H., manuscript preparation and drafting. I.L., M.S., statistical methods, statistical data analysis. C.L., M.M.S., M.W., J.d.P., G.R., R.d.B., M.J.F., C.G., J.d.F., C.C., acquisition of the data and manuscript critique and review. All authors approved the manuscript submitted.
Conflicts of interestThe authors declare that they have no conflict of interest.
The authors want to express a special recognition to the nurses who have participated in the data collection of this study. The authors want also to thank to patients and relatives who participated in the study, without whom the study could not be possible.
The authors would like to express the collaboration of Isabel Millán and Ana Royuela, Biostadisticians of the Instituto de Investigación Puerta de Hierro Majadahonda (Madrid).
In addition to the authors of this paper, the ESCID research group includes the following investigators: Arias S, Cuenca M, Molano M, Zaragoza I, De la Vera E, Lospitao S, Sabell S, Samper M, Bajo R, Merchán R, Roqueiro MJ, Prieto MJ, Bonilla G and Castillo AM.