Adopting a unique Spanish perspective, this study aims to assess healthcare resource utilization (HCRU) and the costs of treating nosocomial pneumonia (NP) produced by methicillin-resistant Staphylococcus aureus (MRSA) in hospitalized adults using linezolid or vancomycin. An evaluation is also made of the renal failure rate and related economic outcomes between study groups.
DesignAn economic post hoc evaluation of a randomized, double-blind, multicenter phase 4 study was carried out.
ScopeNosocomial pneumonia due to MRSA in hospitalized adults.
ParticipantsThe modified intent to treat (mITT) population comprised 224 linezolid- and 224 vancomycin-treated patients.
InterventionsCosts and HCRU were evaluated between patients administered either linezolid or vancomycin, and between patients who developed renal failure and those who did not.
Primary endpointsAnalysis of HCRU outcomes and costs.
ResultsTotal costs were similar between the linezolid- (€17,782±€9,615) and vancomycin-treated patients (€17,423±€9,460) (P=.69). The renal failure rate was significantly lower in the linezolid-treated patients (4% vs. 15%; P<.001). The total costs tended to be higher in patients who developed renal failure (€19,626±€10,840 vs. €17,388±€9,369; P=.14). Among the patients who developed renal failure, HCRU (days on mechanical ventilation: 13.2±10.7 vs. 7.6±3.6 days; P=.21; ICU stay: 14.4±10.5 vs. 9.9±6.6 days; P=.30; hospital stay: 19.5±9.5 vs. 16.1±11.0 days; P=.26) and cost (€17,219±€8,792 vs. €20,263±€11,350; P=.51) tended to be lower in the linezolid- vs. vancomycin-treated patients. There were no statistically significant differences in costs per patient-day between cohorts after correcting for mortality (€1000 vs. €1,010; P=.98).
ConclusionsFrom a Spanish perspective, there were no statistically significant differences in total costs between the linezolid and vancomycin pneumonia cohorts. The drug cost corresponding to linezolid was partially offset by fewer renal failure adverse events.
Analizar la utilización de recursos sanitarios (URS) y los costes de la neumonía nosocomial por Staphylococcus aureus resistente a meticilina en adultos hospitalizados tratados con linezolid o vancomicina. También se evaluó el porcentaje de fallo renal entre dichos pacientes.
DiseñoAnálisis post-hoc de un ensayo clínico fase iv multicéntrico, aleatorizado, doble ciego.
ÁmbitoPacientes adultos, hospitalizados con neumonía nosocomial por Staphylococcus aureus resistente a meticilina.
ParticipantesPacientes tratados con linezolid (224) o vancomicina (224).
IntervencionesDesde la perspectiva española se compararon costes y URS entre pacientes tratados con linezolid o vancomicina y entre los que desarrollaron fallo renal y los que no.
Principales variables de interésAnálisis de costes y URS.
ResultadosLos costes totales fueron similares (p=0,69) en los pacientes tratados con linezolid (17.782±9.615€) o vancomicina (17.423±9.460€). La tasa de fallo renal fue significativamente menor en los tratados con linezolid (4 vs. 15%, p<0,001). Los costes totales fueron mayores en aquellos que desarrollaron fallo renal (19.626±10.840€ vs. 17.388±9.369€, p=0,14). La URS (días de ventilación mecánica: 13,2±10,7 vs. 7,6±3,6, p=0,21; días en UCI: 14,4±10,5 vs. 9,9±6,6, p=0,30; días de hospitalización: 19,5±9,5 vs. 16,1±11,0, p=0,26) y los costes totales (17.219±8.792€ vs. 20.263±11.350€, p=0,51) tendieron a ser inferiores en los pacientes tratados con linezolid que desarrollan fallo renal. Tras corregir el análisis por mortalidad, los costes diarios por paciente fueron similares (1.000 vs. 1.010€; p=0,98).
ConclusionesDesde la perspectiva española, no hubo diferencias en la URS y los costes entre los pacientes con neumonía tratados con linezolid o vancomicina. El coste de linezolid fue contrarrestado por la menor incidencia de fallo renal.
Methicillin-resistant Staphylococcus aureus (MRSA) is a global problem and a common cause of pneumonia in hospitalized patients.1,2 Vancomycin is widely used for treating patients with serious MRSA infections, including hospital-acquired or healthcare-associated pneumonia.3 Linezolid (oxazolidinone) is an alternative treatment option for nosocomial pneumonia (NP) caused by MRSA.3,4 Results from a recently completed phase 4, multicenter, double-blind, randomized clinical trial enrolling patients with MRSA NP5 show that clinical success at end-of-study (EOS) is higher in patients treated with intravenous (IV) linezolid (57.6%) compared to dose-optimized IV vancomycin (46.6%; P=.042; per protocol population).6
In Spain, more than 20% of invasive S. aureus isolates were resistant to methicillin in the year 2012. Results were similar in other European countries, with the majority reporting methicillin resistance in more than 10% and 11 countries reporting methicillin resistance in more than 20% of S. aureus isolates.7 Results from the Extended Prevalence of Infection in the intensive care unit (ICU) (EPIC II) study suggest that MRSA prevalence is even higher in European Intensive Care Units. In this study, the most commonly isolated organism from European ICUs was S. aureus (20.5%) where 49.4% of S. aureus isolates were methicillin resistant.8 Patients with infections caused by resistant compared to nonresistant bacteria have increased mortality, hospital length of stay (LOS), and healthcare costs.9
To our knowledge, few studies have compared the economic outcomes associated with linezolid and vancomycin treatment in patients with MRSA NP from a Spanish perspective10. Additionally, information on the economic impact of renal failure from a Spanish perspective is lacking. To address this gap, we assessed healthcare resource use (HCRU) and costs, including costs incurred due to renal failure, for treating hospitalized adult MRSA NP patients with either linezolid or vancomycin from a Spanish perspective.
MethodsThis is a secondary analysis of a randomized clinical trial of patients treated for nosocomial pneumonia with linezolid as compared to vancomycin.6 While the analysis herein uniquely applies Spanish costs to this trial data, a similar economic study has been previously reported.11 It is still unclear whether their conclusions apply to Spain, which has a different healthcare system and healthcare costing structure.
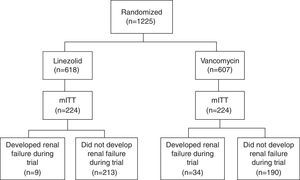
Study cohortsThe study population is composed of patients from a randomized, double-blind, Phase IV global clinical trial, which compared the survival and treatment success rate in patients receiving linezolid vs. vancomycin for NP caused by MRSA.6 The main analysis was performed among the modified intent-to-treat population (mITT), in which patients must have ≥1 doses of study treatments and a confirmed MRSA diagnosis based on a bacteria culture test. Fig. 1 (patient flow chart) shows the cohort selection process starting from the intent-to-treat (ITT) cohort to the mITT cohort, and categorization of study comparison cohorts.6 Detailed data about the study populations and study design of the clinical trial were published elsewhere.6
In addition to the main analysis cohort of mITT patients, the subgroup of patients who developed renal failure during trial follow-up was also evaluated. The criteria for identifying this subgroup of patients included patients who developed acute renal failure defined using RIFLE criteria (Risk, Failure, Injury, Loss, and End-stage kidney disease), started renal replacement therapies (RRT) during follow-up and after the day of randomization, or developed an adverse event of renal failure reported by trial investigators.12 Of note, this subgroup only included patients who developed renal failure during the trial period (until EOS) and after the day of randomization, and excluded patients receiving RRT on the day of randomization or prior to randomization.
Economic variablesHCRUIn this clinical trial, hospital bed-days (including ICU stay, step-down, general ward and long term care), days of mechanical ventilation (MV), study treatment (vancomycin and linezolid) duration, vancomycin serum level monitoring, and RRT durations were collected from randomization until EOS.
Costs from Spanish perspectiveFocusing on a Spanish perspective, unit costs (in 2012 Euros, Table 1) were obtained from the Botplus-Portalfarma database13 for the drugs, and the Oblikue database for other HCRU items (MV, hospital bed-day by type, vancomycin level monitoring and RRT).14 Costs were calculated by multiplying the number or duration of each HCRU item with its corresponding unit cost. Total costs were broken down into cost components, including bed days, MV, study drug (plus drug monitoring costs), and RRT costs. The study was conducted from the perspective of the Spanish National Health System taking into account only direct medical economic costs.
Spanish unit costsa.
| HCRU item | Description | Unit cost (€)a | Unit | Source |
|---|---|---|---|---|
| Study medications | ||||
| Linezolid | Linezolid IV (600mg per dose) | 59.61 | Per 600mg vial | Botplus-Portalfarma11 |
| Vancomycin | Vancomycin IV 15mg/kg per dose | 6.90 | Per 1000mg vial | Botplus-Portalfarma11 |
| Vancomycin level | Laboratory test for monitoring vancomycin levels | 14.55 | Per level | Oblikue database12 |
| Bed cost | ||||
| ICU | Intensive care unit | 1127.25 | Per day | Oblikue database12 |
| Step down | Step down | 851.35 | Per day | Oblikue database12 |
| General ward | General ward | 575.46 | Per day | Oblikue database12 |
| LTC | Nursing home/LTC | 156.15 | Per day | Oblikue database12 |
| Rehabilitation | Rehabilitation | 488.8 | Per day | Oblikue database12 |
| Others | ||||
| Mechanical ventilator | Mechanical ventilator | 17.4 | Per day | Oblikue database12 |
| Hemofiltration | CRRT – hemofiltration | 253.59 | Per HF day | Oblikue database12 |
| Hemodialysis | IRRT – hemodialysis | 184.88 | Per HD day | Oblikue database12 |
Abbreviations: HD: hemodialysis; HF: hemofiltration; LTC: long-term care; ICU: intensive care unit; CRRT: continuous renal replacement therapy; IRRT: intermittent renal replacement therapy.
Economic outcomes were compared within the mITT cohort, and within the subgroup of patients who developed renal failure. To compare patients’ characteristics and economic outcomes between study groups, chi-squared tests (for categorical variables) and Student's t-tests (for continuous variables) were performed whenever appropriate. Fisher's exact tests were performed when ≥1cells had expected counts of <5. Additionally, Wilcoxon Signed-Rank tests were performed to compare the economic outcomes within the subgroup of patients who developed renal failure (as one comparison group had less than 30 patients).
Within the mITT population, we compared costs/HCRU between patients who received linezolid vs. vancomycin, and between patients who developed renal failure and those who did not. Within the subgroup of patients who developed renal failure, economic outcomes were compared between treatment groups. Additionally, total costs per patient-day among those who developed renal failure were assessed between linezolid vs. vancomycin study groups.
Adjusted for survival status at EOS, length of follow-up, and region, costs were examined between treatment groups, and patients who did and did not develop renal failure using generalized linear models with gamma distribution and log link.
ResultsPatient cohortsThe primary analysis cohort (mITT) included 224 linezolid- and 224 vancomycin-treated patients (Fig. 1). Patients’ characteristics between linezolid- and vancomycin-treated patients are similar, including days from randomization till EOS. Exact details were reported in the prior US study.11
A total of 43 patients developed renal failure during the trial, including 9 linezolid- and 34 vancomycin-treated patients (10% vs. 15%, P<.001; Fig. 1). Of note, 3 out of the 405 patients who did not develop renal failure during the trial received RRT at or prior to randomization. As such, they were not included since their renal failure was prevalent rather than incident.
Patients in the mITT population had a mean age of 61.8 years and were 65.6% male and 68.6% white. Except for comorbid renal impairment (58% vs. 28%, P<.001), sepsis/septic shock (30% vs. 12%, P<.001), and diabetes (56% vs. 40%, P=.04), the majority of demographic and clinical variables were similar between patients who developed renal failure and those who did not (Table 2).
Patient characteristics between patients who developed renal failure and those who did nota.
| Variables | Non-Renal Failure (N=405) | Renal Failure (N=43) | P-value |
|---|---|---|---|
| Demographic variables | |||
| Age, mean (SD) | 61.6±18.1 | 64.1±16.8 | .38 |
| Median (range) | 66 (18, 98) | 69 (24, 86) | |
| Male, No. (%) | 266 (66%) | 28 (65%) | .94 |
| Region, No. (%) | .29 | ||
| United States | 261 (64%) | 22 (51%) | |
| Asia | 49 (12%) | 8 (19%) | |
| Europe | 40 (10%) | 4 (9%) | |
| Latin America | 38 (9%) | 7 (16%) | |
| Other | 17 (4%) | 2 (5%) | |
| Follow-up characteristics | |||
| Length since randomization until EOS in days, mean (SD) | 23.6 (10.1) | 20.3 (9.9) | .04 |
| Patients in hospital at the EOS visit, No. (%) | 159 (39%) | 16 (37%) | .79 |
| Patients died at EOS, No. (%) | 53 (13%) | 11 (25%) | .04 |
| Clinical variables | |||
| Co-morbidities, No. (%)b | |||
| Atrial fibrillation | 89 (22%) | 12 (28%) | .39 |
| Chronic obstructive pulmonary disease (COPD) | 119 (30%) | 7 (16%) | .07 |
| Diabetes | 159 (40%) | 24 (56%) | .04 |
| Peripheral vascular disorder (PVD) | 51 (13%) | 5 (12%) | .84 |
| Hyperlipidaemia | 109 (27%) | 13 (30%) | .66 |
| Hypertension | 270 (67%) | 27 (63%) | .56 |
| Renal impairment | 113 (28%) | 25 (58%) | <.001 |
| Sepsis/Septic shock | 48 (12%) | 13 (30%) | <.001 |
| APACHE II score at baseline, mean (SD) | 17.3 (6.2) | 18.8 (6.0) | .12 |
| Baseline Clinical Pulmonary Infection Score, mean (SD) | 9.4 (2.2) | 9.8 (2.0) | .37 |
| Baseline ventilated, No. (%) | 255 (63%) | 31 (72%) | .24 |
| In ICU at or prior to randomization, No. (%) | 189 (84%) | 197 (88%) | .27 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; EOS, end of study; HCRU, healthcare resource utilization; ICU, intensive care unit; mITT, modified-intent to treat; SD, standard deviation.
Patients were followed for a mean duration of 23.3±10.1 days through the EOS. The follow-up duration from randomization until EOS was shorter for patients who developed renal failure (20.3±9.9 days) compared to those who did not (23.6±10.1 days, P=.04; Table 2). More patients also died at EOS among those who developed renal failure compared to those who did not (25% vs. 13%, P=.04; Table 2), which could have contributed to the shorter follow-up duration. 39% of the mITT patients had not been discharged at their EOS visit. Among those patients, 56 were in ICU and 51 were on MV (41 were in both ICU and on MV).
Healthcare resource use and costs from a Spanish perspectiveComparison by linezolid and vancomycin groups (mITT)No statistically significant differences in HCRU and total costs between the linezolid- and vancomycin-treated patients were identified. Patients who used MV (73% vs. 78%, P=.23), patients with ICU stay (86% vs. 89%; P=.32), mean duration of MV use (8.3±9.3 vs. 8.1±9.1 days; P=.84), mean ICU days (10.1±8.8 vs. 10.6±8.7 days, P=.58), and the mean hospital LOS (17.9±9.6 vs. 18.6±9.7 days, P=.45) were similar between the linezolid- and vancomycin-treated patients. The proportion of patients who received RRT (5 [2%] vs. 17 [8%] patients; P=.009), as well as mean RRT days per patient during the follow-up period (0.1±0.8 vs. 0.5±2.1 days; P=.02) differed significantly between the linezolid and vancomycin treatment groups, respectively.
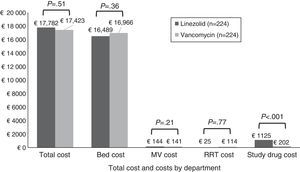
There was no statistically significant difference in total costs between study treatment groups within the mITT population (Fig. 2). No significant differences were found in costs by department except for study drug costs, which were significantly higher for patients receiving linezolid compared to vancomycin therapy. After controlling for survival, duration of follow-up time, and region, patients randomized to linezolid had no significant difference in total costs as compared with vancomycin (cost ratio=1.05; P=.27).
Total costs (by component) per patient by treatment group (mITT population). Note: Costs are given in 2012 Euros. The study drug cost for vancomycin group includes the cost of drug and cost for testing the vancomycin levels. Abbreviations: MV, mechanical ventilation; mITT, modified intent to treat; RRT, renal replacement therapy.
Mean MV (7.8±9.0 vs. 12.0±9.9; P=.004) and ICU days (10.0±8.5 vs. 13.5±9.9; P=.013) were significantly longer in patients who developed renal failure compared to those who did not. However, we did not find a significant difference in hospital LOS (18.2±9.6 vs. 18.8±9.8 days; P=.74). Please note that since 3 out of the 405 patients who did not develop renal failure had RRT at randomization, the mean RRT duration in this study group was not 0 (mean: 0.02) days.
The costs of hospital bed-days (€18,333±€10,092 vs. €16,557±€9,061; P=.23), MV (€210±€172 vs. €135±€157; P=.004), and RRT (€678±€1,144 vs. €5±€69; P<.001) were significantly higher, drug costs were significantly lower (€405±€547 vs. €691±€593; P=.003), and total overall costs were numerically higher (€19,626±€10,840 vs. €17,388±€9,369; P=.14) among patients who developed renal failure compared to those who did not. Patients with renal failure had 19% higher costs after adjustment for survival, duration of follow-up time, and region (Cost ratio=1.19, P=.003).
Comparison by linezolid and vancomycin groups (renal failure subgroup)No differences were found in clinical or demographic variables between patients who received linezolid (N=9) and vancomycin (N=34) within the subgroup of patients who developed renal failure (N=43). Although not statistically significant, the duration of MV (7.6±3.6 vs. 13.2±10.7 days; P=.21), ICU stay (9.9±6.6 vs. 14.4±10.5 days; P=.30), inpatient LOS (16.1±11.0 vs. 19.5±9.5 days; P=.26) and duration of RRT (1.9±3.6 vs. 2.9±4.8 days; P=.77) trended shorter for patients treated with linezolid compared to vancomycin.
Compared to vancomycin (including vancomycin-level monitoring costs), linezolid drug costs were higher (€1,233±€590 vs. €186±€246, P<.001), while total medical costs (€17,219±€8,792 vs. €20,263±€11,350; P=.51) and total costs per patient-day (€1,000±€272 vs. €1,010±€312; P=.98) were numerically lower for linezolid vs. vancomycin patients within the subgroup of patients who developed renal failure. The difference in survival status at EOS between treatment groups was adjusted for by calculating economic cost per person-day.
DiscussionThe current study highlights the economic burden associated with MRSA NP from a Spanish payer's perspective. The results showed that there were no significant differences in treatment costs for linezolid- (€17,782) and vancomycin-treated (€17,423) patients. Significantly higher drug cost for linezolid vs. vancomycin therapy may have been partially offset by fewer renal failure events. Bed costs were the main cost driver, of which ICU costs constituted the highest proportion.
To the best of our knowledge, this is the largest study focusing on a Spanish payer's perspective that evaluates the economic burden of MRSA NP treatments using data from a randomized clinical trial. Prior studies have evaluated the treatment costs of linezolid vs. vancomycin in patients with MRSA NP from a US11 and a China15 perspective. At the outset of this analysis, it was unclear whether the conclusions from the prior US study would remain robust when the Spanish costs were applied, due to fundamental differences in each country's healthcare system. In Spain and most other European countries, healthcare is universally covered and fully funded from taxes obtained from the public sector. This means that health services are mostly free of charge at the point of delivery.16 In contrast, healthcare in the US is not universally covered; no single payer for healthcare exists. China also has a different healthcare system compared to most of the western countries, adopting increasing health care privatization, and decentralization from the central government to provincial and local authorities.17
Although the main conclusions of this study are consistent with the prior US economic analysis,11 there are some notable differences from the Spanish perspective. A key difference in the findings is that the total costs and cost components were lower from a Spanish payer's perspective compared to the US for both treatment groups, which is likely driven by the difference in unit costs. In a cross-national study comparing the US healthcare system with other Organization for Economic Cooperation and Development (OECD) countries, it was suggested that Americans have comparably fewer physician office visits and hospital days. However, the total medical expenditures in the US are twice as high per capita as most other OECD countries, including Spain.18 This conclusion is consistent with our findings from the ZEPHyR trial that patients enrolled in European countries (10%) had over 40% longer LOS than the US (63%) (mean LOS: 23 vs. 16 days) but lower total hospitalization costs for both treatment groups. The incremental difference in total cost between the linezolid and vancomycin groups was the highest in our study (€359), followed by China (¥1,584=€222)15 and US ($107=€102) studies11. As the incremental treatment success rate in linezolid vs. vancomycin is ∼10% (55% vs. 45%), the incremental cost per additional treatment success at base case may be the highest in the current study compared to studies in the US ($16,516 [95% CI: −$68,620 to $164,478]) and in China (¥15,904 [95% CI: −¥161,935 to ¥314,987]).11,15 More details about the comparison of the prior US, China and the current Spanish studies were provided in the Supplemental table.
Several additional modeling studies also evaluated the economic impact of linezolid and vancomycin therapy for patients with MRSA NP from various perspectives in the US and European countries. León et al.10 evaluated the incremental cost-effectiveness ratio of linezolid vs. vancomycin under a decision-analytical framework from a Spanish payer perspective. The study concluded that linezolid was cost-effective against vancomycin, with incremental cost/life-years gained and death avoided below the acceptable threshold.10 Patel et al. published two studies evaluating the cost-effectiveness of linezolid vs. vancomycin from a US and German payer perspective.19,20 In Germany and the US, they suggested that the cost-effectiveness ratio favored linezolid over vancomycin as linezolid-treated patients had greater efficacy and cost-offset by lower treatment failure-related costs in Germany and US.19,20
Although some economic data can be gleaned from the literature, data on the economic burden associated with MRSA pneumonia are limited in Europe (especially in Spain) and varied widely across studies. Results from a Spanish study evaluating data from 27 hospitals found that MRSA bacteremia was associated with a mean inpatient LOS of 25 days, an ICU admission rate of 28.7%, and treatment costs of €11.884 per episode (updated to 2012 Euros).21 A European study (EUVAP) concluded that patients who received inappropriate compared to appropriate empiric treatment had longer ICU stays by approximately 6 days.22 Our study results provide updated economic data on treatments of vancomycin and linezolid for MRSA NP, emphasizing the importance of minimizing total medical costs and improving clinical outcomes through the use of appropriate antibiotics under the Spanish healthcare system and potentially that of other countries.
It is important to understand this study's findings in the context of its limitations. First, the follow-up period for collecting HCRU items and cost calculation through EOS were limited in the study. Length of stay was likely underestimated since ∼40% of patients were hospitalized at the EOS visit and their discharge date was not captured. This is a limitation often observed in clinical studies. In most randomized clinical trials, HCRU data are not usually collected after the EOS visit and are usually truncated when the clinical evaluation ends. In our study, the proportion of patients who remained hospitalized at EOS was balanced between treatment groups, as was time to the EOS visit. Therefore, we would expect the effect on HCRU and cost differences between treatment groups to be minimal. In addition, the regression analysis adjusting for the difference in follow-up, survival, and region showed similar findings as in the descriptive analysis. Second, although we applied unit cost data from Spain to the study results, the results were obtained primarily from patients hospitalized in the US which explains why our LOS was shorter than that reported for other European populations. Given that the patients were randomly assigned into the treatment groups in each region, the difference in HCRU and costs between treatments may not be largely impacted by this limitation. Third, the vancomycin doses used in this clinical trial6 may not reflect dosing patterns in real-world clinical practice since vancomycin dosing was optimized within the randomized clinical trial. Fourth, this analysis was conducted in the mITT population. Consequently, this datum does not represent the HCRU or costs of empiric treatment for patients at risk for MRSA. Lastly, it is likely that this study was underpowered to detect differences in continuous HCRU and cost outcomes between treatment groups, especially within the subgroup of patients who developed renal failure. Therefore, linezolid's benefits in the lower renal failure rate did not translate into a statistically significant difference in total medical cost when compared with vancomycin.
Despite these limitations, our study results add evidence to demonstrate the importance of MRSA NP economic burden from a Spanish payer's perspective. This study provides valuable information by quantifying the economic impact associated with two antibiotic treatments in Spain. Decision makers may use this information to justify the need for strategies aimed at MRSA prevention, and clinicians may use this information to understand the economic burden associated with antibiotic treatment strategies.
ConclusionFocusing on a Spanish perspective, our study provides updated data on the economic burden associated with linezolid and vancomycin treatment in patients with MRSA NP and the impact of renal failure on HCRU and costs. It was found that HCRU and costs were similar for linezolid- and vancomycin-treated patients with MRSA NP during the study period by applying Spanish unit cost data to HCRU data collected from an international randomized clinical trial. Linezolid's high drug acquisition costs were partially offset by fewer renal failure events which impacted ICU, hospital bed, and RRT costs. The potential consequences of developing renal failure include increased mortality and treatment costs. Future research is warranted to further explore the potential impact of linezolid and optimally-dosed vancomycin on HCRU, including the risk of renal adverse events, and the associated economic costs within the Spanish National Health System and other European countries.
Authors’ contributionsDr. Jordi Rello, Dr. Jordi Solé-Violán, Dr. Mercedes Nieto, and Dr. Jean Chastre contributed to study conceptualization and design. Caitlyn T. Solem, Yin Wan, and Xin Gao developed the analysis plan and methodology, and performed the analysis. Marina de Salas-Cansado, Francisco Mesa, and Claudia Charbonneau contributed to study conceptualization and methodology development. All authors listed made substantial contributions to the study in conceptualization and/or study design, analysis and/or data interpretation and manuscript preparation and/or review. All authors read, edited, and approved the final manuscript.
Conflicts of interestThis work was supported by Pfizer, Inc. Pharmerit International received consulting fees from Pfizer, Inc. in connection with this study and development of this manuscript. Caitlyn T. Solem, Yin Wan, and Xin Gao are employees of Pharmerit International. Dr. J Rello received an honorarium from Pfizer Inc. in connection with his work on this study and has been a consultant and an advisory board member and has received honoraria for advice or public speaking for Pfizer Inc. Dr. Mercedes Nieto received an honorarium from Pfizer, Inc. in connection with work on this study, has been a consultant and an advisory board member, and has received honoraria for advice or public speaking for Pfizer, Inc. Dr. Jordi Solé-Violán received an honorarium from Pfizer, Inc. in connection with work on this study, has been a consultant and an advisory board member, and has received honoraria for advice or public speaking for Pfizer, Inc. Jean Chastre received an honorarium from Pfizer, Inc. in connection with work on this study, has been a consultant and an advisory board member, and has received honoraria for advice or public speaking for Pfizer, Inc. Francisco Mesa and Claudie Charbonneau are employees and shareholders of Pfizer, Inc. Marina de Salas-Cansado is a former employee of Pfizer, Inc.
The authors would like to thank Jennifer Stephens from Pharmerit International for her contribution in the study design, analysis plan, and results interpretation and Beth Lesher for editorial assistance. We appreciate the work from the following team members of our original US study using the same clinical trial data: Dr. Mike Niederman from the Winthrop-University Hospital, Richard Chambers, Daniela E. Myers, Seema Haider, and Jim Z. Li from Pfizer, Inc., and Professor Ben van Hout from the University of Sheffield.