Oxygen has been used liberally in ICUs for a long time to prevent hypoxia in ICU- patients. Current evidence suggests that paO2 >300 mmHg should be avoided, it remains uncertain whether an “optimal level” exists. We investigated how “mild” hyperoxia influences diseases and in-hospital mortality.
DesignThis is a retrospective study.
Setting112 mechanically ventilated ICU-patients were enrolled.
Patients or participants112 ventilated patients were included and categorized into two groups based on the median paO2 values measured in initial 24 h of mechanical ventilation: normoxia group (paO2 ≤ 100 mmHg, n = 43) and hyperoxia group patients (paO2 > 100 mmHg, n = 69).
InterventionsNo interventions were performed.
Main variables of interestThe primary outcome was the incidence of pulmonary events, the secondary outcomes included the incidence of other new organ dysfunctions and in-hospital mortality.
ResultsThe baseline characteristics, such as age, body mass index, lactate levels, and severity of disease scores, were similar in both groups. There were no statistically significant differences in the incidence of pulmonary events, infections, and new organ dysfunctions between the groups. 27 out of 69 patients (39.1%) in the “mild” hyperoxia group and 12 out of 43 patients (27.9%) in the normoxia group died during their ICU or hospital stay (p = 0.54). The mean APACHE Score was 29.4 (SD 7.9) in the normoxia group and 30.0 (SD 6.7) in the hyperoxia group (p = 0.62).
ConclusionsWe found no differences in pulmonary events, other coded diseases, and in-hospital mortality between both groups. It remains still unclear what the "best oxygen regime" is for intensive care patients.
El oxígeno durante mucho tiempo se ha utilizado generosamente en la UCI para prevenir la hipoxia de los pacientes. Las pruebas actuales sugieren que debe evitarse una paO2 >300 mmHg, pero aún no se sabe con certeza si existe un "nivel óptimo". Se investigó cómo la hiperoxia "leve" influye en la morbi-mortalidad intrahospitalaria.
DiseñoSe trata de un estudio retrospectivo.
ÁmbitoSe incluyeron 112 pacientes de UCI con ventilación mecánica.
Pacientes o participantesLos 112 pacientes se clasificaron en dos grupos en función de los valores medios de paO2, medidos durante las primeras 24 horas de la ventilación mecánica: pacientes del grupo de normoxia (paO2 ≤ 100 mmHg, n = 43) y pacientes del grupo de hiperoxia (paO2 > 100 mmHg, n = 69).
IntervencionesNo se realizaron intervenciones.
Principales variables de interésEl resultado primario fue la incidencia de episodios pulmonares, los resultados secundarios incluyeron la incidencia de otras disfunciones orgánicas nuevas y la mortalidad intrahospitalaria.
ResultadosLas características basales, como la edad, el índice de masa corporal, los niveles de lactato y las puntuaciones de gravedad de la enfermedad, fueron similares en ambos grupos. No hubo diferencias estadísticamente significativas en la incidencia de episodios pulmonares, infecciones y nuevas disfunciones orgánicas entre los grupos. Pudo observarse que 27 de 69 pacientes (39,1%) del grupo de hiperoxia "leve" y 12 de 43 pacientes (27,9%) del grupo de normoxia fallecieron durante su estancia en la UCI o en el hospital (p = 0,54). La puntuación APACHE media fue de 29,4 (DE 7,9) en el grupo de normoxia y de 30,0 (DE 6,7) en el grupo de hiperoxia (p = 0,62).
ConclusionesNo encontramos diferencias en episodios pulmonares, otras enfermedades codificadas y mortalidad intrahospitalaria entre ambos grupos. Sigue sin estar claro cuál es el "mejor suministro de oxígeno" para los pacientes de cuidados intensivos.
Oxygen therapy is one of the primary supportive care for patients with or at risk of developing hypoxic respiratory failure, who are admitted to the emergency department and intensive care units. To date, no clinically useful biomarker for O2 toxicity is available, and data on the effects of hyperoxia on markers of oxidative stress are equivocal.1
Numerous studies have demonstrated that hyperoxia (paO2 > 100 mmHg) can be harmful and is associated with adverse outcomes.2–6
The lungs are the first organs affected by oxygen toxicity. In previous studies lung damage and gut microbiota alteration were observed during exposure of mice to hyperoxia.7,8 Although paO2 reflects the oxygen supply in the artery, it does not necessarily provide information about the burden of oxygen in the lungs, which can be much higher than systemic levels when a high fraction of oxygen (FiO2) is used. At the same time, alveolar hyperoxia may not result in extremely high paO2 levels due to occasional existing alveolar- capillary diffusion disturbances or intrapulmonary shunting.9
Hyperoxia causes oxidative stress due to the formation of reactive oxygen species (ROS) and inflammation in the lungs,4 which may impair the surfactant system, leading to alveolar collapse and a reduction in pulmonary compliance.10 Excessive oxygen administration can lead to hyperoxic vasoconstriction in the pulmonary system and atelectasis11 as well as impair muco-ciliary clearance and the antimicrobial capacity of immune cells, contributing to the development of secondary ventilator-associated pneumonia (VAP).12 In preterm neonates, hyperoxia is associated with chronic diseases such as bronchopulmonary dysplasia.13
Several studies have shown that hyperoxia is also associated with negative effects on other organ systems, such as the central nervous system and on the cardiovascular system.14,15 Some studies report an increased risk of mortality in critically ill patients associated with hyperoxia, with the most pronounced effects seen at extreme levels of paO2.3,6,17,18 An association between hyperoxia and mortality was also suggested in critically ill children beyond the neonatal period.19
In summary, the previous studies still does not provide definitive information about the optimal paO2 levels to target during the ventilation of critically ill patients.
In this retrospective observational study, the incidence of pulmonary events and other coded diagnoses that typically occur in our critically ill patients admitted to a surgical intensive care unit were examined. Additionally, the study investigated the relationship between oxygen therapy and in- hospital mortality.
The objective of the study was to determine whether hyperoxia leads to a higher occurrence of pulmonary events such as bacterial, viral, or fungal pneumonia, atelectasis, acute respiratory failure, pyothorax, and other coded diseases, ultimately resulting in increased in- hospital mortality compared to normoxia. Furthermore, the study assessed the utility of linking paO2 levels to the onset of coded diseases in ICU- patients.
MethodsStudy designThis retrospective observational cohort study was approved by our Medical Ethics Commission. The study was conducted in accordance with the ethical standards outlined in the latest version of the Helsinki Declaration (2013).20
ParticipantsWe enrolled all adult patients who received treatment in our surgical ICU during the period of 12 months. All adult patients (≥18 years) who had been mechanically ventilated for at least 24 h were screened for inclusion in the study.
The patients in the cohort were categorized into two groups based on their mean paO2 values: the normoxia group with paO2 ≤100 mmHg and the “mild” hyperoxia group with paO2 >100 mmHg.
We excluded patients who were not intubated, patients with acute respiratory distress syndrome (ARDS, as per the Berlin definition21), those on extracorporeal membrane oxygenation (ECMO), patients with severe chronic obstructive pulmonary disease (COPD GOLD IV), cases of brain death, individuals with neutropenia, and pregnant women. Additionally, to prevent potential bias, we excluded cases of ICU readmissions that occurred within the same hospitalization.
Our patients in both groups had various lung diseases. 5 patients in the group with paO2 ≤ 100 mmHg and 11 patients with paO2 > 100 mmHg had lung diseases such as COPD GOLD 1–2, bronchial asthma, and emphysema. One patient had a restrictive ventilation disorder with long-term oxygen therapy (LTOT) due to scoliosis. One patient had a suspected bronchial cancer, while another patient had been treated for bronchial cancer seven years prior. The most common disease was COPD. One patient in each group had LTOT. The other patients were not significantly impaired in their daily lives by their lung disease.
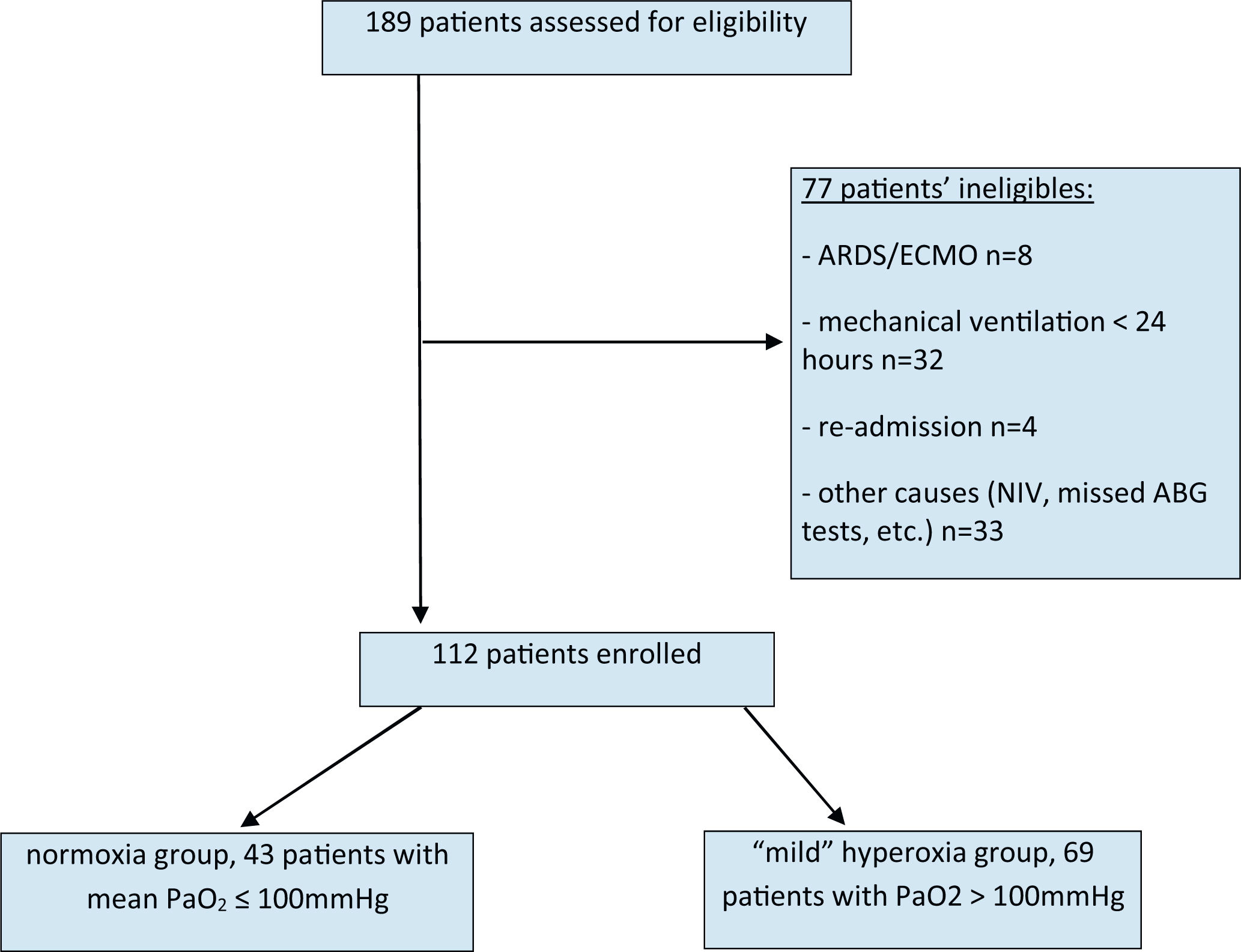
In this observational study involving multiple variables, we analysed and computed the mean values of all paO2 measurements from arterial blood gases (ABGs) taken within the initial 24 h of mechanical ventilation. Depending on the presence of respiratory insufficiency or other clinical conditions, between 9 and 21 ABG tests were conducted within a 24-h period (refer to Fig. 1).
VariablesIn this study, we analysed data from patients recorded in I-SH/ i.s.h.med (the hospital’s electronic patient record system, SAP) who were admitted to our surgical ICU during the period of 12 months. We systematically conducted searches for all relevant DRGs (German Diagnosis Related Groups) that were documented throughout the patient’s ICU stay and that might be influenced by hyperoxia. Specifically, for new pulmonary diagnoses, an inclusive list of various pulmonary events, such as bacterial/viral/fungal infections, pleural effusions, pyothorax, pneumonitis, and atelectasis, was considered.
Further, we compared the values of common ICU scoring systems that reflect the severity of the disease: sequential organ failure assessment (SOFA), Acute Physiology and Chronic Health Evaluation II (APACHE) and Simplified Acute Physiology Score - II (SAPS II) upon admission. Additionally, we considered the maximal value of the SOFA score during the ICU stay, as this can change with the onset of a new infection or organ dysfunction.
The inspired oxygen fraction (FiO2), paO2 from ABGs, positive end-expiratory pressure (PEEP), and lactate averages were defined as the mean values of all measurements within the first 24 h after admission. Furthermore, patients' data included age, body mass index (BMI), and length of stay (LOS) in the ICU.
The primary endpoint of this study was pulmonary events. The secondary outcomes included the incidence of other infections, new organ dysfunctions, and in-hospital mortality in both groups.
Statistical methodsA comprehensive descriptive statistical analysis was conducted, presenting the mean, standard deviation, minimum, median, and maximum values for all continuous data and scores upon admission as well as their maximum during the stay. Binary data were reported with absolute and relative frequencies. Baseline demographics, prognostic variables, scores, and ventilation modalities were compared between the groups using chi-square and Wilcoxon tests, as appropriate.
Cumulative survival probabilities were plotted using the Kaplan–Meier method and compared using the log-rank test. Statistical significance was indicated by a p-value (determined through the Pearson Chi-Square test and two-sided Wilcoxon Test) below 0.05 for patient baseline characteristics and secondary outcomes.
The statistical data analysis was performed using SPSS-Software (IBM ® SPSS ® Statistics Version 23) and Microsoft Excel.
ResultsWe enrolled 112 patients undergoing elective or emergency surgery, organ transplantation, patients with sepsis or septic shock, post-surgical complications, and hemorrhage that required admission to the ICU.
A total of 189 patients were identified as potential study participants. Out of these, 112 met our inclusion criteria and were eligible for analysis (Fig. 1). All patients were assigned to two groups: the normoxia group consisted of patients with paO2 ≤ 100 mmHg (n = 43), and the “mild” hyperoxia group consisted of patients with paO2 >100 mmHg (n = 69).
53 out of 69 patients in the 'mild' hyperoxia group and 33 out of 43 patients in the normoxia group required surgery and were subsequently treated postoperatively in the ICU. The average duration of surgery was 3.6 h in the hyperoxia group and 3.2 h in the normoxia group. Patients were ventilated invasively with pressure control both in the operating room and subsequently in the intensive care unit, following the current ARDS guidelines. According to our study protocol, only patients who were only invasively ventilated were evaluated in the first 24 h in the intensive care unit, ensuring that ventilatory support was not conducted for at least this period.
Primary outcomeThe incidence of pulmonary events related to oxygen therapy was analysed. This included bacterial, viral, and fungal infections, pleural effusions, pyothorax, pneumonitis, and atelectasis. Pleural effusions occurred in 70% of the normoxia group compared to 58% of the hyperoxia group (p = 0.23). Similarly, pneumonia due to sepsis was observed in 12% of the normoxia group and 29% of the hyperoxia group (p = 0.42). The onset of atelectasis, bacterial pneumonia, aspergillosis, herpes simplex virus pneumonia, acute respiratory failure with hypoxia, pyothorax, and aspiration pneumonitis were similar in both groups. There were no statistically significant differences in the values (see Table 2.
Baseline characteristics and clinical parameters of study patients.
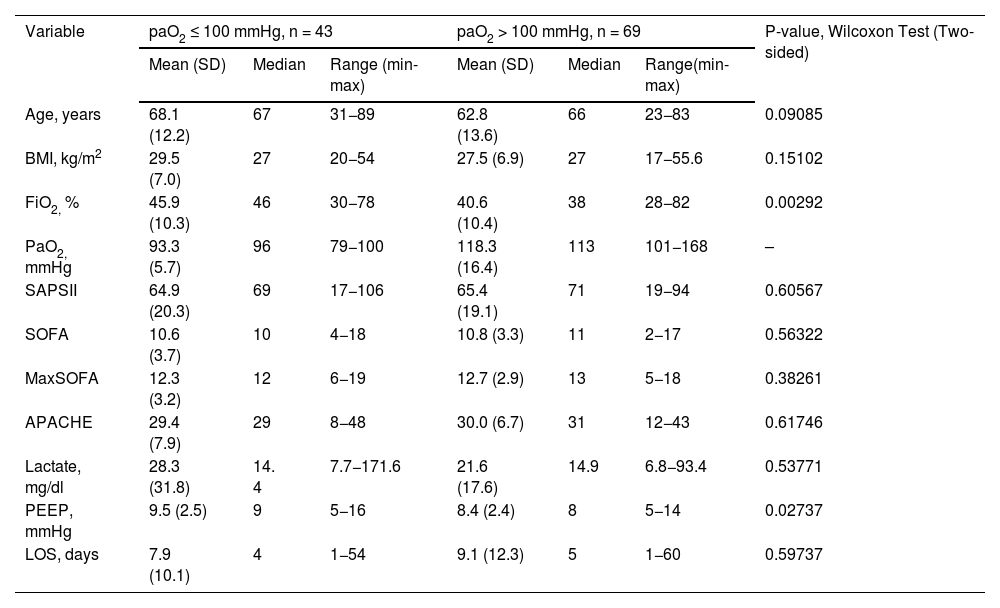
| Variable | paO2 ≤ 100 mmHg, n = 43 | paO2 > 100 mmHg, n = 69 | P-value, Wilcoxon Test (Two-sided) | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Range (min-max) | Mean (SD) | Median | Range(min-max) | ||
| Age, years | 68.1 (12.2) | 67 | 31−89 | 62.8 (13.6) | 66 | 23−83 | 0.09085 |
| BMI, kg/m2 | 29.5 (7.0) | 27 | 20−54 | 27.5 (6.9) | 27 | 17−55.6 | 0.15102 |
| FiO2, % | 45.9 (10.3) | 46 | 30−78 | 40.6 (10.4) | 38 | 28−82 | 0.00292 |
| PaO2, mmHg | 93.3 (5.7) | 96 | 79−100 | 118.3 (16.4) | 113 | 101−168 | – |
| SAPSII | 64.9 (20.3) | 69 | 17−106 | 65.4 (19.1) | 71 | 19−94 | 0.60567 |
| SOFA | 10.6 (3.7) | 10 | 4−18 | 10.8 (3.3) | 11 | 2−17 | 0.56322 |
| MaxSOFA | 12.3 (3.2) | 12 | 6−19 | 12.7 (2.9) | 13 | 5−18 | 0.38261 |
| APACHE | 29.4 (7.9) | 29 | 8−48 | 30.0 (6.7) | 31 | 12−43 | 0.61746 |
| Lactate, mg/dl | 28.3 (31.8) | 14. 4 | 7.7−171.6 | 21.6 (17.6) | 14.9 | 6.8−93.4 | 0.53771 |
| PEEP, mmHg | 9.5 (2.5) | 9 | 5−16 | 8.4 (2.4) | 8 | 5−14 | 0.02737 |
| LOS, days | 7.9 (10.1) | 4 | 1−54 | 9.1 (12.3) | 5 | 1−60 | 0.59737 |
SOFA = Sequential organ failure assessment at admission; MaxSOFA = maximal score during ICU stay; APACHE = Acute Physiology and Chronic Health Evaluation at admission; SAPS-II = Simplified Acute Physiology Score-II at admission; BMI = Body mass index; PEEP = Positive end expiratory pressure; LOS =Length of stay; FiO2 = Fraction of inspired oxygen; PaO2 = Partial pressure of oxygen. Positive end expiratory pressure; LOS = Length of stay; FiO2 = Fraction of inspired oxygen; PaO2 = Partial pressure of oxygen.
DRG – Codes and causes of admission to ICU.
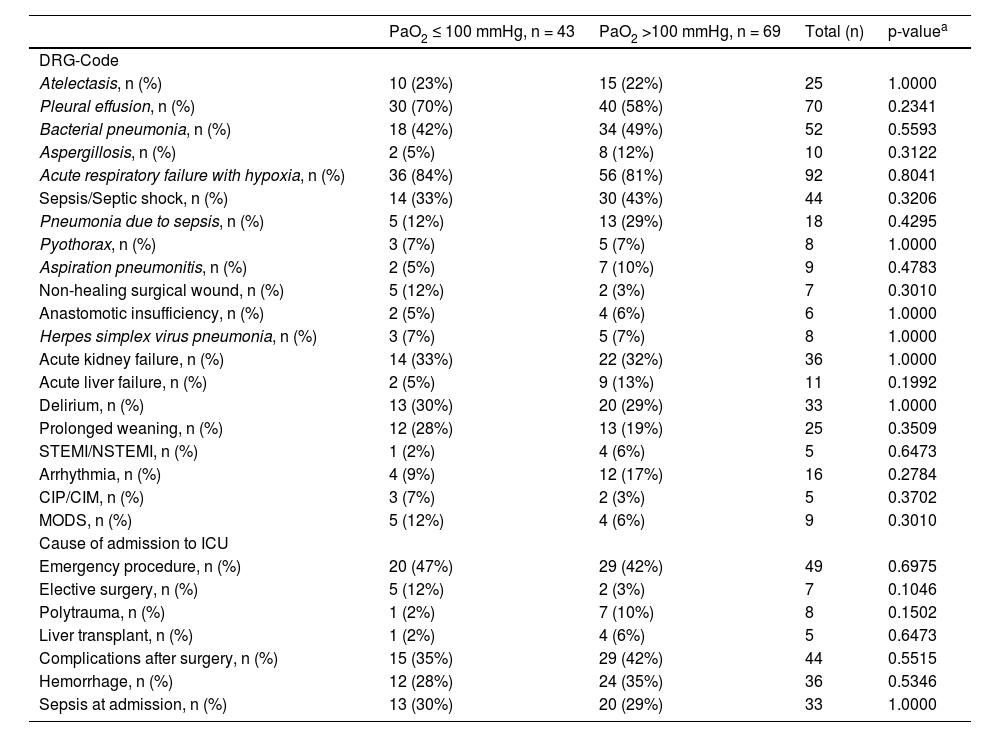
| PaO2 ≤ 100 mmHg, n = 43 | PaO2 >100 mmHg, n = 69 | Total (n) | p-valuea | |
|---|---|---|---|---|
| DRG-Code | ||||
| Atelectasis, n (%) | 10 (23%) | 15 (22%) | 25 | 1.0000 |
| Pleural effusion, n (%) | 30 (70%) | 40 (58%) | 70 | 0.2341 |
| Bacterial pneumonia, n (%) | 18 (42%) | 34 (49%) | 52 | 0.5593 |
| Aspergillosis, n (%) | 2 (5%) | 8 (12%) | 10 | 0.3122 |
| Acute respiratory failure with hypoxia, n (%) | 36 (84%) | 56 (81%) | 92 | 0.8041 |
| Sepsis/Septic shock, n (%) | 14 (33%) | 30 (43%) | 44 | 0.3206 |
| Pneumonia due to sepsis, n (%) | 5 (12%) | 13 (29%) | 18 | 0.4295 |
| Pyothorax, n (%) | 3 (7%) | 5 (7%) | 8 | 1.0000 |
| Aspiration pneumonitis, n (%) | 2 (5%) | 7 (10%) | 9 | 0.4783 |
| Non-healing surgical wound, n (%) | 5 (12%) | 2 (3%) | 7 | 0.3010 |
| Anastomotic insufficiency, n (%) | 2 (5%) | 4 (6%) | 6 | 1.0000 |
| Herpes simplex virus pneumonia, n (%) | 3 (7%) | 5 (7%) | 8 | 1.0000 |
| Acute kidney failure, n (%) | 14 (33%) | 22 (32%) | 36 | 1.0000 |
| Acute liver failure, n (%) | 2 (5%) | 9 (13%) | 11 | 0.1992 |
| Delirium, n (%) | 13 (30%) | 20 (29%) | 33 | 1.0000 |
| Prolonged weaning, n (%) | 12 (28%) | 13 (19%) | 25 | 0.3509 |
| STEMI/NSTEMI, n (%) | 1 (2%) | 4 (6%) | 5 | 0.6473 |
| Arrhythmia, n (%) | 4 (9%) | 12 (17%) | 16 | 0.2784 |
| CIP/CIM, n (%) | 3 (7%) | 2 (3%) | 5 | 0.3702 |
| MODS, n (%) | 5 (12%) | 4 (6%) | 9 | 0.3010 |
| Cause of admission to ICU | ||||
| Emergency procedure, n (%) | 20 (47%) | 29 (42%) | 49 | 0.6975 |
| Elective surgery, n (%) | 5 (12%) | 2 (3%) | 7 | 0.1046 |
| Polytrauma, n (%) | 1 (2%) | 7 (10%) | 8 | 0.1502 |
| Liver transplant, n (%) | 1 (2%) | 4 (6%) | 5 | 0.6473 |
| Complications after surgery, n (%) | 15 (35%) | 29 (42%) | 44 | 0.5515 |
| Hemorrhage, n (%) | 12 (28%) | 24 (35%) | 36 | 0.5346 |
| Sepsis at admission, n (%) | 13 (30%) | 20 (29%) | 33 | 1.0000 |
ICU = intensive care unit; DRG = German Diagnosis Related Groups; PaO2 = partial pressure of oxygen; STEMI/NSTEMI = ST- segment elevation myocardial infarction/non-ST segment elevation myocardial infarction; CIP/CIM = Critical-Illness Polyneuropathy/Critical- Illness Myopathy; MODS = Multi Organ Dysfunction Syndrome.
There were also no statistically significant between-group differences in the incidence of non- pulmonary new infections and organ dysfunctions (DRG Codes) that occurred during the ICU stay, as shown in Table 2. In patients with hemorrhage (p = 0.53) and polytrauma (p = 0.15) as reasons for admission to the ICU, more patients received oxygen therapy resulting in paO2 > 100 mmHg.
Both study groups had similar age, BMI, lactate levels, and severity of disease scores at admission and during their ICU stay, as shown in the baseline characteristics in Table 1. Notably, the PEEP and FiO2 levels were significantly lower in the group of patients with paO2 >100 mmHg (p = 0.03 and p = 0.01, respectively). The “mild” hyperoxia group spent an average of 1.2 day longer in the ICU (p = 0.59).
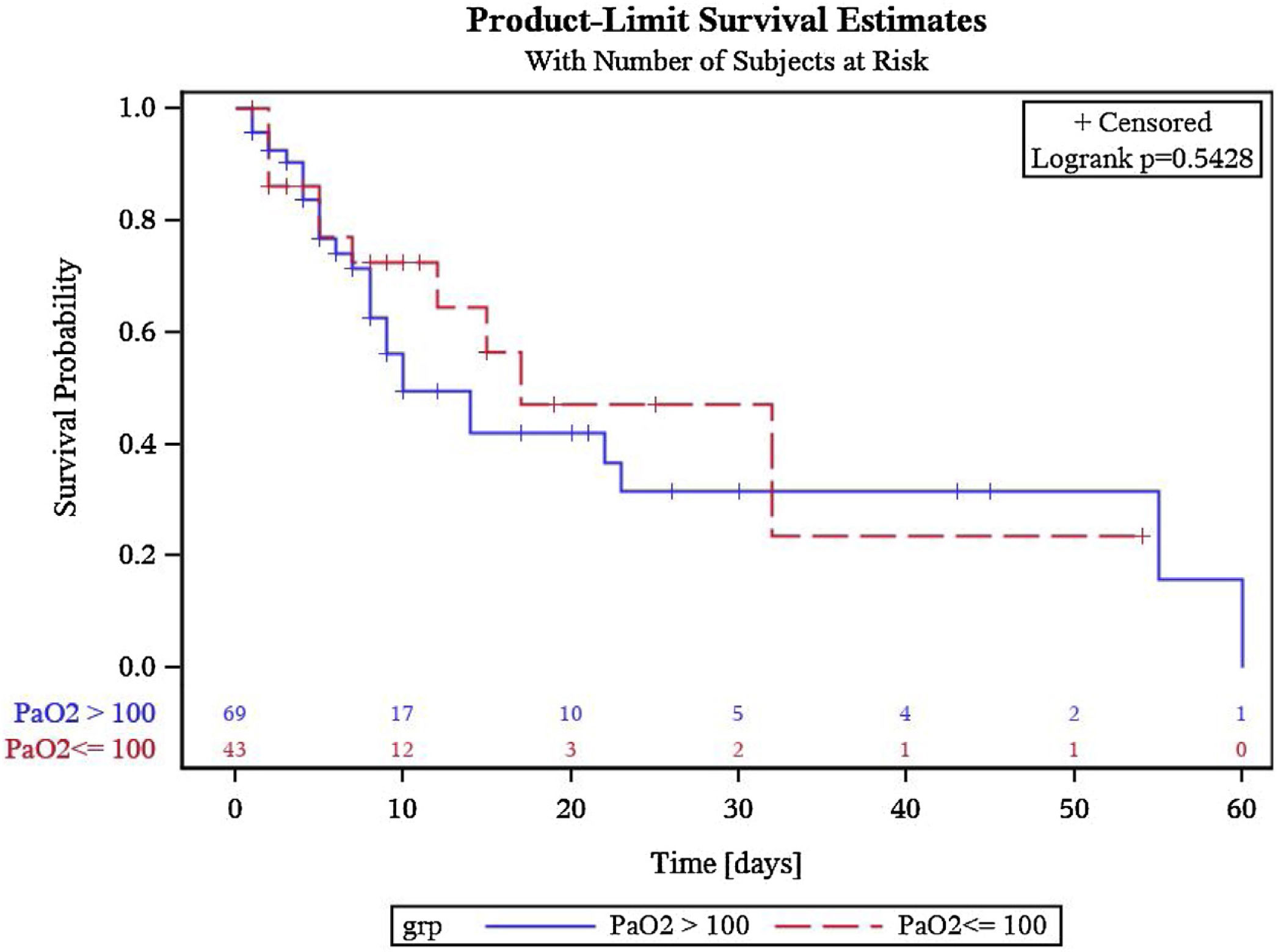
Out of 69 patients in the hyperoxia group, 27 (39. 1%) and out of 43 patients in the group with PaO2 ≤ 100 mmHg, 12 (27. 9%) died during their ICU stay or further hospitalization. The Kaplan–Meier survival analysis presented in Fig. 2 shows no statistically significant differences between the groups in survival estimates (p = 0.5428).
DiscussionIn this retrospective observational cohort study, our aim was to examine the relationship between arterial partial pressure of oxygen, a ventilation parameter, and various outcome variables, such as pulmonary events, the onset of other coded diseases, and in-hospital mortality.
Consistent with previous studies,9,22 we used paO2 as a more precise ventilation parameter for the analysis of the oxygen therapy in invasive ventilated ICU patients. This choice was made due to the S-shaped nature of the oxygen- hemoglobin dissociation curve, where even slight differences in SpO2 can lead to significant variations in paO2.
The highest paO2 observed in the “mild” hyperoxia group was 168 mmHg, a value considerably lower than the paO2 thresholds used in prior studies. In those studies, hyperoxia was typically defined as paO2 of 300 mmHg or higher. Notably, such high paO2 levels were associated with either increased mortality18,23 or worse neurological outcomes.24 In our patient cohort, we specifically examined the impact of oxygen therapy on groups with only “mild” hyperoxia, where there were no extreme differences paO2 in values. Our analysis focused on evaluating disparities in pulmonary events and in-hospital mortality within this context.
Our patient groups were homogenous regarding the severity of the disease (median APACHE score 29 vs. 31) and median paO2 levels (96 mmHg vs. 113 mmHg). Due to this homogeneity, it was likely possible to mitigate the potential effect of sicker patients receiving more oxygen, which could lead to an overestimation of the oxygen’s impact on mortality. On the other hand, nowadays, more clinicians generally recognize the potential deleterious effects of prolonged aggressive oxygen therapy, resulting in excessive paO2 levels.16,17 However, current practice still appears to involve clinicians tolerating a certain degree of hyperoxia to maintain a ‘safety buffer’ against hypoxemia.
Our results are consistent with the systematic review, meta-analysis, and trial sequential analysis conducted by Barbateskovic et al., which showed neither beneficial nor harmful effects of higher versus lower oxygenation strategies. Similarly, no evidence was found indicating that a higher oxygenation strategy versus a lower oxygenation strategy had a profound effect on the all-cause mortality.25
As of the current data, there is no consensus or established definitions for cut-off values, including both the lower and upper limits for partial pressure of oxygen, that can differentiate between beneficial and harmful effects for the patient. In this study, which involved a relatively small patient cohort, it was not feasible to establish such cut-off values. PaO2 values between 70 and 170 mmHg are likely appropriate for critically ill ventilated patients, depending on the clinical condition, given the absence of a dependable measurement of tissue oxygenation for clinical application.
Our findings contrast with those of a previous single-center randomized clinical trial,3 wherein lower mortality was noted among patients receiving conservative oxygen therapy compared to those subjected to usual oxygen therapy. The outcomes of this trial might have been influenced by initial baseline disparities among the study groups, such as age, illness severity, and organ failures. Moreover, the study was prematurely terminated after an interim analysis revealed elevated mortality in one of the study groups, possibly leading to an overestimation of effects.5
ICU- patients with hemorrhage and polytrauma as admission causes were over-represented in the hyperoxia group (28% vs. 35% p = 0.53 and 2% vs. 10% p = 0.15, respectively). These patients often receive oxygen therapy, as clinicians tend to administer oxygen more aggressively in such critical and dynamic situations with a potential increased risk of hypoxia aggravated by blood loss. This could potentially provide an explanation for this discrepancy.16
In the hyperoxia group, bacterial pneumonia occurred more often (49 % vs. 42% in the other group, p- value = 0.5593). Based on our understanding of daily practices in our ICU, we would explain this by the frequent tracheal suctioning of secretions or the performance of bronchoscopy to remove secretions. During these procedures, the fraction of inspired oxygen is typically increased to 100%. Consequently, we might have observed falsely elevated values paO2 in this group.
On the other hand, there is evidence that prolonged aggressive oxygen therapy can impair pulmonary innate immunity and the capacity for bacterial phagocytosis. In a study by Six et al., a clear association between hyperoxia (remarkably defined as paO2 > 120 mmHg) at ICU admission and ventilator- associated pneumonia (VAP) as a secondary pulmonary infection was suggested.12
Studies on animal models have shown an association between hyperoxia and bacterial infection. Hyperoxia-induced suppression of macrophage phagocytosis of Pseudomonas Aeruginosa causes an increased susceptibility to bacterial infection, which can lead to higher mortality.26,27 Another study found that hyperoxia increased mortality in mice with Acinobacter Pneumonia; however, the administration of procysteine was able to improve survival by enhancing the phagocytic activity of alveolar macrophages in mice kept under hyperoxic conditions.28
In the recent study by Damiani et al. involving patients with SARS- CoV-2 viral pneumonia, “the prevalence of hyperoxemia was independently associated with a higher risk of ICU mortality, as well as a greater risk of developing VAP”.29 In our patient cohort only cases of herpes simplex virus pneumonia were observed as viral pneumonia, and the overall incidence of this was low.
Interestingly, with regard to non- pulmonary diagnoses, no cases of mesenteric ischemia occurred in either patient group.
Our retrospective study has demonstrated that relating paO2 to outcomes and coded diseases, particularly in surgical ICU patients, is relatively challenging.
Our evaluation certainly has limitations. Firstly, this is a retrospective single-center study, and the patient cohort, consisting of 112 enrolled surgical critical care patients, is relatively small. However, our patient groups were well-balanced and homogenous in terms of the median APACHE score (29 vs. 31) and the median paO2 levels. Furthermore, a comprehensive analysis was conducted regarding the interaction between all relevant DRG-Codes in intensive care medicine, such as sepsis, septic shock, organ transplantation, delirium, prolonged weaning, and so on.
Secondly, in our study, we focused on mortality during the hospital stay without follow-up, unlike other studies, which means that mortality in both groups might be underestimated.9,30 In this study, we explored a detailed list of different diagnoses typically occurring during an ICU stay and their correlation with paO2. Specifically, pulmonary diseases were investigated, including various lung infections.
ConclusionsIn this study, we observed no differences in pulmonary events, other coded diseases, or in-hospital mortality related to oxygen therapy in “mild” hyperoxia group compared to the normoxia group. However, further prospective multicentre studies involving a larger number of patients are necessary to conduct a precise protocol- based analysis of arterial partial pressure and other ventilation parameters, and to establish a connection between these factors and the outcomes and coded diseases in critically ill patients.
From now, while the “optimal level” remains unknown and may vary based on specific clinical conditions, it is recommended to target PaO2 values within the normal range.
In other words, carefully titrating PaO2 is advised to prevent both hypoxemia and excessive hyperoxemia.
Ethics approval and consent to participateThis retrospective exploratory cohort study received approval from Medical Ethics Commission of the Medical Faculty of Heidelberg University, Heidelberg, Germany (Approval ID: Votum S-357/2020). This study was carried out in compliance with the ethical standards outlined in the latest version of the Helsinki Declaration (2013).30
Consent for publicationRequirement for written informed consent and consent for publication was waived by the Ethics Commission.
Availability of data and materialsAll data are stored for at least 10 years. The datasets analysed during the current study are available from the corresponding author on reasonable request.
Competing interestsThe authors declare that they have no competing interests.
FundingThe participating institutions obtained no public or private funding for this work.
Authors' contributionsLS helped design the work, analysed and interpreted the data, was a major contributor in writing the manuscript, and critically reviewed and revised the final manuscript. DN and FU helped design the work and critically reviewed and revised the final manuscript. TB helped design the work, analysed the data, critically reviewed and revised the final manuscript. MW and AK helped design the work, critically reviewed and revised the final manuscript. MOFK helped design the work, analysed and interpreted the data, drafted the manuscript, and critically reviewed and revised the final manuscript.
Declaration of Generative AI and AI- assisted technologies in the writing processDuring the preparation of this work the authors used ChatGPT in order to improve readability and language. After using this chatbot, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
We thank Sabine Haag for her excellent technical support.