There is controversy about the effects of high plasma bicarbonate concentration ([HCO3−]) and the CO2 response test. We analyzed the relationship between [HCO3−] and the variation in hydrogen ion concentration (pH) for a given change in PaCO2, and its effects upon CO2 response.
DesignA retrospective study was carried out.
SettingTwo intensive care units.
PatientsSubjects with and without chronic obstructive pulmonary disease (COPD), at the beginning of weaning from mechanical ventilation.
InterventionsThe CO2 response was evaluated by the re-inhalation of expired air method, measuring the hypercapnic ventilatory response (ΔVE/ΔPaCO2) and hypercapnic drive response (ΔP01/ΔPaCO2), where VE is minute volume and P0.1 is airway occlusion pressure 0.1s after the initiation of inspiration.
Main outcome measures[HCO3−] and CO2 response.
ResultsA total of 120 patients in the non-COPD group and 48 in the COPD group were studied. COPD patients had higher mean [HCO3−] than non-COPD patients (33.2±5.4 vs. 25.7±3.7mmol/l, p<0.001). In both non-COPD and COPD patients we observed a significant inverse linear relationship between [HCO3−] and pH change per mmHg of PaCO2 (p<0.001), ΔVE/ΔPaCO2 (p<0.001) and ΔP0.1/ΔPaCO2 (p<0.001).
ConclusionsThere is an inverse linear relationship between [HCO3−] and the variation of pH for a given change in PaCO2 and the CO2 response.
Existe controversia en si las diferencias en la concentración plasmática de bicarbonato (CO3H−) modifican la respuesta al incremento de CO2. Hemos analizado la relación entre la CO3H− y la variación en la concentración de iones de hidrógeno (pH) por un incremento agudo de la PaCO2 y entre la CO3H− y la respuesta del sistema respiratorio al incremento de CO2.
DiseñoEstudio retrospectivo.
ÁmbitoDos unidades de cuidados intensivos.
PacientesPacientes con y sin enfermedad pulmonar obstructiva crónica (EPOC) en el inicio de la desconexión de ventilación mecánica.
IntervencionesLa respuesta del sistema respiratorio al incremento de CO2 fue evaluada por el método de reinhalación del aire espirado, midiendo la respuesta ventilatoria a la hipercapnia (ΔVE/ΔPaCO2) y la respuesta del centro respiratorio a la hipercapnia (ΔP0,1/ΔPaCO2), donde VE es el volumen minuto y P0,1 es la presión de oclusión de la vía aérea a 0,1 s del inicio de la inspiración.
Variables de interés principalesCO3H− y respuesta al CO2.
ResultadosFueron estudiados 120 pacientes sin EPOC y 48 con EPOC. Las CO3H− medias en los pacientes sin y con EPOC fueron de 25,7±3,7 y 33,2±5,4 mmol/L, respectivamente (p<0,001). Hallamos, en ambos grupos de pacientes, una relación linear inversa entre la CO3H− y el cambio de pH por mmHg de PaCO2 (p<0,001), el ΔVE/ΔPaCO2 (p<0,001) y el ΔP0,1/ΔPaCO2 (p<0,001).
ConclusionesHay una relación linear inversa entre la CO3H− y la variación en el pH por un incremento agudo de la PaCO2 y entre la CO3H− y la respuesta al CO2.
Elevated plasma bicarbonate concentration is a relatively common entity in critically ill patients that can influence the process of weaning of mechanical ventilation.1 Several factors may contribute to elevated plasma bicarbonate concentration in critically ill patients, such as chronic hypercapnia, permissive hypercapnia in patients with acute respiratory distress syndrome, the use of diuretics and steroids, electrolyte disturbances (hypochloremia, hypokalemia), and hypoalbuminemia, among other factors.2
Elevated plasma bicarbonate concentration may lead to compensatory hypoventilation and subsequent carbon dioxide (CO2) retention to maintain acid-base homeostasis. Also, elevated plasma bicarbonate concentration can blunt the change in hydrogen ion concentration (pH) for a given change in the partial pressure of carbon dioxide (PaCO2), in accordance with the Henderson-Hasselbalch relationship, thereby blunting CO2 response. In spite of this, the effect of plasma bicarbonate concentration on CO2 response is controversial. While some studies including a few number of healthy subjects or stable patients with chronic hypercapnia found that the ventilatory response to CO2 depends on the levels of plasma bicarbonate concentrations,3–6 other studies found that the CO2 response was not affected by bicarbonate levels.7–11
To our knowledge, the effect of plasma bicarbonate concentration on the CO2 response during mechanical ventilation has been studied only in patients with obesity-hypoventilation syndrome.12 If such a relationship between the plasma bicarbonate concentration and CO2 response is confirmed in a general population of critically ill patients, normalizing elevated bicarbonate levels could be a useful tool to reduce the time of weaning of mechanical ventilation by increasing the CO2 response. Whether the same occurs in chronic elevations of plasma bicarbonate levels, as in patients with chronic obstructive pulmonary disease (COPD), and in acutely induced elevated plasma bicarbonate, has not been addressed yet.
The objectives of this study were to analyze the relationship between the plasma bicarbonate concentration and the variation in pH for a given change in PaCO2 and to evaluate the effect of the level of plasma bicarbonate concentration on CO2 response in patients with and without COPD in the weaning of mechanical ventilation.
MethodsPatientsFor the purpose of this analysis we selected cases from a database of patients with mechanical ventilation and CO2 response tests performed. The patients were selected if the CO2 response test was performed on the first day of meeting criteria for a spontaneous breathing trial. The patients were grouped in COPD and non-COPD. COPD was diagnosed according pulmonary function tests by the forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) ratio and the FEV1 expressed as percentage of predicted. We excluded those patients admitted in the intensive care unit (ICU) with acute respiratory failure due to acute injury of the central nervous system, neuromuscular disease or patients with chronic hypercapnia due to other reason than COPD.
Patients were retrospectively and non-consecutively studied in two medical-surgical ICUs, at the beginning of the weaning of mechanical ventilation. Hospital research committee approved the study. Informed consent was obtained in all cases from patients or closest relatives. Most of the patients of this study have been included in previous studies of our group.13–15
ProtocolPatients were studied when the physician in charge considered that they had clinical stability and fulfilled criteria for a spontaneous breathing trial. These criteria included that the patient was hemodynamically stable, without sedation awake and able to obey oral commands, had core temperature below 38.3°C and a ratio of the partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) above 150mm Hg with a positive end-expiratory pressure (PEEP) of ≤8cm H2O.
When the patients were clinically stable and ready for a spontaneous breathing trial, respiratory neuromuscular function was evaluated by measurement of maximal inspiratory pressure (PImax), maximal expiratory pressure (PEmax), and CO2 response test. All these measurements were carried out in the semirecumbent position at 30 degrees. We continuously recorded electrocardiogram, heart rate, pulse oximetry, and invasive systemic blood pressure.
Measurements and proceduresMaximal inspiratory and expiratory pressureMuscular respiratory strength was assessed with the PImax and PEmax measurements. PImax and PEmax were measured, after 1–2min of spontaneous breathing, with an external pressure transducer via a unidirectional valve (Hans Rudolph, Kansas City, MO) connected to the endotracheal tube. PImax was obtained at residual volume, by occluding the inspiratory port of the unidirectional valve, whereas PEmax was measured at total lung volume, by occluding the expiratory port.16 After 20–25s of occluded inspiration or expiration, the most negative or positive pressure values were recorded for PImax or PEmax test. Two measurements were performed and the highest value was used for analysis.
CO2 response testTo increase the CO2 we used the method of the re-inhalation of expired air13,17 by inserting a corrugated tube between the Y-piece and the endotracheal tube that increased the dead space with a volume similar to the tidal volume (VT) obtained with a pressure support of 7cm H2O in each patient. The increase of dead space was made with a continuous corrugated tube (CORR-A-FLEX II® 22mm Tubing, Hudson RCI®, Temecula, USA).
Baseline values for CO2 response test were obtained after applying 5min of pressure support ventilation with a pressure of 7cm H2O without PEEP, and FiO2 was set at 1.0 to prevent hypoxemia for patients’ security and to avoid hypoxic stimuli. Then, respiratory rate, airway occlusion pressure 0.1s after the beginning of inspiration (P0.1), and minute volume (VE) were recorded from the ventilator, and an arterial blood sample was withdrawn. Thereafter, we initiated the CO2 response test by increasing dead space maintaining the same ventilatory support, and when the exhaled CO2 (measured through capnography) had increased by above 10mm Hg, we measured again the respiratory rate, P0.1 and VE, and withdrew another arterial blood sample. Once the CO2 response test was finished the added dead space was removed and the patient was returned to his original assisted ventilation mode.
We studied the following derived indexes of CO2 response test: the hypercapnic ventilatory response (ΔVE/ΔPaCO2), defined as the ratio of the change in VE (ΔVE) to the change in PaCO2 (ΔPaCO2) and the hypercapnic drive response (ΔP0.1/ΔPaCO2), defined as the ratio of the change in P0.1 (ΔP0.1) to ΔPaCO2. The changes in VE, P0.1, and PaCO2 were calculated as the difference between the value at the end of the CO2 response test and the baseline value. P0.1 was measured by means of the built-in system of the Dräger ventilator (Evita 2 Dura or Evita 4, Dräger, Lübeck, Germany),18,19 and P0.1 was calculated as the mean of 5 measurements at each point of the study. Arterial blood gases were measured with a blood gas analyzer (IL-1650, Instrument Laboratory, Izasa, Spain).
Data collection and definitionsWe recorded the following clinical variables: gender, age, height, weight, the Simplified Acute Physiological Score (SAPS) II, length of mechanical ventilation before the study day, ICU and in-hospital length of stay, and in-hospital mortality.
We considered the study day as the day that the CO2 response test was performed. Length of mechanical ventilation before the study day was defined as the number of days between the beginning of mechanical ventilation and the day the CO2 response test was performed. Patients were followed-up until discharge from our two hospitals.
Statistical analysisCategorical data are expressed as number and percentages. Continuous variables are expressed as mean±standard deviation or as median and interquartile ranges (IQR). Differences between groups were compared with t test and chi-square test. Non-COPD and COPD patients were grouped into tertiles according to the baseline level of plasma bicarbonate concentration. Trend analysis among the tertiles of plasma bicarbonate concentration and changes of pH by changes of PaCO2, minute volume, CO2 response, tidal volume and respiratory rate, were conducted with Jonckheere–Terpstra test. Statistical analysis was performed with specific statistics software (SPSS 19.0, SPSS, Chicago, IL).
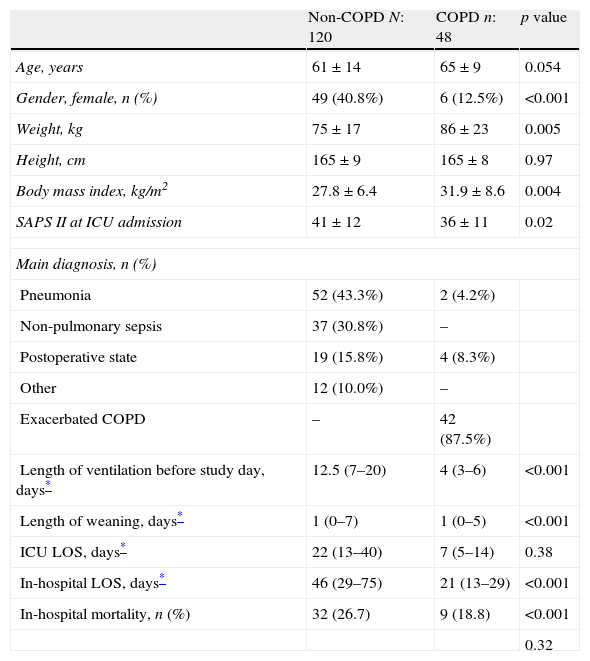
ResultsOne hundred and sixty-eight patients were studied, 120 patients in the non-COPD group and 48 in the COPD group. The COPD group had a mean FEV1/FVC ratio of 54±12% and of the FEV1 of 37±16%. Compared with non-COPD patients, COPD patients were mostly men, with higher body mass index and lower severity disease (Table 1). The main diagnoses of non-COPD patients were pneumonia and non-pulmonary sepsis (Table 1). Non-COPD patients had longer duration of ventilation before the study day, and longer ICU and in-hospital length of stay (Table 1).
Clinical characteristics of 168 patients.
| Non-COPD N: 120 | COPD n: 48 | p value | |
| Age, years | 61±14 | 65±9 | 0.054 |
| Gender, female, n (%) | 49 (40.8%) | 6 (12.5%) | <0.001 |
| Weight, kg | 75±17 | 86±23 | 0.005 |
| Height, cm | 165±9 | 165±8 | 0.97 |
| Body mass index, kg/m2 | 27.8±6.4 | 31.9±8.6 | 0.004 |
| SAPS II at ICU admission | 41±12 | 36±11 | 0.02 |
| Main diagnosis, n (%) | |||
| Pneumonia | 52 (43.3%) | 2 (4.2%) | |
| Non-pulmonary sepsis | 37 (30.8%) | – | |
| Postoperative state | 19 (15.8%) | 4 (8.3%) | |
| Other | 12 (10.0%) | – | |
| Exacerbated COPD | – | 42 (87.5%) | |
| Length of ventilation before study day, days* | 12.5 (7–20) | 4 (3–6) | <0.001 |
| Length of weaning, days* | 1 (0–7) | 1 (0–5) | <0.001 |
| ICU LOS, days* | 22 (13–40) | 7 (5–14) | 0.38 |
| In-hospital LOS, days* | 46 (29–75) | 21 (13–29) | <0.001 |
| In-hospital mortality, n (%) | 32 (26.7) | 9 (18.8) | <0.001 |
| 0.32 | |||
SAPS: simplified acute physiology score; COPD: chronic obstructive pulmonary disease.
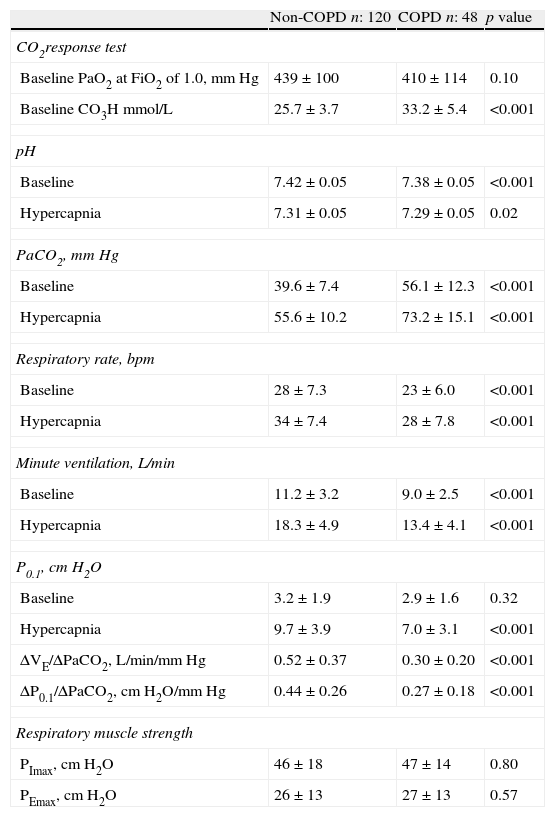
Between non-COPD and COPD groups there was no difference in the added dead space used to increase the PaCO2. Mean values were 414±70mL and 392±68mL respectively (p=0.08). The baseline values of CO2 response test, with pressure support ventilation of 7cm H2O and FiO2 of 1.0 showed a higher plasma bicarbonate concentration and PaCO2 and lower minute volume, respiratory rate and pH in COPD group compared with the non-COPD group (Table 2). No differences were found in mean PaO2 and baseline P0.1 values between groups (Table 2). The ventilatory and central CO2 response was lower in COPD patients than in non-COPD. No differences were found in respiratory muscle strength assessed by PImax and PEmax between groups (Table 2).
CO2 response test (baseline and hypercapnia values of arterial blood gases and ventilatory parameters) and respiratory muscle strength (PEmax and PImax values).
| Non-COPD n: 120 | COPD n: 48 | p value | |
| CO2response test | |||
| Baseline PaO2 at FiO2 of 1.0, mm Hg | 439±100 | 410±114 | 0.10 |
| Baseline CO3H mmol/L | 25.7±3.7 | 33.2±5.4 | <0.001 |
| pH | |||
| Baseline | 7.42±0.05 | 7.38±0.05 | <0.001 |
| Hypercapnia | 7.31±0.05 | 7.29±0.05 | 0.02 |
| PaCO2, mm Hg | |||
| Baseline | 39.6±7.4 | 56.1±12.3 | <0.001 |
| Hypercapnia | 55.6±10.2 | 73.2±15.1 | <0.001 |
| Respiratory rate, bpm | |||
| Baseline | 28±7.3 | 23±6.0 | <0.001 |
| Hypercapnia | 34±7.4 | 28±7.8 | <0.001 |
| Minute ventilation, L/min | |||
| Baseline | 11.2±3.2 | 9.0±2.5 | <0.001 |
| Hypercapnia | 18.3±4.9 | 13.4±4.1 | <0.001 |
| P0.1, cm H2O | |||
| Baseline | 3.2±1.9 | 2.9±1.6 | 0.32 |
| Hypercapnia | 9.7±3.9 | 7.0±3.1 | <0.001 |
| ΔVE/ΔPaCO2, L/min/mm Hg | 0.52±0.37 | 0.30±0.20 | <0.001 |
| ΔP0.1/ΔPaCO2, cm H2O/mm Hg | 0.44±0.26 | 0.27±0.18 | <0.001 |
| Respiratory muscle strength | |||
| PImax, cm H2O | 46±18 | 47±14 | 0.80 |
| PEmax, cm H2O | 26±13 | 27±13 | 0.57 |
COPD: chronic obstructive pulmonary disease; P0.1: occlusion pressure at 100ms; ΔVE/ΔPaCO2: hypercapnic ventilatory response; ΔP0.1/ΔPaCO2: hypercapnic drive response; PImax: maximal inspiratory pressure; PEmax: maximal expiratory pressure.
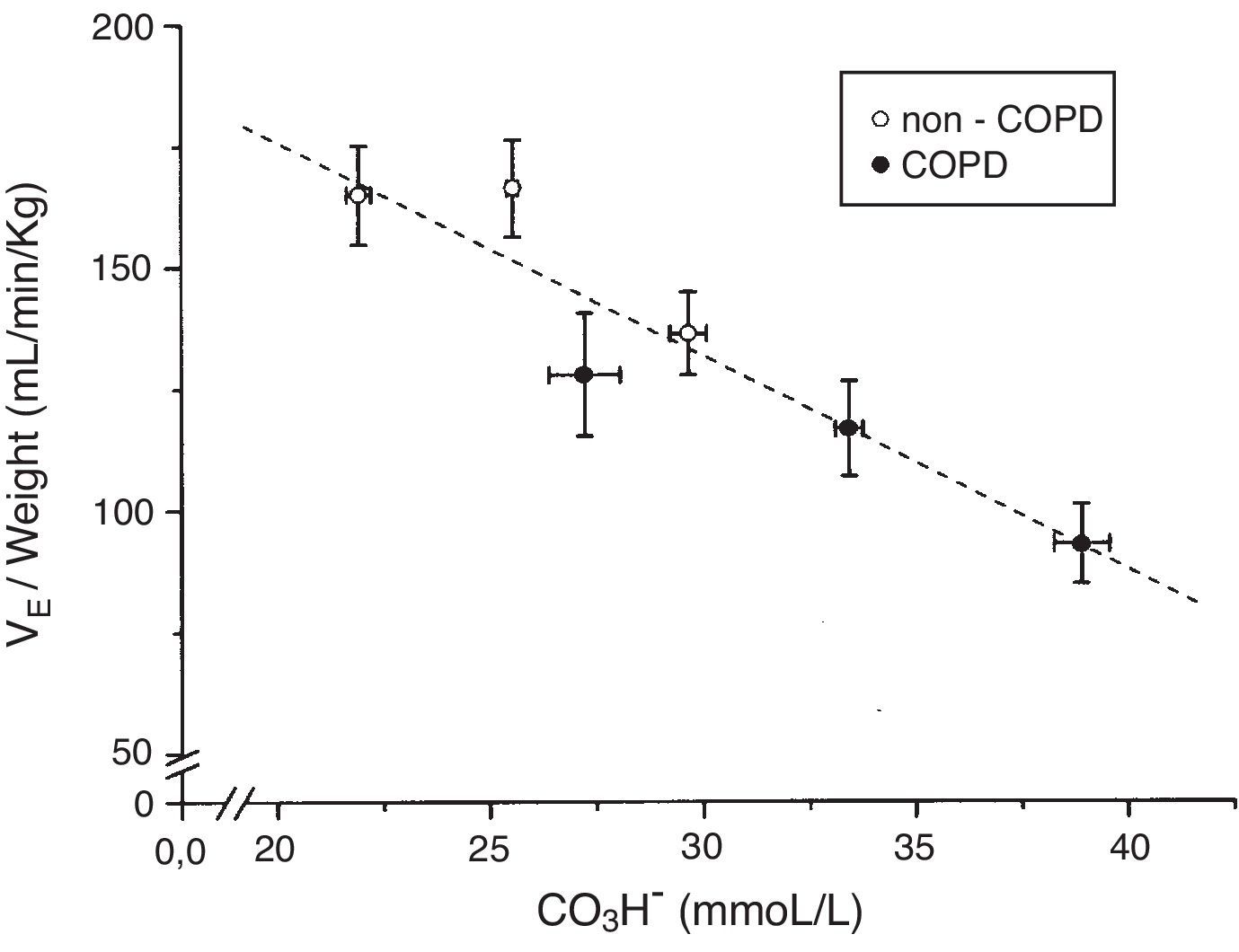
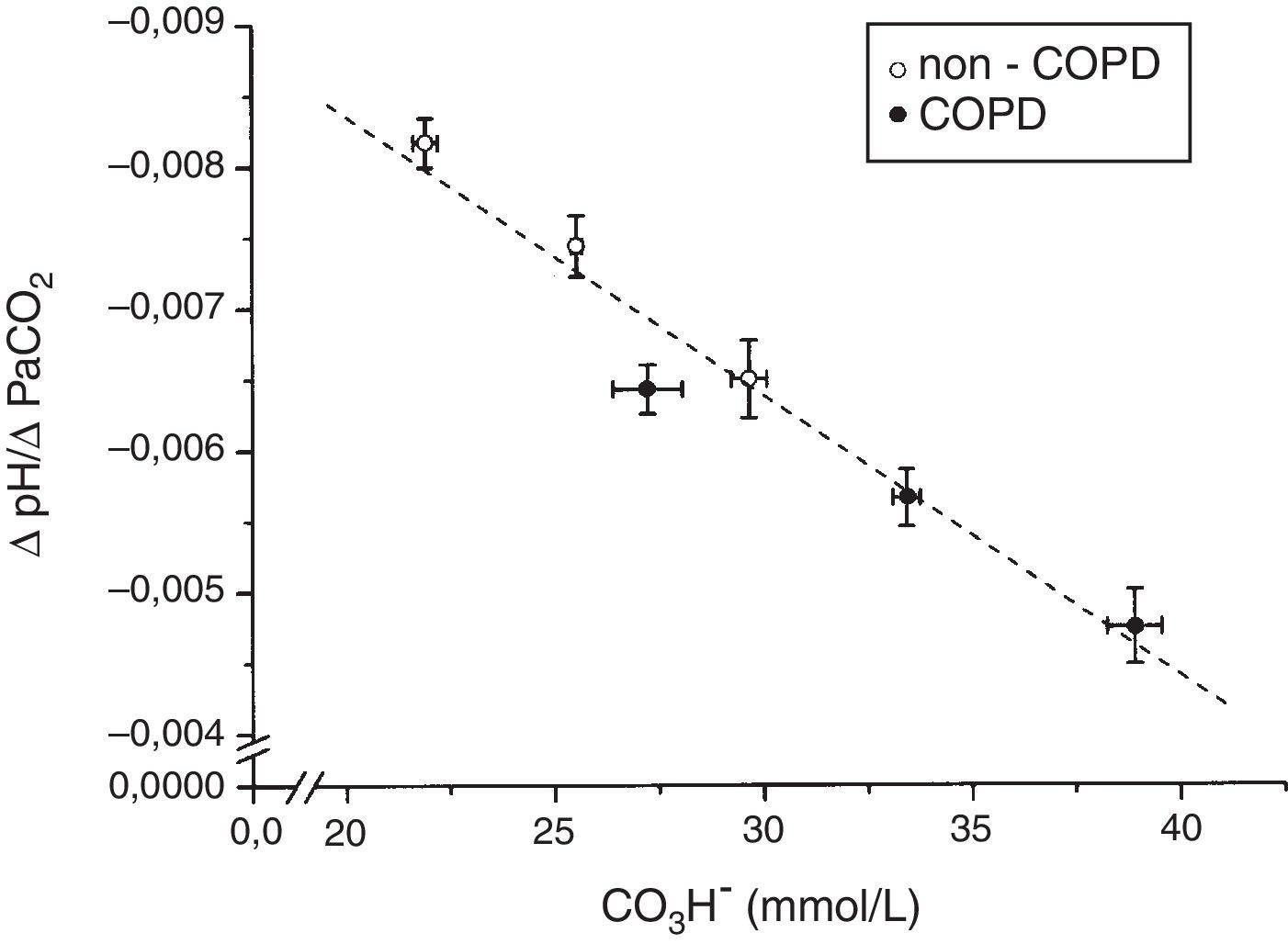
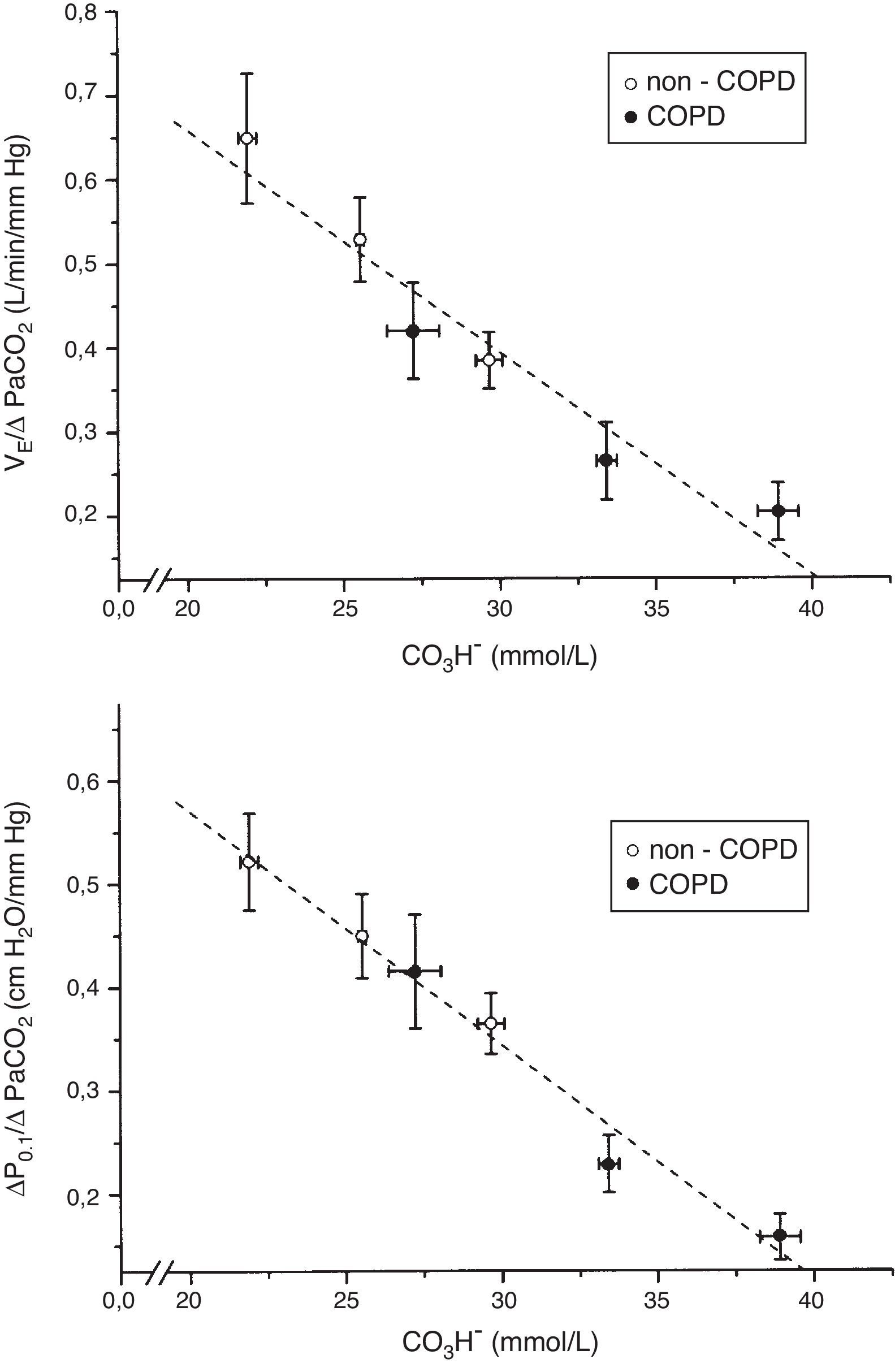
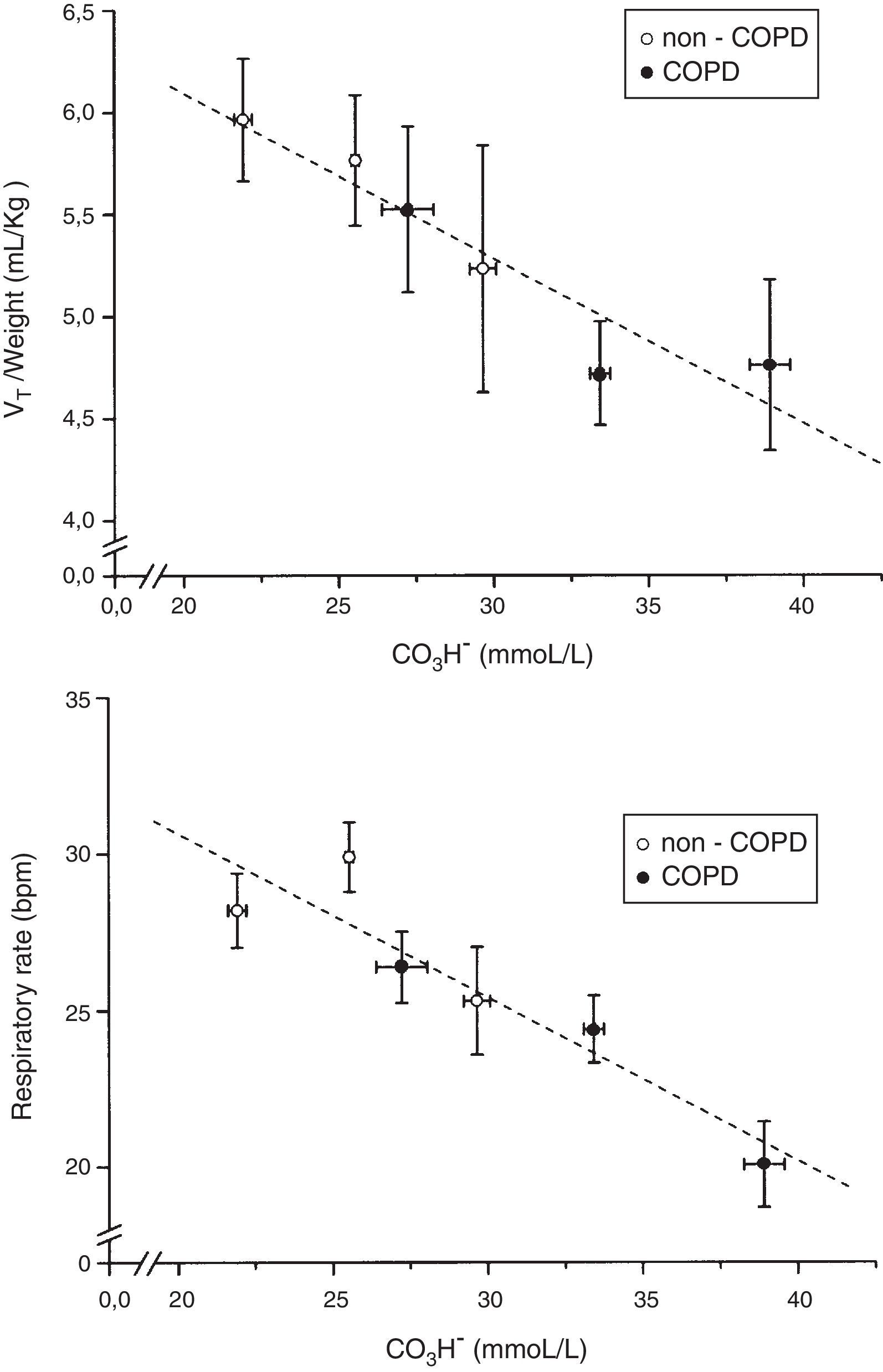
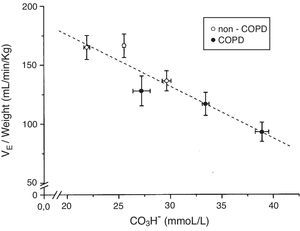
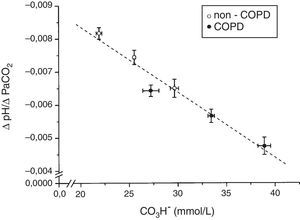
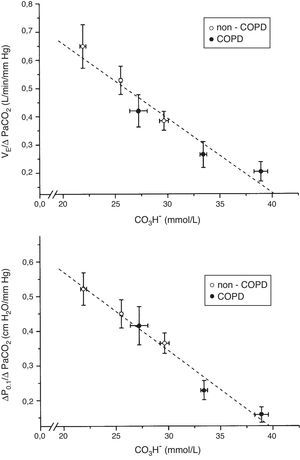
The tertile ranges of plasma bicarbonate concentration were 17.1 to 24.1, 24.1 to 26.6 and 26.6 to 37.5mmol/L for non-COPD patients, and 19.7 to 30.9, 30.9 to 35.5 and 35.5 to 43.7mmol/L for COPD patients. We found a significant inverse linear relationship between plasma bicarbonate concentration grouped in tertiles, in non-COPD and COPD groups of patients, and baseline minute volume (p<0.001) (Fig. 1). The same inverse linear relationship in both groups of patients were found between the tertiles of plasma bicarbonate concentration and pH change per mm Hg of PaCO2induced by CO2response test (p<0.001) (Fig. 2), and between the tertiles of plasma bicarbonate concentration and hypercapnic ventilatory response (p<0.001) and hypercapnic drive response (p<0.001) (Fig. 3).
Relationship between plasma bicarbonate concentration grouped in tertiles and baseline minute volume expressed by body weight in mL/min/kg, in non-COPD (white circles) and COPD patients (black circles). Dashed lines show the linear relationship among groups (p<0.001 for each variable). Values are expressed as mean and standard error.
Relationship between plasma bicarbonate concentration grouped in tertiles and hypercapnic ventilatory response (ΔVE/ΔPaCO2) (top), and hypercapnic drive response (ΔP0.1/ΔPaCO2) (bottom), in non-COPD (white circles) and COPD patients (black circles). Dashed lines show the linear relationship among groups (p<0.001 for each variable). Values are expressed as mean and standard error.
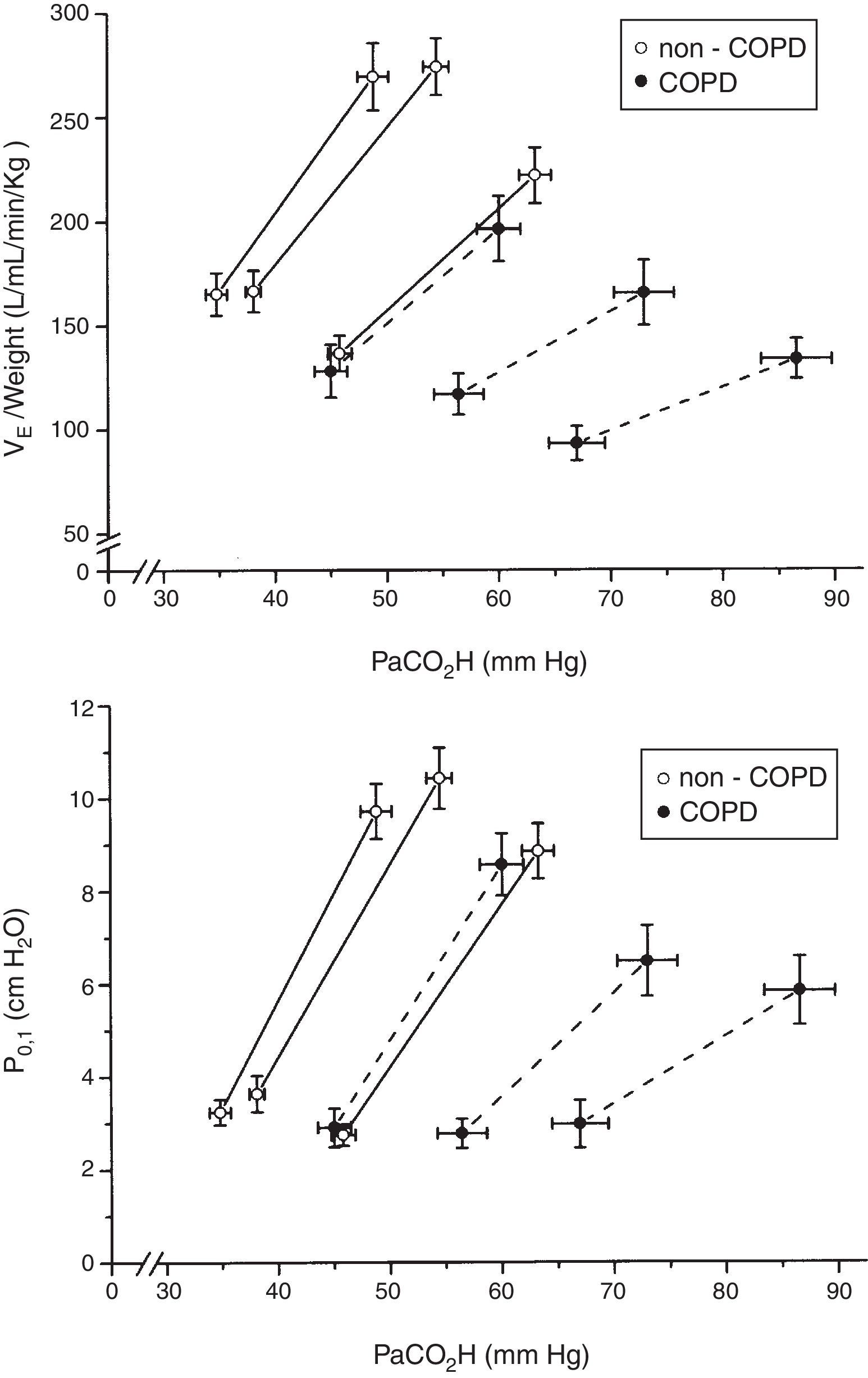
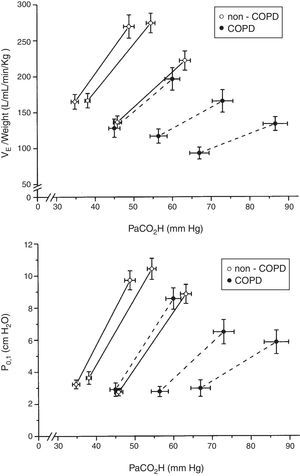
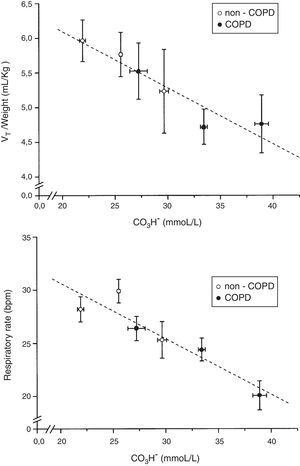
When plotted minute volume and P0.1 against PaCO2, in non-COPD and COPD patients grouped by tertiles of plasma bicarbonate concentration, the slope of the CO2 response flattened with high levels of plasma bicarbonate concentration (Fig. 4). Of note, baseline values of minute volume decreased with the increased level of plasma bicarbonate, without changes in P0.1. Minute volume decreases through decrease in tidal volume (p=0.003) and respiratory frequency (P<0.001) (Fig. 5).
Relationship between plasma bicarbonate concentration grouped in tertiles and tidal volume (VT) in mL/kg of body weight and respiratory rate in breath per minute (bpm), in non-COPD (white circles) and COPD patients (black circles). Dashed lines show the linear relationship among groups. Values are expressed as mean and standard error.
In this study we found an inverse relationship between the plasma bicarbonate concentration and the variation in pH for a given change in PaCO2. Indeed, the most interesting finding was that the response to CO2 also inversely depends on the level of plasma bicarbonate concentration, both in non-COPD and COPD patients, during the weaning of mechanical ventilation.
These results are in agreement with previous studies in healthy subjects3,4 and chronic hypercapnia secondary to kyphoscoliosis or skeletal muscle disease.5,6 In these studies the variations in plasma bicarbonate concentration were induced by oral administration of sodium bicarbonate and ethacrynic acid, or ammonium chloride. Our results are also in accordance with a previous study with obesity-hypoventilation syndrome patients during the weaning of mechanical ventilation.12 In that study we found the same inverse relationship between plasma bicarbonate concentration and CO2 response. We also found that lowering plasma bicarbonate concentration by acetazolamide administration increased the CO2 response in a small subset of patients.12
One possible explanation for several previous studies that did not find the relationship between the plasma bicarbonate concentration and CO2 response was that the changes in the bicarbonate concentrations were small and in some cases only toward acidosis.7–11 Changes in the slope of the CO2 response are more evident when the patients reach high levels of plasma bicarbonate concentration, as occurred in our study.
The ventilatory adaptation to an increase of plasma bicarbonate concentration is primarily achieved by decrease of tidal volume in subjects without lung disease,6 while we found a decrease in both tidal volume and respiratory rate. This discordance may be related with the fact that they were studied with a pressure support of 7cm H2O. Indeed, Brochard et al.20 found that the ventilation with a pressure support of 10cm H2O during weaning of mechanical ventilation resulted in significant improvements in tidal volume with a decreased respiratory rate compared to spontaneous ventilation.
The clinical significance of this relationship between the level of plasma bicarbonate concentration and baseline minute volume and CO2 response relies on the fact that modifying the plasma bicarbonate concentration we can change the baseline minute volume, the CO2 response, and the work of breathing. Thus, in patients with COPD we could increase the minute volume and the CO2 response by reducing the plasma bicarbonate concentration. Reducing elevated levels of plasma bicarbonate concentration is simple with the administration of acetazolamide, as observed in our study of patients with obesity-hypoventilation syndrome.12 Ongoing clinical trials evaluating the use of acetazolamide to facilitate the weaning of mechanical ventilation will address this issue. However, one must bear in mind several issues when considering this potential treatment. First, when reducing elevated plasma bicarbonate concentration there is an inherent risk of increasing the work of breathing in excess. Second, in non-COPD patients ventilated with high minute volume and with difficulty of weaning of mechanical ventilation, increasing the plasma bicarbonate concentration can reduce the minute volume and therefore the work of breathing.
Along with the plasma bicarbonate concentration, the CO2 response depends on other factors such as the family,21 age,22 and circadian rhythm.23 In this regard, we note that approximately 15% of healthy subjects have decreased response to CO2. These individuals are likely to develop CO2 retention when additional respiratory problems arise, such as obesity, obstructive lung disease or status asthmaticus.21,24,25 The reduced CO2 response in COPD patients cannot be attributed to hyperoxia. In these patients, the increase in the PaCO2 by hyperoxia is due to changes in the ventilation-perfusion distribution26,27 and to the increase in dead space by the Haldane effect on PaCO2.28Likewise, the high PaO2observed in our patients may be explained by the FiO2of 1 used in the study to correct hypoxemia in all lung units regardless of their ventilation-perfusion ratio except with a pure shunt.
The main limitation of this study was the difficulty to interpret the results of the CO2 response tests due to the wide range of normal values in healthy subjects.29,30 In addition, the coefficients of variation are also wide, from 17.9% for the ventilatory response (ranged from 8.3 to 26.3%)31 and about 60% for the P0.1 during CO2 rebreathing trials.32 Other limitations of our study were the way in which we measured CO2 response with the ventilator, instead of with the conventional method.33 The Evita ventilator tends to overestimate high P0.1 values and to underestimate low P0.1 values.19 Another possible limitation is air trapping due to the high minute volume that occurs with hypercapnic stimulation or by effect of bronchoconstriction in patients with COPD. However, Conti et al.34 found that reliable measurement of P0.1 can be obtained during pressure-support ventilation in patients with variable levels of intrinsic PEEP.
In conclusion, we observed an inverse relationship between the plasma bicarbonate concentration and the variation in hydrogen ion concentration for a given change in PaCO2, and we found that elevated levels of plasma bicarbonate reduced the CO2 response both in COPD and non-COPD critically ill patients during weaning of mechanical ventilation.
Conflict of interestAll authors declare that they have no conflict of interest.