Cerebral vasospasm, one of the main complications of subarachnoid hemorrhage (SAH), is characterized by arterial constriction and mainly occurs from day 4 until the second week after the event. Urotensin-II (U-II) has been described as the most potent vasoconstrictor peptide in mammals. An analysis is made of the serum U-II concentrations and mRNA expression levels of U-II, urotensin related peptide (URP) and urotensin receptor (UT) genes in an experimental murine model of SAH.
DesignAn experimental study was carried out.
SettingExperimental operating room of the Biomedicine Institute of Seville (IBiS), Virgen del Rocío University Hospital (Seville, Spain).
Participants96 Wistar rats: 74 SAH and 22 sham intervention animals.
InterventionsDay 1: blood sampling, followed by the percutaneous injection of 100μl saline (sham) or blood (SAH) into the subarachnoid space. Day 5: blood sampling, followed by sacrifice of the animals.
Main variables of interestWeight, early mortality, serum U-II levels, mRNA values for U-II, URP and UT.
ResultsSerum U-II levels increased in the SAH group from day 1 (0.62pg/mL [IQR 0.36–1.08]) to day 5 (0.74pg/mL [IQR 0.39–1.43]) (p<0.05), though not in the sham group (0.56pg/mL [IQR 0.06–0.83] day 1; 0.37pg/mL [IQR 0.23–0.62] day 5; p=0.959). Between-group differences were found on day 5 (p<0.05). The ROC analysis showed that the day 5 serum U-II levels (AUC=0.691), URP mRNA (AUC=0.706) and UT mRNA (AUC=0.713) could discriminate between sham and SAH rats. The normal serum U-II concentration range in rats was 0.56pg/mL (IQR 0.06–0.83).
ConclusionThe urotensinergic system is upregulated on day 5 in an experimental model of SAH.
El vasoespasmo cerebral, una de las principales complicaciones secundarias a hemorragia subaracnoidea (HSA), se caracteriza por una constricción arterial que tiene lugar principalmente entre el día 4 y la segunda semana. La urotensina-II (U-II) ha sido definida como el péptido con mayor capacidad vasoconstrictora en mamíferos. Quisimos analizar los niveles séricos de U-II, así como los niveles de expresión de los genes de U-II, péptido relacionado con urotensina y receptor de urotensina, en un modelo murino experimental de HSA.
DiseñoEstudio experimental.
ÁmbitoQuirófano experimental del Instituto de Biomedicina de Sevilla, Hospital Universitario Virgen del Rocío.
ParticipantesNoventa y seis ratas Wistar: 74 con inyección percutánea de sangre (HSA), 22 con inyección percutánea de 100μL de salino (Sham).
IntervencionesDía 1: extracción de muestras de sangre. Posteriormente, inyección percutánea de 100μL de salino (Sham) o de sangre (HSA) en el espacio subaracnoideo. Día 5: extracción de muestras de sangre y sacrificio del animal.
Principales variables de interésPeso, mortalidad precoz, niveles séricos de U-II, valores de ARNm de U-II, péptido relacionado con urotensina y receptor de urotensina.
ResultadosObservamos un incremento en los niveles de U-II sérica en el grupo HSA desde el día 1 (0,62pg/mL [RI 0,36-1,08]) al día 5 (0,74pg/mL [RI 0,39-1,43]) (p<0,05); pero no observamos tal diferencia en el grupo Sham (0,56pg/mL [RI 0,06-0,83] día 1; 0,37pg/mL [RI 0,23-0,62] día 5) (p=0,959). Se encontraron diferencias en los niveles de U-II entre ambos grupos al quinto día (p<0,05). El análisis de curvas ROC demostró que la U-II sérica al quinto día (AUC=0,691), ARNm de péptido relacionado con urotensina (AUC=0,706) y ARNm de receptor de urotensina (AUC=0,713) podían discriminar entre ratas Sham y HSA. Además, definimos un rango de normalidad para los niveles de U-II séricos en ratas: 0,56pg/mL (RI 0,06-0,83).
ConclusiónEste estudio demuestra por primera vez que el sistema urotensinérgico ve incrementada su expresión en el quinto día en un modelo de HSA.
Spontaneous subarachnoid hemorrhage (SAH) has an annual incidence rate of 4–28 cases per 100,000 people.1,2 SAH accounts for about 80% of all nontraumatic extravasated bleeding into the subarachnoid space, 5% of stroke deaths and over a quarter of potential life years lost due to stroke.1,3,4 Approximately 15% of SAH patients die after aneurysmal rupture. Another 25–50% die within a month of the bleeding. Of those who survive, 40% present disabling sequelae.5,6 It is estimated that cerebral vasospasm (CVS) is responsible for neurological deterioration, and even death, in 15–20% of patients with SAH.5,7 CVS is characterized by diffuse and long-lasting arterial constriction. Several vasoconstrictor proteins have been shown to contribute to this narrowing process.8–11 However, Urotensin-II (U-II), defined as the most potent vasoconstrictor peptide in mammals according to Ames et al. research study, is not among these biomarkers.12
U-II is an 11-amino-acid peptide with a cysteine disulfide bond, derived from the polypeptide precursor known as prepro-urotensin-II (preproU-II).13 Proteolytic cleavage of the C-terminal fragment from this precursor is required for biological activity. The result of this proteolysis is an undecapeptide with a cyclic hexapeptide sequence, fundamental for this hormonal action.14 Once the active form of the peptide is generated, U-II mediates its biological action by interacting with a specific plasma membrane G-protein coupled receptor identified as GRP14, or UT.12 This receptor binds different U-II sequences, including the U-II fragment (4–11) and U-II (5–11), as well as the urotensin-related peptide (URP), which derives from a different precursor.15 URP is shorter (octapeptide), but exhibits the cyclic hexapeptide core sequence. U-II expression has been found throughout the heart, kidney, urogenital system and nervous system.16–18 Tian et al. described its expression, as well as UT expression, in the cytoplasm of neurons and in vascular endothelial cells from rats.19 Our research group recently found that high doses of U-II (10μM) in intact rat cerebral arteries evoke arterial contraction. However, in depolarized vascular smooth muscle, lower U-II concentrations (0.1 and 1μM) cause dose-dependent arterial contraction.20 This effect could facilitate arterial CVS in vascular pathophysiological processes such as SAH, where sustained depolarization is present.21
The objective of the present study was to determine the normal range for U-II serum levels and analyze U-II serum and mRNA expression values for the urotensinergic system genes (U-II, URP and UT) in an experimental murine model of SAH; with the purpose of using these molecules as biomarkers of arterial vasoconstriction in SAH.
Materials and methodsProcedures were performed in the experimental operating room of the Biomedicine Institute of Seville (Instituto de Biomedicina de Sevilla [IBiS]/CSIC University of Seville), at the Virgen del Rocío University Hospital, Seville, Spain. This research project was overseen and approved by our hospital's Animal Experimentation Committee. It met all the legal requirements and ethical standards for research established by current legislation (Directive 2010/63/EU).
Animals and samplesWistar rats weighing 300–350g were used. The procedure was performed on males, to eliminate the cyclic cardioprotective effects of the estrous cycle. After the procedure, the animals were housed in independent cages, in a dry area away from infectious sources and surgical areas, and kept on a 12:12h light/dark cycle. A stable room temperature was set between 23°C and 27°C. Food and water was provided without restrictions, before and after surgery.
Animals were anesthetized by intraperitoneal injection of the following preparation: 50mg ketamine hydrochloride (Ketolar©, Pfizer; 50mg/mL), 2mL Xylazine (Rompun© 2%, Bayer; 20mg/mL) and 1mg atropine (Atropina Bayer©, 1mg/mL). The optimal dose for analgesia and sedation was established at 0.025mL per gram of body weight.20,22 Adequate depth of anesthesia in the spontaneously breathing rats was ensured by the absence of corneal reflex and withdrawal reflex after pressure on the hind legs. Cardiac and respiratory rates were measured during the procedure.
Procedures for the two animal modelsSAH group: The SAH model was executed as previously described by our research group.23 The animals were put in prone position with their heads fixed to a stereotaxic frame (Stoelting©). A non-heparinized syringe was used to obtain 100μL of autologous blood from the proximal part of the tail (1mL BD Plastipak©). This was injected into the intracisternal space via cisterna magna, after extraction of the same volume of CSF.
Sham group: Identical procedures were executed with one exception: 100μL of isotonic saline was administered in place of blood after CSF extraction.
On day 5 post-surgery, the animals were sacrificed with a lethal dose of ketamine hydrochloride (0.01mL/kg) (Ketolar©, Pfizer, 50mg/mL) injected into the intraperitoneal cavity.20,22
Blood draws were performed on day 1 prior to the execution of the animal model and on day 5, prior to the sacrifice. Animals were placed in a supine position for blood sample collection. Using binocular lens, an inguinal incision was made, freeing up layers of the abdominal wall, to expose the femoral vein. For serum isolation, 0.8mL of venous blood was collected in a VACUETTE® serum separator tube containing aprotinin (0.6TIU/mL of blood). An additional extraction was performed on day 5, after the lethal injection of ketamine. Each animal's entire blood volume was extracted by cardiac puncture and collected in a VACUTAINER® CPT™ tube (Cell Preparation Tube) (BD, Franklin Lakes, NJ), for mononuclear cell isolation. Brains were removed and examined macroscopically to analyze the presence or absence of pathological findings.
The VACUETTE® tubes were centrifuged at 1600×g for 10min at room temperature. The separated sera were frozen in aliquots at −80°C prior to their analysis. VACUTAINER® CPT™ tubes were centrifuged at 1900×g for 20min at room temperature. Mononuclear cells were collected from the processed CPT by gently inverting the collection tube several times, and drawing off the mononuclear cells containing plasma with a pipette into a tube containing 10mL of saline. Following the manufacturer's instructions, this tube was centrifuged at 300×g for 5min at 4°C. Mononuclear cells were washed twice with saline as described in the previous step and finally pelleted by centrifugation at 16,000×g for 5min at 4°C in a sterile Eppendorf tube.
Measurement of Urotensin-II serum levelsU-II serum levels were measured by fluorescent enzyme immunoassay (EIA) produced by Phoenix Pharmaceuticals, Inc. For peptide extraction, serum samples were previously loaded into a SEP-COLUMN containing 200mg of C18 (Cat. No. RK-SEPCOL-1) following the manufacturer's indications. Data on cross-reactivity was provided by the manufacturer: 100% with rat UT-II and 11% with URP. No cross-reactivity was found with rat endothelin-I, C-type natriuretic peptide-22, calcitonin gene-related peptide, adrenomedullin, atrial natriuretic peptide, bradykinin, angiotensin II or brain natriuretic peptide.
Nucleic acid extractionThe pelleted mononuclear cells were lysed by repetitive pipetting with 750μL of TRIsure™ Isolation Reagent (Bioline). The samples were incubated for 5min at room temperature to ensure the complete dissociation of nucleoprotein complexes. We then added 300μL of chloroform and vigorously shook the Eppendorf for 15s. This step was followed by 2–15min of incubation on ice, and 15min of centrifugation at 12,000×g, to separate the solution into three phases. The upper colorless phase contained the RNA fraction—it was carefully removed and drawn into an Eppendorf containing 500μL of isopropanol. After 5–10min of incubation, samples of this new mixture were incubated for 2h at −20°C and then centrifuged at 12,000×g for 10min at 4°C to form an RNA pellet. This pellet was isolated and washed twice with 1mL of ethanol. Finally, the pellet was resuspended in 30μL of RNAs-free water.
Prior to cDNA synthesis, extracted RNA concentration and purity (OD260/OD280) were quantified by spectrophotometry at 260–280nm (NanoDrop, PeqLab Technology, Erlangen, Germany). An OD260/OD280 ratio between 1.7 and 2.0 indicates reasonable RNA purity. Samples above or below this purity range were discarded.
One μg of RNA from each sample was retrotranscripted into cDNA with the QuantiTect Reverse Transcription Kit (Qiagen), following the manufacturer's protocol. This protocol includes treating the samples with gDNA wipeout buffer, to eliminate genomic DNA contamination.
Real-time PCRTo conduct a polymerase chain reaction (PCR), half of the lymphocyte samples were selected from both animal groups by applying block randomization. This procedure also ensured that the two subgroups were of equal size. The remaining samples were used for other analyses (unpublished).
Commercially available Real-Time ready assays for the target genes (U-II, URP and UT), and the 18 ribosomal RNA housekeeping genes, were obtained lyophilized in 384-well PCR plates, with forward and reverse primers (400nM) and fluorescently labeled hydrolysis probes (200nM), from Universal Probe Library (Roche Applied Science). Gene expression analysis was performed using real-time PCR with final reaction volumes of 10μL (5μL LightCycler® 480 Probes Master, 2μL water [PCR Grade], 0.5μL RealTime ready Assay and 2.5μL cDNA sample). Thermal cycling on the LightCycler® 480 used the following program: one cycle at 95°C for 10min for enzyme activation and denaturation, followed by 45 cycles of 95°C for 10s, 60°C for 30s and signal detection at 72°C for 1s, with detection and cooling at 40°C for 30s. The samples subjected to the 45 cycles were assayed in duplicate.
Gene expression levels were evaluated using the threshold cycle (Ct) method. This data was normalized to the 18 ribosomal RNA reference gene and analyzed according to the 2−ΔCt method.
Statistical analysisQuantitative variables were presented as medians (interquartile range [IQR]: P25-P75), since they followed a non-normal distribution. We executed the non-parametric Wilcoxon test to analyze within-group differences, and the Mann Whitney U test to verify between-group differences. If significant differences were obtained, 95% confidence intervals (CI) were calculated. Spearman's rank correlation was used to test the association between biomarkers analyzed in this study. Receiver Operator Characteristic (ROC) curve analysis was performed on U-II serum values and on U-II, URP and UT 2−ΔCt values to allocate rats in the Sham or SAH groups. The resulting area under the curve (AUC) was used to establish a cut-off point for animal classification, choosing the point which reached the greatest sum of sensitivity plus specificity. Statistical significance was defined as p<0.05. Statistical analyses were performed using SPSS software (Version 20.0, Chicago, IL, USA).
ResultsA total of 96 animals underwent surgery. They were classified as follows: 22 Sham and 74 SAH. Four animals (including one Sham animal) died within 5 days of the surgical intervention.
The macroscopic evaluation of the brains showed that one SAH rat presented an intercisural hematoma.
Urotensin-II serum levelsWhen analyzing U-II serum levels, we observed the following results: the SAH rats suffered an increase on U-II serum values from day 1 (0.62pg/mL [IQR 0.36–1.8]) to day 5 (0.74pg/mL [IQR 0.39–1.43]) (p=0.04). In the Sham group, no significant differences in U-II serum levels were found between day 1 (0.56pg/mL [IQR 0.06–0.83]) and day 5 (0.37pg/mL [IQR 0.23–0.62]) (p=0.959).
Between-group differences in U-II serum levels were found on day 5 (p=0.008) but not on day 1 (p=0.174).
Based on U-II serum levels on day 1 in the Sham group, we were able to define the normal median basal serum level for U-II in rats: 0.56pg/mL (IQR 0.06–0.83).
U-II, URP and UT mRNA expressionFor conducting PCR, half lymphocyte samples were randomly selected from both animal groups. The remaining samples were stored for possible future analysis. Thus, we selected 37 SAH samples and 11 Sham samples. Three of the SAH group samples did not have sufficient RNA for inclusion in the analysis.
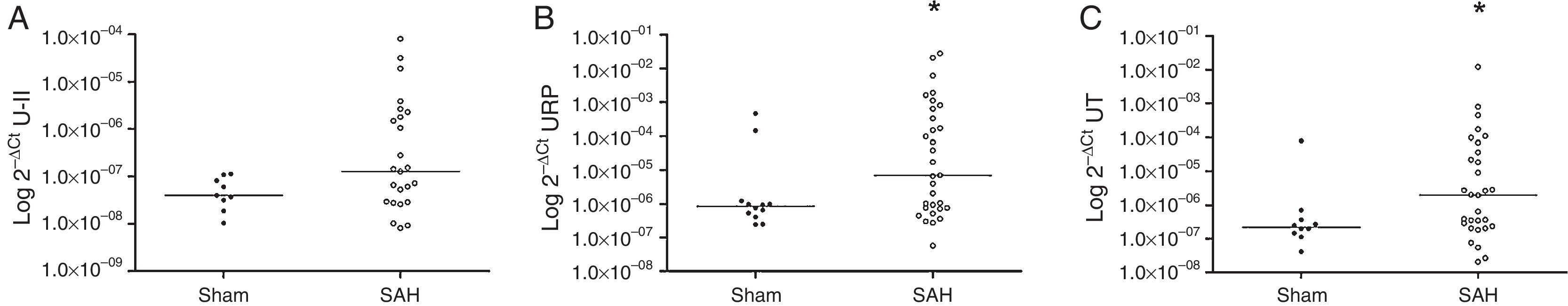
The results showed that the SAH rats had higher expression levels for URP and UT genes: URP 2−ΔCt=7.07×10−6 (IQR 7.48×10−7–6.40×10−3) SAH vs. URP 2−ΔCt=8.38×10−7 (IQR 4.37×10−7–11.58×10−7) Sham, p=0.037; UT 2−ΔCt=1.97×10−6 (IQR 2.26×10−7–4.49×10−5) SAH vs. UT 2−ΔCt=2.22×10−7 (IQR 1.37×10−7–4.50×10−7) Sham, p=0.046 (Fig. 1). U-II expression levels did not reach statistical significance: U-II 2−ΔCt=1.28×10−7 (IQR 2.84×10−8–2.04×10−6) SAH vs. U-II 2−ΔCt=4.02×10−8 (IQR 2.55×10−8–9.64×10−8) Sham, p=0.175.
In the correlation analysis, in the SAH group, all the quantified gene expressions correlated positively with each other: U-II vs. URP r=0.786, p<0.001; U-II vs. UT r=0.727, p<0.001; URP vs. UT r=0.906, p<0.001. Additionally, URP mRNA showed a positive correlation with U-II serum levels on day 1 (r=0.490; p=0.024) and with U-II serum levels on day 5 (r=0.409; p=0.038). In the Sham group, just correlation between U-II 2−ΔCt and UT 2−ΔCt was found (r=0.767; p=0.016).
Receiver operating characteristics curvesROC curves were calculated for U-II serum levels and for U-II, URP and UT mRNA expression. The analysis showed that day 5U-II serum levels, URP mRNA and UT mRNA could discriminate between Sham and SAH rats. The AUC for these variables were: AUC=0.691; 95%CI: 0.565–0.817; for day 5U-II serum levels; AUC=0.706; 95%CI: 0.543–0.868; for URP; and, AUC=0.713; 95%CI: 0.540–0.887; for UT (Fig. 2).
When examining the coordinates of the ROC curve for day 5 U-II serum levels, we observed that the best cut-off, chosen as the one which reached the greatest sum of sensitivity plus specificity, was the value previously defined as the normal median basal serum level for U-II in rats: 0.56pg/mL, with 66.2% sensitivity and 76.2% specificity, 2.15 positive likelihood ratio (LR+), 0.42 negative likelihood ratio (LR−). In our series, the best cut-offs for gene expression were 2−ΔCt=3.77×10−7 for URP, and 2−ΔCt=1.62×10−6 for UT. These cut-offs were applied in our model to determine the accuracy of the variables. URP mRNA displayed 61.3% sensitivity and 83.3% specificity, 3.67 LR+, 0.46 LR−; and UT mRNA showed 60% sensitivity and 80% specificity, 3 LR+, 0.5 LR−.
DiscussionThis is the first study carried out in an experimental murine model to demonstrate that U-II serum levels are significantly elevated on fifth day after SAH, date that coincides with the usual CVS period in both rats24 and humans.25 Moreover, we found that the three urotensinergic system genes were up-regulated after in vivo brain exposure to blood injected into the subarachnoid space. We were also able to define the normal range for U-II serum levels in rats.
The fact that U-II is widely recognized as a peptide with vasoactive properties,26,27 yet no research exists on its role in SAH and its complications, led us to investigate urotensinergic genes in this pathology. In doing so, we detected changes not only in U-II serum level, but in the expression of URP and UT genes as well. We also uncovered the ability of these three genes to discriminate SAH rats. Although no research studies have examined the role of the urotensinergic system in SAH, a recent paper suggested its implication in arterial vasospasm development, based on U-II contraction activity in both intact and depolarized arteries.20 In the present study, the fact that rats with SAH suffer a delayed increase in their gene expression and protein production levels compared to baseline and Sham animals, points to urotensinergic activation in SAH. The increase in plasmatic U-II, limited to the SAH group and confined to the usual CVS period in both rats24 and humans,25 opens the door to a wide range of possibilities for the study of U-II as a biomarker for cerebral arterial vasoconstriction and even the possibility of constituting a new therapeutic target.
The results concerning the correlation between quantified gene expression levels coincides with Yoshimoto et al., who pointed out U-II's function as an autocrine/paracrine.28 Based on our observations, we could highlight this peptide's autocrine activity (correlation between three gene expressions of the urotensinergic system, within the lymphocyte) and its possible paracrine activity (correlation between lymphocyte gene expression, specifically URP, and protein serum levels). Nevertheless, we should note that although there are other sources of U-II release throughout the organism, we only measured mRNA production in the lymphocyte.
U-II has been proposed as a biomarker for various diseases in humans, since its concentration results in a variety of clinical and experimental pathologies, including renal and heart failure, essential and portal hypertension, diabetes, and liver cirrhosis.29 But before using U-II quantification to assess disease onset or progression, we must first establish a normal range for its physiological serum concentration. Several authors have attempted this, and while most agree that U-II serum concentration is found in the picomolar range in species that have been studied, the reports do not agree on the protein's exact concentration range.30–33 On the other hand, some authors suggest that the physiological concentration of U-II is under detectable limits.34 This lack of consensus stems from the absence of interchangeability between the analytical assays employed for U-II quantification.35 These methods include radioimmunoassay, radioreceptor assays, enzyme immunoassays and ELISA.36 The main obstacle is that these methods use antibodies for, but not limited to, U-II (e.g. U-II metabolites). In nonhuman U-II plasma quantification, there is an additional problem: despite the high conservation of this protein throughout the species, the exact sequence varies between them.36 As a result, specific antibodies must be designed for each species. In this study, we solved this problem by using specific biochemical reagents for Rattus norvegicus, in both ELISA and PCR execution. As a result, we were able to define the normal range for U-II serum levels in rats measured by fluorescent enzyme immunoassay (EIA, Phoenix Pharmaceuticals, Inc.). This range of normal values is a significant finding, given that it could serve as a reference for assessing U-II serum levels not only in SAH but in other pathologies as well.
The major strengths of this study rest in the utilization of specific biochemical reagents for the species analysis. Additionally, we performed a very easy and reproducible murine model, which would allow other researchers to reproduce our experiments and continue with this line of investigation. Finally, this animal model of SAH allowed us to study biomarker variation in vivo, and thereby study the progression of the disease in real time.
There were some limitations to the present study. The main limitation was the lack of human analysis. We consider that experimental analysis is important prior to human studies, but we recognize that once demonstrated the up-regulation of the urotensinergic system genes in a SAH model, it is necessary to analyze the same biomarkers in SAH human pathology. We analyzed two blood samples, at baseline and on day 5 after surgery, but did not analyze biomarker evolution throughout the development of the disease. By analyzing more blood samples prior to sacrifice, we could define the kinetics of metabolite production. This research could also benefit from the analysis of other tissues, such as brain tissue, which would provide information on tissue status and their potential gene production.
ConclusionOurs is the first study carried out in an experimental murine model to demonstrate that U-II protein serum levels, as well as U-II, URP and UT mRNA expression levels are up-regulated on the fifth day after a percutaneous SAH, usual CVS period in both rats24 and humans.25 Furthermore, the three genes showed a high discriminative ability to identify SAH rats. These findings suggest that measurements of gene expression or production, depending on the gene, could serve as biomarkers for SAH onset and progression. Additional studies on SAH patients would be necessary to ascertain the U-II correlation with CVS development, and subsequently determine if this tendency is also observed in humans. These noninvasive analyses could offer physicians early and accurate information on a patient's condition. Furthermore, these results open a new research line for the design of new therapeutic targets for the treatment of the SAH pathology.
FundingThis study was funded by a grant from the Instituto de Salud Carlos III (Spanish National Health System) (10/02044), Consejería de Igualdad, Salud y Políticas Sociales de Andalucía, Spain (PI-0136-2012) and Consejería de Igualdad, Salud y Políticas Sociales de Andalucía, Spain (PI-0002-2013).
Authors contributionThe manuscript has been read and approved by all of the authors and each author believes that the manuscript represents honest work.
Conflict of interestThe authors declare no conflicting interest in this work.
This study was funded by a grant from the Instituto de Salud Carlos III (Spanish National Health System) (10/02044), Consejería de Igualdad, Salud y Políticas Sociales de Andalucía, Spain (PI-0136-2012) and Consejería de Igualdad, Salud y Políticas Sociales de Andalucía, Spain (PI-0002-2013).



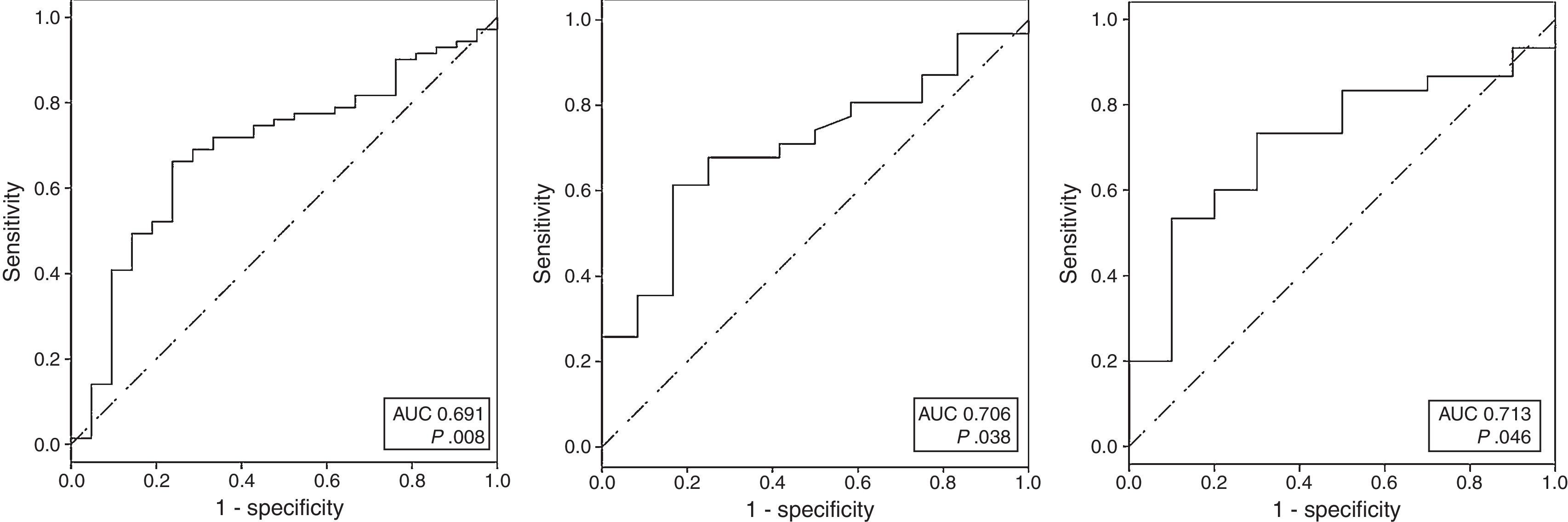

![ROC analysis comparing sensitivity to 1-specificity of serum U-II on fifth day (A), URP mRNA (B) and UT mRNA (C) to discriminate SAH rats. (A: AUC=0.691 [95%CI: 0.565–0.817], p=0.008; AUC=0.706 [95%CI: 0.543–0.868] p=0.038; C: AUC=0.713 [95%CI: 0.540–0.887] p=0.046). ROC analysis comparing sensitivity to 1-specificity of serum U-II on fifth day (A), URP mRNA (B) and UT mRNA (C) to discriminate SAH rats. (A: AUC=0.691 [95%CI: 0.565–0.817], p=0.008; AUC=0.706 [95%CI: 0.543–0.868] p=0.038; C: AUC=0.713 [95%CI: 0.540–0.887] p=0.046).](https://static.elsevier.es/multimedia/02105691/0000004100000008/v1_201710301105/S0210569116302583/v1_201710301105/en/main.assets/thumbnail/gr2.jpeg?xkr=1dZuESKpnCAWr3yCSGZ24A==)


