In continuous renal replacement therapy (CRRT), the contact of blood with the extracorporeal circuit activates both the intrinsic and extrinsic pathways of plasma coagulation and of the platelets, leading to thrombin production, platelet aggregation, thrombus formation, and fibrin deposition. For these reasons, anticoagulation is necessary in CRRT to prevent clotting of the circuit and to maintain the permeability of the membrane to ensure appropriate daily dosing of the technique. The volume of blood circulating in the extracorporeal circuit ranges from 150ml to 250ml, depending on the surface area of the hemofilter. Repeated clotting without the possibility of blood return results in significant blood loss leading to anemia and multiple transfusions. Time in which the technique is interrupted (downtime) reduces the total dose administered respect to the prescribed dose, increases nursing workload, and increases costs. Catheter dysfunction is a common cause of circuit clotting. Choosing the right catheter and vascular access is fundamental to ensure adequate circuit function. This article reviews different strategies to prolong the patency of the circuit, discussing non-pharmacological strategies and the different anticoagulants used in CRRT, including unfractionated heparin, low-molecular-weight heparin, citrate, thrombin inhibitors, and platelet aggregation inhibitors.
Vascular access for continuous renal replacement therapiesThe treatment of acute kidney failure with CRRT requires temporary vascular access. In the early days, CRRT used arteriovenous techniques, but these have been supplanted by venovenous techniques through double-lumen dialysis catheters. Despite the high quality of current catheters, catheters play a role in a high proportion of increased morbidity from CRRT due to complications during insertion, dysfunction (stenosis and/or thrombosis), and infection. The ideal catheter should be rapidly and easily accessible, should enable adequate flow, should be long lasting, and should keep complications to the minimum. This review will discuss the characteristics of catheters, the site and method of inserting them, and their complications.
Characteristics of cathetersCatheters can be characterized by their geometry, material, inner diameter, and length. Geometry: The catheters used nowadays have a double lumen with or without separate distal tips. The proximal lumen is used for the access line and the distal lumen is used for the return line. One of the problems with these catheters is recirculation (the mixing of access and return blood), which decreases the effectiveness of the technique. Increasing the distance (2–3cm) between the access lumen and the return lumen and a design with lateral holes, both help to reduce recirculation.1 The lumen of the catheter can have different designs (coaxial, split, double D, double O), although this does not seems to significantly influence the life of the circuit.2Materials: Nowadays polyurethane and silicone are used; both these materials are very flexible and cause little damage to the vessel wall. In critical patients, polyurethane is more common. Polyurethane catheters can last for several weeks; their main complication is thrombosis. Silicone catheters are used for long-term dialysis, usually with subcutaneous tunneling; this method of canalization makes them longer lasting (they can even last for months) and less prone to infection. Diameter: The inner diameter of a catheter determines how much blood can flow through it. Catheter diameter is usually measured in French (F) units, with 11–14F being the most appropriate for CRRT.3Length: The length of the catheter to be placed depends on the blood vessel used for the approach. If placed in the superior vena cava system through the jugular vein or right subclavian vein, it should measure 15–18cm. If placed in the inferior vena cava system through the femoral vein, it should measure 24–28cm3.
Insertion and siteVenous accesses used for CRRT in critical patients should be easy and quick to insert as they are usually canalized at the bedside during the ICU stay. The catheter must be inserted under conditions of maximum asepsis, normally using percutaneous canalization or the Seldinger technique. Unlike chronic dialysis, CRRT for acute kidney failure in critical patients normally does not last for more than two weeks.4 For this reason, the guidelines developed through the initiative Kidney Disease: Improving Global Outcomes (KDIGO)5 recommend placing nontunneled catheters initially, and then replacing them with tunneled catheters if dialysis must be prolonged or if the patient requires frequent changes in venous access.5 The choice of insertion site must aim to reduce complications such as infection, thrombosis, stenosis, and catheter malfunctioning. The most common sites of insertion are the femoral vein, internal jugular vein, and subclavian vein. Whether the right internal jugular or the femoral vein should be the first choice is a matter of controversy. The KDIGO5 and the European Renal Best Practice (ERBP) of the European Dialysis Association6 recommended avoiding femoral access when jugular access is available due to a supposed increased risk of infection. However, several studies, all of which were observational, have yielded discrepant results regarding the incidence of complications between femoral and jugular access,7–9 so the choice of insertion site remains a matter of individual preference, given the low level of scientific evidence. In 2008, the Cathedia study was published.10 This prospective randomized multicenter trial comparing access through the femoral and internal jugular veins in critical patients undergoing CRRT found that canalization through the internal jugular vein did not reduce the risk of infection compared with femoral canalization except in patients with a high body mass index. In 2012, the same group published a study11 with the patients in the Cathedia study who had required a second catheter in a site different from the first one. They found no differences between the two sites in catheter malfunction, colonization, or the efficacy of dialysis. In 2014, a single-center observational study evaluating 419 femoral catheters and 82 in other sites found that the rates of colonization and infection were similar, and the risk of infection was related to longer ICU stays, older age, and obesity.12 In 2016, Bellomo et al.13 analyzed the information about catheters obtained from the RENAL study,4 in which 1508 patients were randomized and information about the catheter was available for 1399 (93%). The femoral vein (especially the right) was chosen in 937 (67%) patients, followed by the jugular (25%) and the subclavian (8%). The patients in whom the femoral vein was chosen had more severe disease and weighed less. The impact of the insertion site on the dose of treatment was limited, unlike the impact of catheter diameter, which was greater in femoral catheterizations than catheterizations in other sites. The authors concluded that the diameter of the catheter was more important for treatment dose than the site of catheterization.
The left jugular vein follows a tortuous course toward the right atrium, and this can result in inadequate flow and filter malfunction; for this reason, this insertion site should be considered only after the right jugular and femoral veins.3 The subclavian vein would be the last choice because it is prone to stenosis that would make it impossible to create an arteriovenous fistula if chronic dialysis were necessary.3 Likewise, the possibility of stenosis of the iliac vein means that the femoral vein should be avoided if the patient might need a kidney transplant.
Ultrasound guidance for central venous catheter placement has been recommended for years. The advantages are fewer complications such as bleeding, pneumothorax, and arterial wall puncture; moreover, ultrasound guidance makes it possible to avoid cannulation in thrombotic regions, reduces the incidence of catheter-related thrombosis, and results in lower costs by reducing the iatrogenic effects associated with blind insertion.14
ComplicationsThe mechanical complications of catheter insertion include hematomas, pseudoaneurysms, vascular dissection, and arterial puncture; these complications occur in up to 5% of insertions. Other, less common complications include hemothorax, pneumothorax, pneumopericardium, retroperitoneal bleeding, tracheal puncture, chylothorax, anesthetic blockade of the brachial plexus, fat emboli, cardiac arrhythmias, and atrial rupture.15 Chest X-rays are mandatory after catheter insertion in the jugular vein or subclavian vein. While the catheter is in use, the main complications are malfunctioning, thrombosis, and infection. Malfunction and thrombosis: These complications are caused by intraluminal thrombosis, catheter kinking, or malpositioning with occlusion of the catheter holes against the vessel wall. Malfunction manifests as increased pressure in the venous and arterial lines that results in inadequate treatment. Polyurethane catheters are more prone to thrombosis. The catheters are often sealed with heparin when not in use. One recent study comparing sealing with heparin versus sealing with citrate found that citrate was better at restoring flow to obstructed catheters and had a lower incidence of infection and hemorrhagic complications. Infection: Infection results from the contamination of the catheter lumen from the skin and its entrance into the blood flow or even from the hematogenous dissemination of pathogens from other foci of infection. The most common germs are coagulase negative Staphylococci and Staphylococcus aureus follow by gram-negative bacilli (particularly Pseudomonas aeruginosa). The incidence ranges from 3.8 to 6.6 episodes per 1000 days for nontunneled catheters and 1.6 to 5.5 episodes per 1000 days for tunneled catheters.16 As for any other catheter, the risk factors for bacteremia are the frequency of catheter manipulation, the patient's severity, emergency insertion, the time the catheter remains in place, and the operator's experience. Various studies mentioned above have failed to show a higher incidence of infection in femoral catheters compared to jugular catheters. Among the fundamental measures to prevent infection, the most important are extreme asepsis during insertion, ultrasound guidance for insertion, keeping manipulation to a minimum (exclusive use for dialysis), and appropriate training for staff. The administration of local antibiotics to prevent infection is currently not recommended because it can favor fungal infection and resistance to antibiotics.5
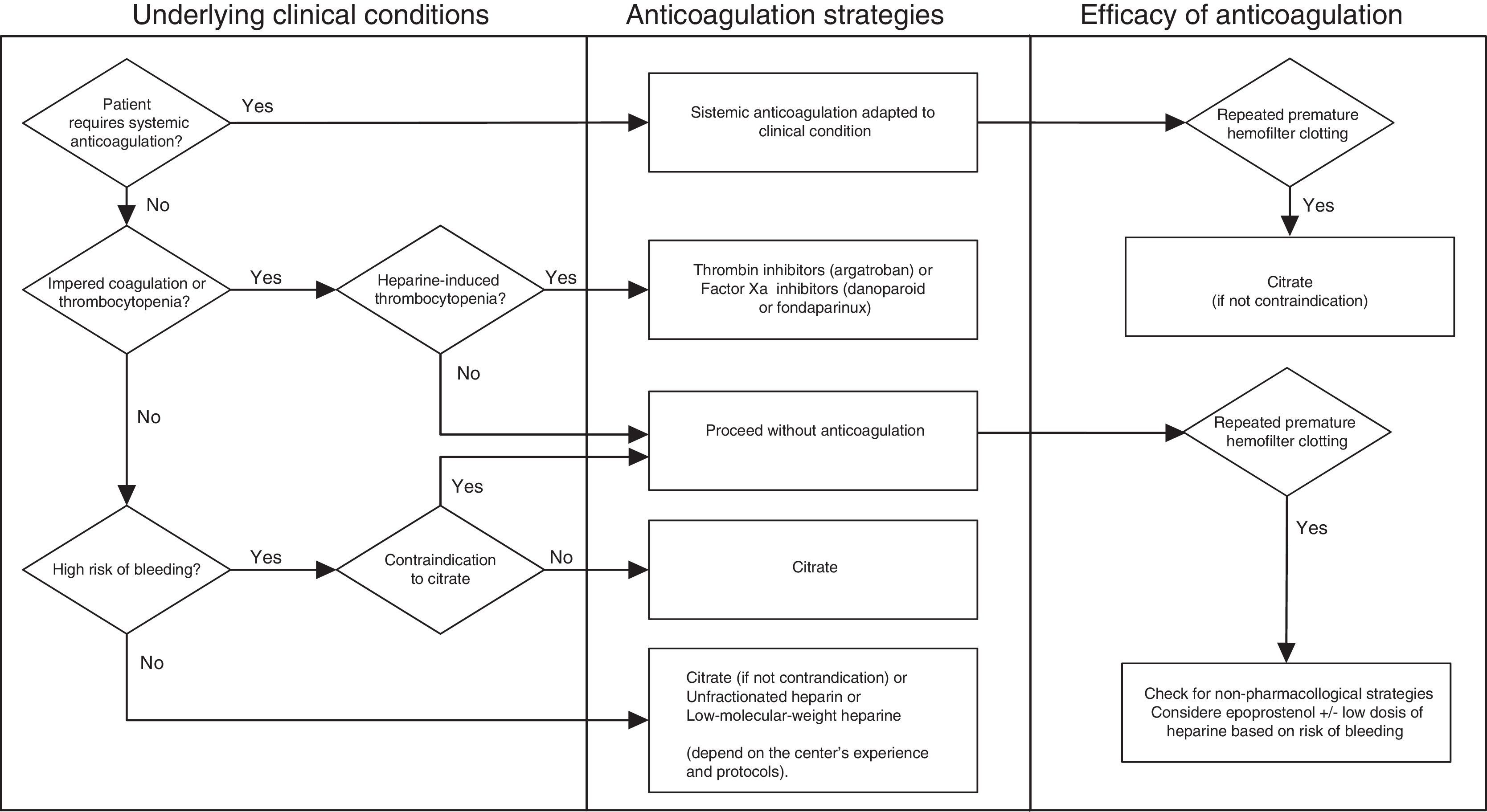
Optimization of patency in CRRT circuitsMaintaining the patency of the circuit during CRRT usually requires a pharmacological anticoagulation strategy. The ideal anticoagulant should have the following properties: (a) high antithrombotic potential with low risk of bleeding, (b) anticoagulation limited to the extracorporeal circuit, (c) quick and easy monitoring at the point of care, (d) possibility of prolonged use without undesired secondary effects, accumulation, or tachyphylaxis, and (e) an antidote that can reverse the effects immediately. Below we present the different strategies for optimizing the patency of the CRRT circuits (Fig. 1).
Non-pharmacological strategiesIt is important for the CRRT to incorporate biocompatible materials and to be designed in way that limits the turbulent flow of blood. The biocompatibility of the membrane is especially important for preventing hemofilter coagulation. Negatively charged membranes have a greater capacity for adsorption of proteins and formed elements of blood, so they might not last as long as neutral membranes. Hemofilters with larger membrane surfaces present less resistance to blood flow, and this may also help them to last longer. Moreover, clotting is more likely to occur when blood flow through the circuit is interrupted or is slower. Other measures that favor the rheology of the circuit include prescribing a filtration fraction <25% in convection techniques, prioritizing diffusion rather than convection, and predilution to limit the concentration of blood in the filter by reducing the viscosity of the blood. The response to alarms must be rapid. The correct adjustment of the level in the bubble-trapping capsule can reduce the effect of contact between blood and air and minimize the risk of coagulation at this point. Establishing a protocol for the technique and ensuring that nursing staff are highly trained guarantee the best outcomes. The intermittent infusion of saline solution has not proven beneficial in prolonging the life of the circuit. Finally, as discussed above, it is very important to choose the best vascular access and the catheter with the best characteristics to ensure the correct flow of blood through the circuit and keep it from coagulating.17,18
We also need to consider situations in which the circuit will not require any type of anticoagulation, such as coagulopathies or platelet abnormalities. There are no studies that can establish evidence for specific cutoffs for determining the platelet count, activated partial thromboplastin time (aPTT), International Normalized Ratio (INR), fibrinogen, or other coagulation factors that would indicate the possibility of carrying out CRRT without anticoagulation. In recent years, experts have recommended the following values: thrombocytopenia with platelet count <50×109/L, aPTT >60s, INR >2, and disseminated intravascular coagulation. Similarly, patients with acute kidney failure who require systemic anticoagulation for their underlying disease (e.g., valve replacement, atrial fibrillation, or thromboembolic disease) do not usually require additional anticoagulation for CRRT.
Pharmacological strategiesAnticoagulation for CRRT circuits with heparinIn 2007, the Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. kidney) investigators showed that up to 60% of CRRT procedures were done without any type of anticoagulation. When anticoagulation was prescribed, unfractionated heparin was the most commonly used.19 In Spain, a survey done in 51 ICUs confirmed that 96% of the centers used heparin, 39.2% used prostacyclin, and only 5.8% used citrate.20
Unfractionated heparinThis mixture of sulfated polysaccharides with molecular weights varying between 2kDa and 30kDa is the anticoagulant most commonly used in CRRT. Unfractionated heparin achieves its anticoagulant effect by bonding with antithrombin, favoring its bonding with thrombin (FII), and to a lesser extent by inhibiting activated factor X. To inhibit thrombin, it must bind both with thrombin itself and with antithrombin, although it does not need to bind with antithrombin to inhibit activated factor X. Its half-life ranges from 30min to up to 3h in cases with renal dysfunction. Its major advantages are (a) it is inexpensive; (b) prescribing physicians and the nurses in charge of the procedure are familiar with it; (c) it is easy to administer and to monitor (through aPTT); and (d) its effects can be reversed with protamine. There is no universally accepted protocol for its use, although various algorithms have been proposed for its administration during CRRT.21 It is usually present in the solution primed from the system in variable concentrations (1000–5000IU per liter). Before connection to CRRT and after the evaluation of the patient's coagulation, a bolus (30IU/kg body weight) is usually administered through the arterial line; the recommended maintenance dose is 5–10IU/kg/h.
The main problems associated with heparin are its dosing, bleeding, resistance, and heparin-induced thrombocytopenia. The problem with dosing heparin is that there is no direct relation between the dose of heparin and the aPTT, the duration of the filter, or hemorrhagic complications. Dosage is monitored through the aPTT, targeting levels between 1.5 and 2.0 times the baseline value (45–60s). The dose must be reduced drastically in patients with disseminated intravascular coagulation or thrombocytopenia. Every increase of 10s in the aPTT causes a 25% decrease in clots in the filter. The values of aPTT are partly dependent on the levels of coagulation factors associated with critical illness such as the variable clearing that the heparin itself undergoes when it is administered before filtering. Hemorrhagic events are the most common complication, affecting 10–50% of cases, depending on the type of population and the degree of anticoagulation.22 Hemorrhagic complications are not strictly related to the dose of heparin administered, but they are strictly related to the aPTT. The risk of bleeding increases by 50% for each increment of 10s in the aPTT. The incidence of bleeding complications is underestimated because minor events often go unreported; major bleeding events are defined as intracranial hemorrhage, retroperitoneal hemorrhage, or hemorrhages that result in death or the transfusion of ≥2 units of packed red blood cells.
Resistance to heparin is based on two pillars: (a) On the one hand, low levels of antithrombin III (AT-III), because this protein is consumed when the sepsis coagulation cascade is activated, and greater proteolytic degradation mediated by granulocyte elastase, the effect of which is increased by heparin itself. (b) On the other hand, the expression of heparin-binding proteins decreases heparin's effective concentration and thereby its anticoagulant effect.23 Some centers opt for the administration of AT-III to favor anticoagulation and the durability of the circuit, but the cost-effectiveness remains to be determined. Acquired AT-III deficiency (AT-III <70%) can be supplemented by an initial bolus [AT-III bolus=Weight×(100−initial AT-III activity %)/1.5], following a continuous infusion (40UI/kg/d). The doses should be secondarily adjusted to maintain an AT-III activity level between 80% and 100%.24
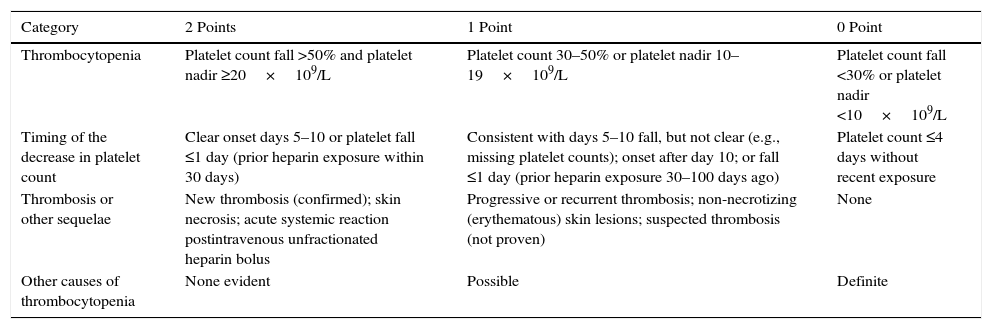
The incidence of heparin-induced thrombocytopenia (HIT) in ICU patients is very low, ranging from 0.3% to 0.5%.25 The frequency of HIT in critically ill patients receiving heparin anticoagulation for CRRT still remain to be determined in prospective studies.26,27 It is advisable to determine the levels of antibodies to heparin platelet factor 4 (H/PF4) after calculating the 4T score (Table 1).28 The most susceptible patients are those with a decrease >50% on the fourth day of receiving heparin. HIT should not be confused with the reversible thrombocytopenia that occurs after CRRT is started and ends when CRRT is stopped.26
Clinical estimation of the probability of HIT (The 4Ts score).28
| Category | 2 Points | 1 Point | 0 Point |
|---|---|---|---|
| Thrombocytopenia | Platelet count fall >50% and platelet nadir ≥20×109/L | Platelet count 30–50% or platelet nadir 10–19×109/L | Platelet count fall <30% or platelet nadir <10×109/L |
| Timing of the decrease in platelet count | Clear onset days 5–10 or platelet fall ≤1 day (prior heparin exposure within 30 days) | Consistent with days 5–10 fall, but not clear (e.g., missing platelet counts); onset after day 10; or fall ≤1 day (prior heparin exposure 30–100 days ago) | Platelet count ≤4 days without recent exposure |
| Thrombosis or other sequelae | New thrombosis (confirmed); skin necrosis; acute systemic reaction postintravenous unfractionated heparin bolus | Progressive or recurrent thrombosis; non-necrotizing (erythematous) skin lesions; suspected thrombosis (not proven) | None |
| Other causes of thrombocytopenia | None evident | Possible | Definite |
The 4Ts score is the sum of the values for each of the 4 categories. Scores of 1–3 are considered to correspond to a low, 4–5 intermediate, and 6–8 high probability of HIT.
Low-molecular-weight heparins with 18 saccharide units have a lower molecular weight (3600–6500kDa) than unfractionated heparin; therefore, they cannot simultaneously bond to thrombin and antithrombin, so the antithrombin activity is lost but the anti-factor Xa activity is maintained. Low-molecular-weight heparins are more effective than unfractionated heparin at decreasing fibrin deposits in the filter, and they start to act faster, activating fewer platelets and leukocytes. They are less dependent on antithrombin, bond less with proteins and cells, cause less activation of platelet factor-4, and result in a lower incidence of heparin-induced thrombocytopenia.29 These characteristics, together with more predictable pharmacokinetics, make low-molecular-weight heparins safer and more reliable. Nevertheless, they are more expensive than unfractionated heparin, and monitoring their effects is more complex – not all centers can routinely determine anti-factor Xa levels. The different molecules of low-molecular-weight heparins have distinct pharmacokinetics, which is not interchangeable with their counterparts. The bioavailability, half-life, or clearance in plasma and degree of accumulation in acute kidney failure can vary significantly among the different molecules. A recent systematic review30 concluded than no accumulation effect occurred with tinzaparin and dalteparin, but significant accumulation effects occurred with enoxaparin, bemiparin, and certoparin, even at prophylactic doses. The range of anti-factor Xa for their use has not been defined and is extrapolated from intermittent dialysis (iHD) between 0.2IU/ml and 0.4IU/ml. Unlike in iHD, the studies done in acute patients undergoing CRRT were done a long time ago, included few patients, and had heterogeneous objectives and designs, so it is difficult to draw conclusions. Low-molecular-weight heparins have been compared with unfractionated heparin31,32; different doses of low-molecular-weight heparins have been compared33; and different low-molecular-weight heparins have been compared with each other34 and with different anticoagulation strategies.35 Two recent studies comparing the incidence of bleeding and the duration of the filter between enoxaparin and unfractionated heparin reported contradictory results. Garcés et al.32 found similar filter life and anti-factor Xa ≥1.0IU/ml with enoxaparin, although the incidence of bleeding was higher. On the other hand, in a prospective controlled randomized crossover trial, Joannidis et al.31 with anti-factor Xa levels between 0.25IU/ml and 0.30IU/ml found that the low-molecular-weight heparins (0.15mg/kg pre-filter bolus and 0.05mg/kg/h maintenance perfusion) conferred longer filter life without increasing the incidence of bleeding and were also a cost-effective option. For all these reasons, it is difficult to interpret low-molecular-weight heparins for use in CRRT. Both the dose and the anti-factor Xa target must be tailored in function of the low-molecular-weight heparin chosen and the patient's clinical characteristics.
Anticoagulation with heparin-protamine (regional heparinization)First reported in the 1960s, regional anticoagulation combines pre-filter unfractionated heparin and post-filter protamine to counteract its effects (1mg protamine/100IU heparin). Its main indication was for patients in whom systemic anticoagulation was contraindicated. Its use has declined in parallel with the rise of citrate and increased knowledge about the side effects of protamine. Regional heparinization exposes patients to the risk of heparin-induced thrombocytopenia and the dose-dependent side effects of protamine: hypotension decreased cardiac output, anaphylaxis, pulmonary vasoconstriction with the risk of right ventricular failure, leukopenia, and a paradoxical effect on coagulation. This paradoxical effect is due to transient thrombocytopenia as well as platelet dysfunction (lower reactivity and thrombin-mediated aggregation). There is also a risk of a rebound anticoagulation effect because the half-life of heparin is longer than that of protamine; this effect is dose dependent and increases slightly with prolonged administration. Heparin-protamine complexes are taken up by the reticuloendothelial system, which fractionates them, rereleasing the heparin. This makes it difficult to titrate the appropriate doses of protamine to reverse its effects. It is more complex to monitor because the aPTT must be measured before and after the administration of the heparin and after reversion with protamine. Protamine should not be used with heparin-lined filters because it reverses the effects of the heparin. For all these reasons, regional anticoagulation with heparin-protamine is not recommended.36
Regional anticoagulation with citrateThis type of anticoagulation is based on the pre-filter administration of a fluid with a certain concentration of citrate. Citrate chelates with the calcium in the blood and forms a citrate-calcium complex, thus impeding the formation of clots because calcium is a cofactor necessary for the activation of the coagulation cascade. The chelated calcium that is lost in the circuit is later replaced to maintain the ionized calcium in the patient's serum at normal levels. Most of the citrate administered is also removed through the effluent. The amount of citrate that passes into the patient's bloodstream is metabolized through the Krebs cycle in the liver, muscle tissue, and renal cortex. Thus, the circuit is anticoagulated without affecting the patient's coagulation and the life of the hemofilter is significantly prolonged, minimizing the risk of bleeding.
Regional anticoagulation with citrate has been in use in intermittent dialysis for more than 20 years, where it has reduced bleeding events in patients with a high risk of bleeding. In recent years, different manufacturers have incorporated software in their CRRT monitors to simplify the calculations necessary for the administration of citrate and to enable automatic calcium compensation. This advance, together with the commercialization of specific fluids for anticoagulation with citrate, has reduced the complexity of this approach, increased its safety, and decreased its cost.37,38 There is currently a wide consensus that regional anticoagulation with citrate should be the first-choice option for anticoagulation in patients with high risk of bleeding.22,39,40,5
The main complications of citrate are alterations in the acid–base balance, hypocalcemia, and hypercalcemia. In the patient's body, the citrate–calcium complex is metabolized mainly in the liver, but the kidneys and skeletal muscle also contribute. Citrate is converted into sodium bicarbonate (1mmol of citrate is converted into 3mmol sodium bicarbonate), so the net result of the metabolism of the citrate–calcium complex is the incorporation of an alkali (sodium bicarbonate), which affects the acid–base balance. Hypocalcemia can result from insufficient replacement of the calcium that is chelated by the citrate, and hypercalcemia can result from excessive replacement (whether due to error or inadvertently maintaining continuous calcium infusion after suspending anticoagulation with citrate). Citrate also chelates magnesium, so magnesium levels must be closely monitored and supplemented to avoid hypomagnesemia. If the administration of citrate is excessive and the patient can metabolize it, the immediate consequences are hypernatremia and metabolic alkalosis.
Citrate intoxication occurs when the patient cannot metabolize the citrate administered; this occurs in ischemic hepatitis (even before it is reflected in increased liver enzymes) or in decreased hepatic blood flow or severe peripheral hypoperfusion (cardiogenic shock and/or septic shock). It has been suggested that regional anticoagulation with citrate might be contraindicated in these patients. In this case, we would observe metabolic acidosis (with high anion gap) and a steep drop in the levels of ionic calcium in a patient with high total calcium, hypophosphatemia, and hypomagnesemia. The gold standard for the diagnosis for this complication is the direct measurement of the concentration of citrate in plasma; however, this determination is not usually available in daily practice. The laboratory determination used instead is the ratio of total calcium to ionic calcium. The normal value of this ratio is 2 (total calcium is normally the double of ionic calcium) and values ≥2.5 indicate intoxication. In these patients, the classical liver function parameters such as the transaminases and bilirubin are poor predictors of the development of citrate intoxication; the best predictors of this complication are serum lactate (≥3.4mmol/l) and prothrombin time (≤26% or ≥33s).41 An increasing number of publications show adequate tolerance to anticoagulation with citrate in patients with compensated cirrhosis of the liver or acute liver failure40,42,43 since these patients do not have more metabolic complications. More frequent monitoring (every 4h) and higher target ranges of ionized calcium in the circuit (0.3–0.5mmol/l) are necessary to guarantee patient safety. This strategy is able to guarantee acceptable filter life by decreasing the contribution of citrate and the risk of intoxication.44
We can conclude that there are no absolute contraindications for the use of citrate, although it must be employed with particular caution in clinical contexts characterized by severe liver failure/liver transplant or in situations of severe lactic acidosis secondary to hepatic hypoperfusion and severe intracellular hypoxia (septic or cardiogenic shock).
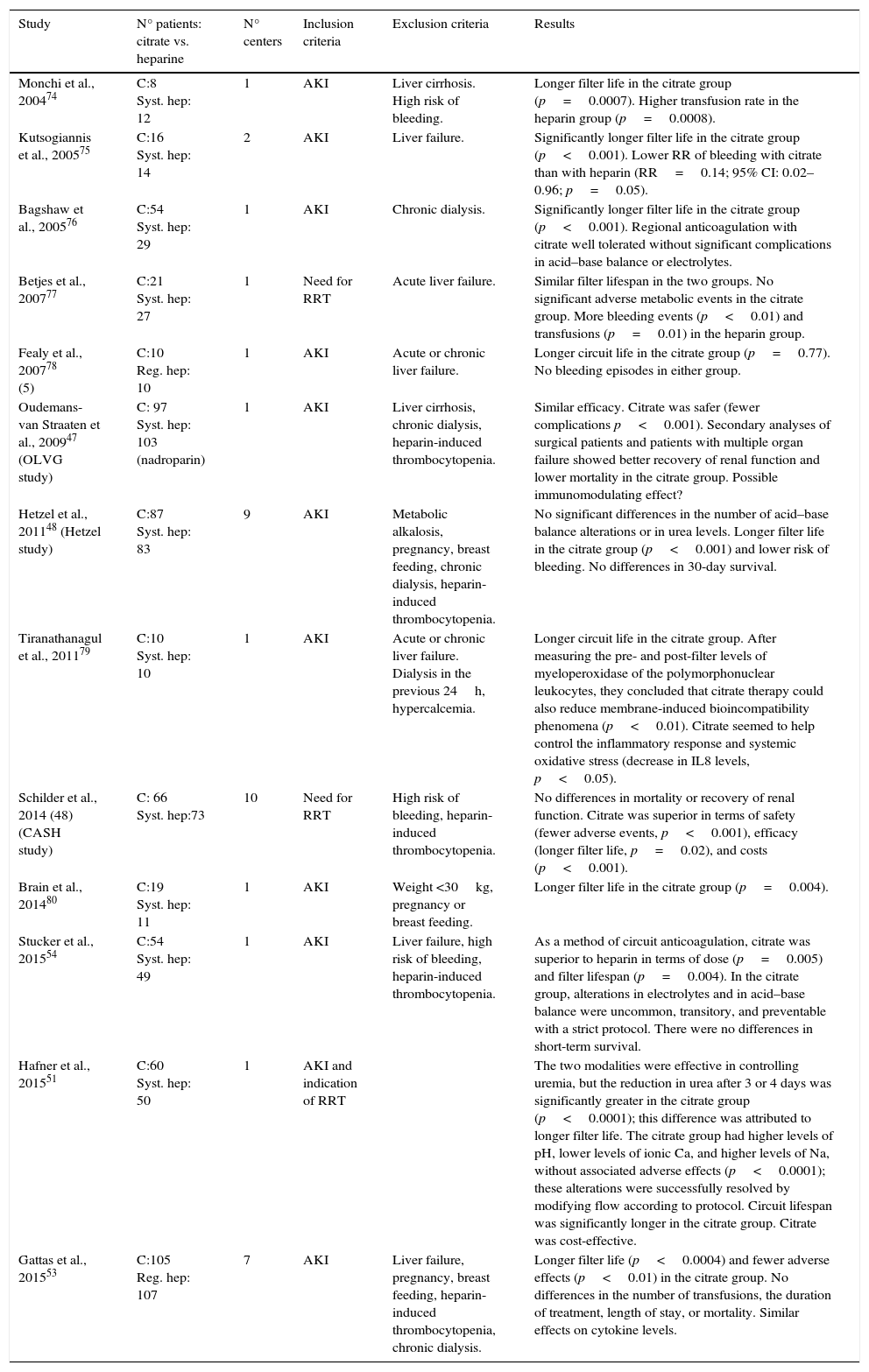
Studies that compare citrate with other modalitiesThere is a growing body of literature to support favorable results for regional anticoagulation with citrate in CRRT. The strong points of this approach versus other anticoagulation strategies are the increased life of the filter and the decreased number of hemorrhagic complications. Table 2 shows some of the most relevant and most recent studies that compare regional anticoagulation with citrate against other anticoagulation strategies.
Studies comparing regional anticoagulation with citrate versus other anticoagulation strategies.
| Study | N° patients: citrate vs. heparine | N° centers | Inclusion criteria | Exclusion criteria | Results |
|---|---|---|---|---|---|
| Monchi et al., 200474 | C:8 Syst. hep: 12 | 1 | AKI | Liver cirrhosis. High risk of bleeding. | Longer filter life in the citrate group (p=0.0007). Higher transfusion rate in the heparin group (p=0.0008). |
| Kutsogiannis et al., 200575 | C:16 Syst. hep: 14 | 2 | AKI | Liver failure. | Significantly longer filter life in the citrate group (p<0.001). Lower RR of bleeding with citrate than with heparin (RR=0.14; 95% CI: 0.02–0.96; p=0.05). |
| Bagshaw et al., 200576 | C:54 Syst. hep: 29 | 1 | AKI | Chronic dialysis. | Significantly longer filter life in the citrate group (p<0.001). Regional anticoagulation with citrate well tolerated without significant complications in acid–base balance or electrolytes. |
| Betjes et al., 200777 | C:21 Syst. hep: 27 | 1 | Need for RRT | Acute liver failure. | Similar filter lifespan in the two groups. No significant adverse metabolic events in the citrate group. More bleeding events (p<0.01) and transfusions (p=0.01) in the heparin group. |
| Fealy et al., 200778 (5) | C:10 Reg. hep: 10 | 1 | AKI | Acute or chronic liver failure. | Longer circuit life in the citrate group (p=0.77). No bleeding episodes in either group. |
| Oudemans-van Straaten et al., 200947 (OLVG study) | C: 97 Syst. hep: 103 (nadroparin) | 1 | AKI | Liver cirrhosis, chronic dialysis, heparin-induced thrombocytopenia. | Similar efficacy. Citrate was safer (fewer complications p<0.001). Secondary analyses of surgical patients and patients with multiple organ failure showed better recovery of renal function and lower mortality in the citrate group. Possible immunomodulating effect? |
| Hetzel et al., 201148 (Hetzel study) | C:87 Syst. hep: 83 | 9 | AKI | Metabolic alkalosis, pregnancy, breast feeding, chronic dialysis, heparin-induced thrombocytopenia. | No significant differences in the number of acid–base balance alterations or in urea levels. Longer filter life in the citrate group (p<0.001) and lower risk of bleeding. No differences in 30-day survival. |
| Tiranathanagul et al., 201179 | C:10 Syst. hep: 10 | 1 | AKI | Acute or chronic liver failure. Dialysis in the previous 24h, hypercalcemia. | Longer circuit life in the citrate group. After measuring the pre- and post-filter levels of myeloperoxidase of the polymorphonuclear leukocytes, they concluded that citrate therapy could also reduce membrane-induced bioincompatibility phenomena (p<0.01). Citrate seemed to help control the inflammatory response and systemic oxidative stress (decrease in IL8 levels, p<0.05). |
| Schilder et al., 2014 (48) (CASH study) | C: 66 Syst. hep:73 | 10 | Need for RRT | High risk of bleeding, heparin-induced thrombocytopenia. | No differences in mortality or recovery of renal function. Citrate was superior in terms of safety (fewer adverse events, p<0.001), efficacy (longer filter life, p=0.02), and costs (p<0.001). |
| Brain et al., 201480 | C:19 Syst. hep: 11 | 1 | AKI | Weight <30kg, pregnancy or breast feeding. | Longer filter life in the citrate group (p=0.004). |
| Stucker et al., 201554 | C:54 Syst. hep: 49 | 1 | AKI | Liver failure, high risk of bleeding, heparin-induced thrombocytopenia. | As a method of circuit anticoagulation, citrate was superior to heparin in terms of dose (p=0.005) and filter lifespan (p=0.004). In the citrate group, alterations in electrolytes and in acid–base balance were uncommon, transitory, and preventable with a strict protocol. There were no differences in short-term survival. |
| Hafner et al., 201551 | C:60 Syst. hep: 50 | 1 | AKI and indication of RRT | The two modalities were effective in controlling uremia, but the reduction in urea after 3 or 4 days was significantly greater in the citrate group (p<0.0001); this difference was attributed to longer filter life. The citrate group had higher levels of pH, lower levels of ionic Ca, and higher levels of Na, without associated adverse effects (p<0.0001); these alterations were successfully resolved by modifying flow according to protocol. Circuit lifespan was significantly longer in the citrate group. Citrate was cost-effective. | |
| Gattas et al., 201553 | C:105 Reg. hep: 107 | 7 | AKI | Liver failure, pregnancy, breast feeding, heparin-induced thrombocytopenia, chronic dialysis. | Longer filter life (p<0.0004) and fewer adverse effects (p<0.01) in the citrate group. No differences in the number of transfusions, the duration of treatment, length of stay, or mortality. Similar effects on cytokine levels. |
AKI, acute kidney injury; C, citrate; Syst. hept, systemic heparin; Reg. hep, regional heparin (heparin-protamine); RR, relative risk; CI, confidence interval; RRT, renal replacement therapy.
Oudemans et al.45 did a comparative analysis of the large randomized multicenter studies published through 2014 (CASH46 from 2014, OVLG47 from 2009, and Hetzel48 from 2011). In the CASH study46 citrate was compared to systemic anticoagulation with heparin in 139 patients. No differences were found in mortality or in recovery of renal function, but citrate was superior in terms of safety (fewer adverse effects, p<0.001), efficacy (longer filter life, p=0.02), and costs (p<0.001). The OLVG study47 compared citrate with nadroparin in 200 patients. The efficacy of the two drugs was similar, but citrate was safer (fewer complications, p<0.001). In a secondary analysis in subgroups, citrate was particularly beneficial after surgery and in sepsis with multiple organ failure, where renal recovery and mortality were improved. The improved survival was attributed to a possible immunomodulating effect of citrate. This hypothesis is supported by experimental data showing that anticoagulation with citrate impedes the degranulation of polymorphonuclear leukocytes and the activation of thrombocytes induced by dialysis; these processes depend on ionic calcium and are blocked by the administration of citrate.49,50 In the Hetzel study,48 citrate was compared against systemic heparin in 170 patients. No significant differences were found in the number of alterations in the acid–base balance or in the reduction of the levels of urea. The group that received citrate had longer filter life and a lower risk of bleeding. No differences in 30-day mortality were found. Hafner et al.51 also compared citrate against systemic heparin. Both modalities were effective in controlling uremia, although the reduction in urea was larger in the citrate group; the authors attributed this difference to longer hemofilter life. The citrate group had slight alterations in the levels of pH, ionic calcium, and sodium; however, these alterations were not associated with adverse events and were all successfully resolved by modifying fluids according to protocol. In the economic analysis, citrate was cost-effective. In a meta-analysis of six randomized controlled trials with a total of 488 patients, Wu et al.52 found that patients receiving citrate had a lower risk of bleeding without an increased incidence of alkalosis compared to patients in whom the circuits were anticoagulated with heparin. The efficacy and filter life were similar in the two groups. The authors concluded that citrate is at least as safe as heparin and significantly cheaper. Gattas et al.53 recently carried out a prospective randomized multicenter trial comparing citrate versus heparin-protamine in 212 patients. The results showed longer filter lifespan and fewer adverse events in the citrate group. There were no differences in the number of transfusions, the duration of CRRT, length of stay, or mortality. There were also no differences in cytokine levels. Stucker et al.54 found that citrate was superior to heparin as a method of anticoagulation in terms of dose and filter lifespan. Alterations in electrolytes and acid–base balance in the citrate group were uncommon, short-lived, and preventable with a strict protocol. There were no differences in short-term survival. Bai et al.55 recently published a meta-analysis including the randomized controlled trials that compared heparin versus citrate in CRRT. The meta-analysis included a total of 992 patients in 11 studies; two of these used regional heparinization, and the rest used systemic heparinization. In the citrate group, there was a significant reduction in the risk of circuit loss and filter failure compared to the groups receiving regional heparinization or systemic heparinization. The risk of bleeding in the citrate group was significantly lower than in the systemic heparinization group and similar to that observed in the regional heparinization group. No differences were observed in survival. These authors concluded that given the low risk of circuit loss and filter failure, regional anticoagulation with citrate should be considered a better method of anticoagulation than heparin in critical patients without contraindications.
In summary, regional anticoagulation with citrate can be considered efficacious and safer than anticoagulation with heparin. Citrate is associated with increased hemofilter lifespan and a lower risk of bleeding and multiple transfusions. Regional anticoagulation with citrate administered under strict protocols by properly trained staff is not associated with significant adverse events. More economic studies are necessary to evaluate its cost-effectiveness. The anti-inflammatory effects of citrate during CRRT and its impact on survival are controversial and should be evaluated in future studies.
Thrombin inhibitorsDirect thrombin inhibitors (lepirudin, argatroban, bivalidurin) have proven efficacy as anticoagulants in critical patients with heparin-induced thrombocytopenia. However, most of the studies that support their use in CRRT are limited, retrospective, observational, and small. Heparin-induced thrombocytopenia results from the formation of antibodies against the heparin-platelet factor 4 complex, which secondarily activates the platelets and coagulation and eventually leads to increased thrombin formation.56 The probability of heparin-induced thrombocytopenia can be predicted with the 4T score, which includes the degree of thrombocytopenia, time since the onset of the drop in platelet levels, presence of thrombosis or acute systemic symptoms, and presence of other causes of thrombocytopenia. Although the incidence of this complication in critical patients is low (0.3–0.5%),25 it is associated with increased morbidity and mortality if its diagnosis is delayed, due to the prothrombotic situation it generates. In patients undergoing CRRT, heparin-induced thrombocytopenia can be suspected when the circuit becomes clotted early and repeatedly, even when citrate is used.57,58 Suspicion of heparin-induced thrombocytopenia requires treatment with heparin or heparin derivatives to be discontinued. No randomized studies have indicated the optimal anticoagulant in cases of heparin-induced thrombocytopenia. This choice will depend on local availability and experience in monitoring. Although citrate can be used for regional anticoagulation, these patients need thromboprophylaxis. These drugs inhibit free thrombin and thrombin bound in thrombi, and they do not react with antibodies against heparin. There are important differences in the pharmacokinetics of the three most studied direct thrombin inhibitors, and these are explained here below.
LepirudinThis drug is metabolized by the kidneys. In patients with acute kidney failure, the half-life can be prolonged up to 50h, with the risk of the drug accumulating and increasing the risk of bleeding.59 It is usually monitored through the aPTT, although the relationship between the levels of lepirudin and the aPTT is not linear. The most accurate test is ecarin clotting time, but this test is not available in all centers. Some cases of anaphylaxis have been reported. Lepirudin is perfused intravenously (0.005–0.01mg/kg/h) and the dose is adjusted in function of the time to ecarin clotting.60 There is no specific antidote, but factor VIIa can be used in case of bleeding.61 In 2014, Treschan et al.62 published the results of a randomized double-blind study (ALicia) comparing lepirudin and argatroban in 28 critical postoperative patients with suspected heparin-induced thrombocytopenia and need for CRRT. The two drugs were equally efficacious in terms of circuit survival, but the lepirudin group had more episodes of bleeding.
ArgatrobanA derivative of l-arginine, argatroban bonds reversibly to the catalytic site of thrombin without the need for a cofactor. It is metabolized by the liver (70%) and has a short half-life (31–59min), so it may be appropriate for critical patients with acute kidney failure without liver failure who need CRRT.63 The recommended dose, based on a prospective study of 30 patients with heparin-induced thrombocytopenia, acute kidney failure, and CRRT, is a bolus of 100μg/kg followed by the perfusion of 1μg/kg/min; the dose is adjusted to reach a target pre-filter aPTT of 1.5–3 times the baseline level.59 In patients with liver dysfunction, the half-life of the drug increased, making it necessary to reduce the dose, as in patients with multiple organ dysfunction (0.2–0.6μg/kg/min). There was an inversely proportional relation between the maintenance dose and severity scales; the closest correlation was with the Sequential Organ Failure Assessment (SOFA) (r=−90, p<0.001, dose μg/kg/min=2.18–0.09×SOFA).59 Argatroban brought about an increase in filter lifespan comparable to that achieved with unfractionated heparin, but argatroban did not increase the risk of bleeding after heart surgery.64 There is no specific antidote for argatroban, but an ex vivo study showed that factor VIIa can be used in cases of bleeding.61 The KDIGO guidelines recommend using argatroban during CRRT before other thrombin inhibitors or factor Xa inhibitors in patients with heparin-induced thrombocytopenia that do not have severe liver failure.5
BivalidurinBivalidurin reversibly inhibits thrombin. It has a short half-life (25min) and has the additional advantage over argatroban that its metabolism is enzymatic, so it can be used in patients with heparin-induced thrombocytopenia who have hepatic and renal dysfunction. Although the prescribing information does not list this indication, retrospective studies with few patients on the efficacy, safety, and dosing in these patients have shown good results.65 The dose in patients on CRRT is 0.03–0.05mg/kg/h, which is adjusted in function of the pre-filter aPTT (1.5–2.5 times the baseline level). One prospective randomized double-blind study in 10 patients showed that the lifespan of the hemofilter was prolonged significantly compared to unfractionated heparin and to no anticoagulation.66 Although no specific antidote is available, in cases with bleeding, factor VIIa can be administered.61
Platelet aggregation inhibitorsProstacyclin (PGI2) is derived from the arachidonic acid in the vascular tissues and inhibits the final pathway of all mechanisms of cellular activation that lead to the adhesion, activation, liberation, and aggregation of the platelets. This effect is mediated by an increase in the intracellular levels of cyclic adenosine monophosphate (cAMP) secondary to the activation of the membrane's specific adenyl cyclase receptors.67
EpoprostenolThe vasodilatory effects of this drug are short lasting (half-life 2min) whereas its antiplatelet effects last longer (2h). Some studies have analyzed the use of prostacyclin alone68,69 or combined with unfractionated heparin. The lifespan of the circuits was longer when it was used with unfractionated heparin (PGI2 4–5ng/kg/min with 2–3IU/kg/h unfractionated heparin).70 Herrera et al.71 reported that circuit lifespan was similar with epoprostenol and unfractionated heparin but the risk of bleeding with epoprostenol was lower. Hemodynamic instability was the most common adverse effect associated with PGI2, and it was directly related to the dose used. The main drawback is the risk of arterial hypotension, and this can be minimized by progressively increasing the dose. The cost has come down since a generic version of this drug became available.
NafamostatNafamostat, a prostacyclin analog, is a synthetic serine protease inhibitor that inhibits various coagulation factors (thrombin, factor Xa, factor XIIa, kallikrein) and components of the complement system, as well as platelet aggregation. It has a low molecular weight and a short half-life (5–8min). It is eliminated through the circuit, limiting systemic anticoagulation and lowering the risk of bleeding. Few data (only from Japan and Korea) are available about its use in CRRT because it is not available in the United States or Europe. Retrospective72 and prospective73 studies have demonstrated the efficacy and safety of nafamostat (fewer bleeding episodes and transfusions). However, Baek et al.72 founder longer filter lifespan without anticoagulation than with nafamostat (27.5 vs. 19.8h), and Lee et al.73 found a nonsignificant trend toward longer circuit lifespan with nafamostat than with no anticoagulation. Moreover, significant secondary effects such as anaphylaxis, agranulocytosis, and hyperkalemia have been reported.
Finally in Table 3 are summarized the main key points of this review about vascular access and circuit patency in continuous renal replacement therapy.
Key points.
| 1. Non-tunneled polyurethane catheters with a double lumen are the appropriate vascular access for CRRT in acute kidney failure. Tunneled silicone catheters represent an alternative that is generally reserved for longer treatments or for patients with problematic venous access. |
| 2. The right internal jugular vein or the femoral veins are the access sites of choice. The subclavian vein should be avoided due to the high risk of thrombosis and stenosis. |
| 3. The location, diameter, geometry, and length of the catheter should be tailored to physical and clinical characteristics of the patient. |
| 4. Dialysis catheters should be placed under ultrasound guidance to minimize the iatrogenic effects of insertion. Malfunction, thrombosis, and infection are complications that can be prevented by proper care of the catheter. |
| 5. When not in use, catheters should be sealed with an antithrombotic solution. |
| 6. Non-pharmacological measures are useful strategies for optimizing the patency of the circuit. These include correct venous access, appropriate settings of blood flow and filtration fraction, prior dilution, favoring diffusion over convection, ensuring thorough training of staff, using protocols, and monitoring the technique closely. |
| 7. Unfractionated heparin continues to be the anticoagulation strategy most frequently employed in CRRT. It is an effective anticoagulation strategy, but there are problems with dosing and increased risk of bleeding. There may also be a certain degree of resistance to heparin, and this strategy can result in heparin-induced thrombocytopenia. |
| 8. Despite repeated clotting of the filter, forgoing anticoagulation of the circuit continues to be a common option in patients with high risk of bleeding in centers where regional anticoagulation with citrate is not available. |
| 9. Regional anticoagulation with citrate has proven very effective. It is associated with increased filter lifespan and decreased risk of bleeding and need for multiple transfusions. This strategy should be the first choice in patients with increased risk of bleeding. |
| 10. Regional anticoagulation with citrate requires strict protocols and thorough staff training to ensure patient safety. |
| 11. In patients without high risk of bleeding, citrate, unfractionated heparin, and low-molecular-weight heparins have all proven efficacious. The choice of strategy will depend on the patient's clinical condition and contraindications, as well as on the center's experience and protocols. |
| 12. Unfractionated heparin or low-molecular-weight heparins are probably the best alternatives in patients with low risk of bleeding in whom citrate administration is contraindicated. |
| 13. In patients with heparin-induced thrombocytopenia, the administration of thrombin inhibitors (argatroban) or factor Xa inhibitors (danaparoid or fondaparinux) is highly recommended. |
| 14. Regional anticoagulation with heparin-protamine is not currently recommended. The evidence available about the safety and efficacy of epoprostenol and other platelet aggregation inhibitors and thrombin inhibitors is insufficient to recommend their use in routine practice. |
Vascular access placement and filter patency (anticoagulation) is a fundamental issue strictly related to dialysis delivery and should be carefully evaluated for each patient and CRRT modalities. Non-tunneled polyurethane catheters with a double lumen are the appropriate vascular access for CRRT in acute kidney failure. The vein used for the catheter insertion should be chosen taking into account the patients’ clinical characteristics. The catheter should be placed under ultrasound guidance and with adherence to infection-control policies. Citrate anticoagulation may be used for all patients without contraindications. Heparin as a first choice is still feasible in patients without high risk of bleeding, especially for units with less or no experience in citrate anticoagulation. In patients with heparin-induced thrombocytopenia, the administration of thrombin inhibitors (argatroban) or factor Xa inhibitors (danaparoid or fondaparinux) is highly recommended. The evidence available about the safety and efficacy of thrombin inhibitors and platelet aggregation inhibitors is insufficient to recommend their use in routine practice.
FundingThere has been no funding for the realization of this writing.
Conflict of interestThe authors declare that they have no conflicts of interest.