The increase in bacterial resistance to antibiotics is one of the greatest threats to global health. This problem is especially relevant in critical care units, where the number of isolates of multiresistant species grow each year1 reducing the number of alternatives available to treat serious infectious diseases such as sepsis, meningitis or nosocomial pneumonia. Moreover, despite promises and efforts to create new antimicrobials, currently there are little effective developments in the pharmaceutical market.
The spread of multidrug-resistant microorganisms (MDRM) is due to the failure of the measures to prevent cross-transmission but also to the continuous genesis of new resistances. It has been repeatedly shown that the generation of resistance is closely related to exposure to antimicrobials.2 In this sense, what is most striking is that even 30–50% of prescriptions of antibiotics may be unnecessary.3
All this has sponsored the development of so-called Antimicrobial Stewardship Programs (ASP) (Programas de Optimización de Antimicrobianos (PROA) in Spanish). These programs include a set of activities intended to optimize the antimicrobial treatment, ensuring the best clinical outcome for the patient but avoiding where possible the development of antimicrobial resistance. The latter objective is largely based on the elimination of all those unfair treatments and on the replacement of broad-spectrum drugs when possible. This type of programs is being implemented in hospitals around the world, proving to be a useful tool in reducing the consumption of antimicrobials and the reduction of bacterial resistance. However, its implementation in critical care units has an added difficulty because of several factors such as patient severity, high MDRM prevalence and pharmacokinetic–pharmacodynamic particularities. However, several works in critical patients have shown the success of the ASP. Examples are the results obtained by Elligsen et al. who achieved a 23% reduction in consumption of antimicrobials and also succeeded an improvement in sensitivity to meropenem.4 Also noteworthy is the work of Rimaway et al., who, in addition to a reduction in broad-spectrum antibiotics consumption, achieved a diminution in the days of mechanical ventilation and length of stay in the unit.5 Other studies published in critically ill patients have shown a significant reduction in the use of antimicrobials.
Nevertheless, we do not yet have sufficient scientific evidence to show a positive impact of ASP on the evolution of critically patients and their ecological environment. In fact, in a recent review Mertz et al. underscore this fact by the low methodological quality of the studies published to date.6 We should note the great difficulty of achieving an effect on hospital stay or mortality due to the multitude of factors that influence the prognosis of critically ill patients. Furthermore, an ASP applied exclusively in the critical care unit could hardly have an effect on the occurrence of bacterial resistance due to the continuous movement of patients with the rest of the hospital. However, given the impact of these programs on antimicrobial consumption and the relationship between antibiotic use and resistance, ASP should be implemented in all critical care units.
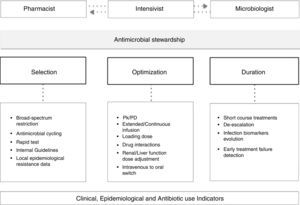
ASP format may be different but should be directed to the early stages of antibiotic treatment, should include joint assessment between the pharmacist and the medical care and should conclude with a feedback to the doctor who prescribed the treatment. ASP in ICUs should be led by a specialist in hospital pharmacy in tandem with an intensivist specially dedicated to the field of infection, but must have the support of other specialties such as microbiology and infectious as well as a clear institutional support.7 Factors that should be evaluated include drug de-escalation to a lower therapeutic spectrum, duration of treatment, pharmacokinetic–pharmacodynamics characteristics and possible interactions (Fig. 1). By feedback to the prescriber the ASP will also achieve a progressive educational effect.
Antimicrobial de-escalationIn much of the infectious processes the antimicrobial spectrum of the initially chosen drugs can be safely reduced following MDRM rule out in the microbiological analysis. This strategy has proven to be safe for patient outcome.8 In fact, broad-spectrum antimicrobial agents should be reserved for patients with risk factors for MDRM infection (empirical treatment) or before the isolation of a MDRM in clinically representative samples (targeted therapy).
Antimicrobials may also be de-escalated in terms of its number. The combination of antibiotics is one strategy that increases the likelihood of achieving an appropriate empirical treatment. However, once an etiologic diagnosis has been achieved, the combination should be restricted to those cases where the characteristics of the host, the infection, the drug or the microorganism make unlikely to achieve the pharmacokinetic–pharmacodynamic target. An example of this situation could be a severely ill patient with a ventilator-associated pneumonia caused by Pseudomonas aeruginosa susceptible exclusively to colistin and fosfomycin; both drugs have a low lung penetration, P aeruginosa is a typically difficult to eradicate microorganism and the patient is in a critical condition. In this example monotherapy would have little chance of effectiveness and otherwise there would be a high risk of induction of new resistances.
In any case, it must be noted that in most of clinical trials and published meta-analysis, combination therapy has shown little benefit over monotherapy and has proved in many cases an increase in drug toxicity.9 ASP teams should carefully evaluate the desirability for combined treatment and the duration thereof.
Antimicrobial treatment durationShorten the duration of antimicrobial treatment is one of the main tasks of the ASP team as prolonged treatments are a key element in the genesis of resistance. The prolonged antibiotic treatment often leads to colonization by MDRDM and the possible emergence of recurrent of infection.
Most of the episodes of nosocomial pneumonia or bacteraemia in immunocompetent patients have a good outcome after seven days of treatment and stopping treatment is safe at that time.10
Over the past several years, biomarkers have been studied in relation to infection in critically ill patients, among them procalcitonin. Procalcitonin has been studied in several clinical trials and, although the results have not been entirely homogeneous, this biomarker seems to be useful to safely shorten antibiotic treatment.11
Pharmacokinetic/pharmacodynamics optimizationThe critical patient suffers major pathophysiological changes that alter the plasma and tissue concentrations of most antimicrobials. Acute renal failure, liver failure and reduced local blood flow can cause an accumulation of drugs and therefore toxicity. Moreover, more frequent is the increase in the volume of distribution of hydrophilic antimicrobials (due to volume overload and oedema) causing a decrease in plasma concentrations and consequent therapeutic failure.12
These pharmacokinetic changes must be added to the progressive increase of the minimum inhibitory concentration (MIC) to various antibiotics of many microorganisms, mainly for Gram-negative species, making it more difficult to obtain an adequate antimicrobial exposure in pharmacodynamic terms.13
ASP teams should include experts in pharmacokinetic/pharmacodynamic variables when advising on the most appropriate drug and dosage for critically ill patients.
Communication with the attending physicianCommunication (feedback) with the prescribing physician is key element for the ASP success. Feedback can be set by technological means or by personal interview but in any case must be based on mutual respect, attitude to dialogue and on the establishment of agreements if necessary.
Only in the absence of cooperation or in situations of high clinical or epidemiological risk, the mode of action of the ASP team could be that the imposition or restriction of antimicrobial treatments and in these cases the team will need an institutional support.
ConclusionsASPs in intensive care units, in coordination with nosocomial infection control teams, are able to reduce and optimize antimicrobial therapies. The need to preserve antimicrobials given the lack of alternatives and the expansion of MDRM makes it essential to implement such programs in all critical care units. To ensure the success of these programs it is required the participation of a multidisciplinary team, the use of educational measures, an adequate system of periodic indicators and results and an adequate feedback to the clinical staff of the unit.
Conflict of interestThe authors declare no conflict of interest.