Severe hemorrhage is prevalent in a significant proportion of trauma, surgical, and obstetric patients. It leads to acute severe anemia and coagulopathy which are usually treated with transfusion of blood components. Coagulopathy is present in approximately one third of patients presenting with trauma bleeding upon hospital admission or those developing postpartum hemorrhage.1 As coagulopathy leads to poor clinical outcomes, it should always be suspected in patients with hemorrhagic shock and an international normalized ratio (INR)≥1.2, platelet count<100,000μL, fibrinogen level (Clauss)<1,5g/L, A5-EXTEM≤35–37mm, and/or A5-FIBTEM<8mm1 (Figs. 1 and 2).
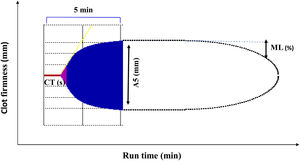
ROTEM (rotational thromboelastometry) trace displaying the clinically most important parameters. Applicable to EXTEM (extrinsic coagulation pathway), INTEM (intrinsic coagulation pathway), HEPTEM (effect of heparin, cardiac surgery), and FIBTEM (dynamic fibrinogen contribution to clot firmness). CT, clotting time (s); A5, clot amplitude (mm) obtained 5min after clotting time; ML, maximum lysis percentage. (%) decrease of maximum clot firmness during run time. See Ref.5 for a more detailed description of ROTEM traces.
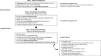
Current evidence-based algorithm for treating severe bleeding and coagulopathy used at Hospital “Virgen del Rocío,” Seville, Spain. Reproduced with permission of the Institutional Transfusion Committee. Based on Refs.1–3,5
In-hospital and out-of-hospital patients with severe hemorrhage are immediately checked for basic clinical signs (clinical and ROTEM management goals: temperature>35°C, pH>7.3, calcium>1mmol/L, lactate<2mmol/L, hemoglobin>7g/dL, A5-EXTEM≥35mm, A5-FIBTEM≥12mm, CT-EXTEM<60s, and ML<3%). Massive hemorrhage protocol is quickly activated by phone, according to the prefixed activation criteria. Overall, initially a 1:1:1 ratio-driven delivery is used for patients with bleeding trauma and postpartum hemorrhage (1 red blood cell (RBC), 1 thawed fresh frozen plasma (FFP), and 1 platelet pool). Thawed plasma is always available at the hospital blood bank, to avoid administration delays. However, when ROTEM results become available, a ROTEM-guided-therapy with coagulation factors concentrates is always preferred. If clotting tests or ROTEM values are normal, no further plasma is transfused. Patients who present with severe hemorrhage (e.g., cardiac surgery, liver transplantation) are usually managed according to a goal-directed strategy based on point-of-care ROTEM parameters and coagulation factor concentrates (mainly fibrinogen concentrate administration) from the onset of bleeding. Anyway, after the initial management of coagulopathy with hemostatic resuscitation, if hemorrhage persists, patients are managed using a goal-directed strategy. Notably, coagulopathy correction is a step-by-step process. First: Treat possible hyperfibrinolysis; Second: In bleeding cardiac surgery, a CT-INTEM/CT-HEPTEM ratio>1.2 should be achieved with protamine. Third: Correct hypofibrinogenemia. Fourth: Correct thrombocytopenia. Fifth: Correct thrombin-generating factors deficit.
The massive hemorrhage management strategy is based on prompt bleeding control, blood loss replacement, and rapid diagnosis and effective treatment of coagulopathy. In fact, early hemostasis is independently associated with a decreased incidence of 30-day mortality and, therefore, it should be considered both as a treatment endpoint and as a potential quality indicator. From a practical point of view, massive hemorrhage and coagulopathy can be treated with hemostatic resuscitation and/or with a goal-directed therapy strategy (GDT) based on point-of-care (POC) testing with viscoelastic hemostatic assays (VHAs). The standard coagulation assays (SCAs) would be a valid alternative when VHAs are unavailable.1–3
Hemostatic resuscitation is the basis of most massive transfusion protocols involving resuscitation with blood components resembling whole blood.1,2 It aims at controlling coagulopathy and restoring blood losses, while maintaining circulating volume and avoiding the complications of aggressive crystalloid fluid resuscitation. It involves a prefixed ratio-driven administration of blood components (packed red blood cells, fresh frozen plasma (FFP), and platelet pools). However, the main disadvantage of this strategy is the large quantities of blood components (mostly FFP) “blindly” transfused (non-directed by clotting tests) with a prolonged turnaround time, due to thawing and administration of FFP, that might delay the initiation of specific hemostatic therapy. Prolonged turnaround time may be shortened by replacing FFP transfusion by fibrinogen concentrate, and by using POC devices that measures aPTT and INR quickly.4
GDT, the second strategy to treat the coagulopathy, relies on the administration of coagulation factor concentrates, mainly fibrinogen concentrate or cryoprecipitate, guided by VHAs.5 Unlike a prefixed ratio-driven strategy, GDT relies on guided administration of pro-hemostatic drugs, with reduced turnaround time (Table 1).
Advantages and disadvantages of applying point-of-care viscoelastic hemostatic assays (TEG: thromboelastography or ROTEM: rotational thromboelastometry).
| Advantages: |
| - Used as point-of-care, with rapid availability of results (within 10–15min) |
| - Reduce transfusion requirements and, probably, mortality rate |
| - Detect the timing and extent of fibrinolysis (Early detection of the onset and extent of fibrinolysis?) |
| Performed in whole blood, unlike the conventional coagulation tests which are performed in plasma. |
| Disadvantages: |
| - Do not detect platelet inhibition (except for platelet mapping of TEG) |
| - Role in detecting the effect of new anticoagulants not well-defined |
| - Do not detect inherited bleeding disorders (von Willebrand, hemophilia) |
| - Require adequate technical and interpretation training |
| - Require periodic calibration. |
Which approach should be used to treat coagulopathy is a matter of continued controversy. A reasonable strategy (Fig. 2) could be:
Step 1: Administer tranexamic acid1,2 (strong evidence) within the first 3h of bleeding onset in patients with trauma1,2 (crash studies), postpartum hemorrhage1,2 (woman study), and when hyperfibrinolysis is strongly suspected (VHAs diagnoses). Moreover, 2g of fibrinogen concentrate could also be administered (low grade of evidence) in patients with uncontrollable hemorrhage to avoid the delay associated with thawing and administration of plasma.1,2
Step 2: A second phase based on hemostatic resuscitation follow should immediately (Fig. 2). It is aimed at early control of coagulopathy, and must be conducted irrespectively of coagulation test results.
Step 3: Once the results of the VHAs/SCAs tests are available, any “blind” treatment should be abandoned, and coagulopathy should be controlled by a GDT, based on VHAs and specific therapy with blood components (plasma and platelets) and prohemostatic drugs (additional doses of tranexamic acid, fibrinogen concentrate or cryoprecipitate, and prothrombin complex concentrate). If VHAs are unavailable, SCAs could be used instead.
The commonly used SCAs in clinical practice, PT, INR, aPTT, fibrinogen (Clauss), and platelet count may provide inadequate information about clinically significant coagulopathy, suffer from slow turnaround time (not a POC), and lack the ability to guide individual therapy. Unlike SCAs, VHAs (TEG/ROTEM) are POC testing that rapidly assesses viscoelastic clot strength, with the potential of an ongoing real-time process, rather than reflecting individual steps of the coagulation cascade (Table 1). Additionally, TEG/ROTEM can detect the timing and extent of fibrinolysis, which cannot be accurately estimated by SCAs.
A Cochrane Systematic Review (1493 participants)6 concluded that TEG/ROTEM use significantly reduced transfusion rate and overall mortality, which was confirmed by a systematic review and meta-analysis7 (38 studies with 13 randomized controlled trials (RCTs)) comparing guided-TEG/ROTEM with non-guided TEG/ROTEM blood products transfusion in trauma, surgical, and critically ill patients with acute, severe hemorrhage. By using the GRADE methodology,7 TEG/ROTEM-guided transfusions led to fewer numbers of patients being exposed to blood product in all studied populations and to reduced transfusion requirements in trauma and surgical ones. However, the mean difference in transfused units was always ≤1 (questionable clinical significance), and the authors acknowledged low quality of evidence and publication bias. The use of ROTEM/TEG POC-testing also reduces the transfusion requirements during cardiac surgery8 and postpartum hemorrhage.9
Despite the theoretical advantage of VHAs/POC tests over SCAs, a systematic review aimed to summarize the published evidence evaluating VHAs-guided resuscitation strategy in trauma patients did not find significant differences in transfusion rates and mortality between the VHA-guided and control groups.10 The recently published multi-center RCT11 ITACTIC (Implementing Treatment Algorithms for the Correction of Trauma-Induced Coagulopathy) also failed to demonstrate any benefit to include VHAs in the major hemorrhage managing protocol.
The following limitations may account for these controversies. First, prolonged empirical delivery of balanced hemostatic therapies before applying a guided strategy probably influences the clinical outcomes by delaying targeted hemostasis management by VHAs. Second, the VHAs-based cutoffs for infusing coagulation factors varies among studies. Third, the clinical efficacy depends on the clinical setting. Targeted therapy with VHAs can be applied earlier in hospitalized patients, such as those undergoing cardiac surgery, increasing the effectiveness of VHAs in this clinical setting when compared to trauma patients, for whom it is initiated later.
Upon patient admission to hospital, all actions to control critical bleeding and coagulopathy must be centered on the institutional protocol for management of massive hemorrhage. This protocol should be known in detail by the members of all departments caring these patient populations, and should include clear, easy-to-follow algorithms developed for rapid clinical decision-making to minimize the turnaround time. GDT should be initiated as soon as possible based on VHAs1,5 (Fig. 2), or SCAs, if VHAs are unavailable. Delaying guided therapy and continuing with hemostatic resuscitation may worsen the clinical outcome.
There is some evidence of the superiority of VHAs over SCAs for GDT, yet it has not been clearly documented and depends on the clinical situation and the rapid availability of these assays. More RCTs using clear-cut action protocols and well-defined cutoffs for making effective clinical decisions are needed in order to compare clinical outcomes (reduction in mortality, bleeding volume, and transfused units) of bleeding patients managed with hemostatic resuscitation vs. GDT, and with VHAs vs. SCAs strategies, guided by algorithms.AbbreviationsA5-EXTEMClot amplitude (mm) obtained 5min after clotting time, assessed in extrinsic coagulation pathway in ROTEM assay (EXTEM)A5-FIBTEMClot amplitude (mm) obtained 5min after clotting time, assessed in dynamic fibrinogen contribution to clot firmness in ROTEM assay (FIBTEM)FFPFresh frozen plasmaGDTGoal directed therapyINRInternational normalized ratiopRBCPacked red blood cellsPOCPoint of careRCTRandomized controlled trialROTEMRotational thromboelastometrySCAsStandard coagulation assaysTEGThromboelastographyVHAsViscoelastic hemostatic assays
AbbreviationsA5-EXTEM Clot amplitude (mm) obtained 5min after clotting time, assessed in extrinsic coagulation pathway in ROTEM assay (EXTEM) Clot amplitude (mm) obtained 5min after clotting time, assessed in dynamic fibrinogen contribution to clot firmness in ROTEM assay (FIBTEM) Fresh frozen plasma Goal directed therapy International normalized ratio Packed red blood cells Point of care Randomized controlled trial Rotational thromboelastometry Standard coagulation assays Thromboelastography Viscoelastic hemostatic assays
The authors declare that they have no conflicts of interest.
FundingThis research was supported by the Fondo de Investigación Sanitaria (FIS)PI 15/00512, Instituto de Salud Carlos III, Ministry of Health, Government of Spain, and European Regional Development Fund (ERDF).
We thank Proff Manuel Múñoz Gómez, University of Málaga, for their expertise and assistance throughout all aspects of our study and for their help in critical reading and English wording of the manuscript.