There has been an increase in the use of veno-venous (VV) and veno-arterial (VA) extracorporeal membrane oxygenation (ECMO) in recent years. Nevertheless, ECMO is not a healing treatment; it only affords bridging cardiocirculatory and/or respiratory support until the patient disease condition improves or some other type of treatment or a transplantation is performed. In this context, adequate pharmacological management plays an important role in the clinical course of the patient, and sedoanalgesic is a key element in this regard.1 However, the optimum management of analgesia and sedation in ECMO has not been clearly defined, and daily practice varies considerably from one center to another.2–5
In addition to the usual pharmacokinetic alterations found in critical patients, consideration is required of the changes induced by ECMO,6–9 and which are mainly a consequence of the following:
- 1-
Drug adsorption or sequestration within the circuit. The tubing of the system is the main site where this occurs, though there is a lack of comparative data on the different types of tubing used (impregnated or not with heparin, albumin, etc.).6,7 The oxygenator device can also exert an influence, though its impact is possibly less relevant.7 Adsorption is also conditioned by the physicochemical characteristics of the administered drug. The greater the lipophilicity of the latter and its binding to plasma proteins, the greater the possibility of sequestration (Table 1). After hours of ECMO, saturation may occur at the drug sequestration sites within the circuit, giving rise to the risk of toxicity if high drug dosing is maintained for a prolonged period of time once the adsorption phenomenon has been saturated.6,8 Furthermore, the circuit may act as a reservoir, releasing the sequestered drug once its administration has ceased; this in turn would prolong the residual sedoanalgesic effect and could affect attempted weaning from ECMO or mechanical ventilation (MV).6–8
Table 1.Pharmacokinetic characteristics of analgesics and sedatives, and changes observed in studies with ECMO.
Drugs Log P Protein binding (%) Vd (L/kg) Considerations Published studies Fentanyl 3.8–4.1 80–85 4 (3–8) Losses 67–97% in 24 h • Shekar K et al. Crit Care 2012;16:R194 (ex vivo) Up to 70% lost in first hour • Harthan AA et al. J Pediatr Pharmacol Ther 2014;19:288–95 (ex vivo) Morphine 0.9 35 5 No significant losses (<10–20%) in 24 h • Shekar K et al. Crit Care 2012;16:R194 (ex vivo) • Harthan AA et al. J Pediatr Pharmacol Ther 2014;19:288−95 (ex vivo) Remifentanil 1.5–1.7 70 0.35 Increased Vd and clearance in ECMO influenced by gender (↑ women) and rpm of pump (lineal) • Yang S et al. Sci Rep 2017;7:16276 (in vivo ECMO VA) Midazolam 3.9 97 1–3 Losses 81–89% in 24 h • Shekar K et al. Crit Care 2012;16:R194 (ex vivo) Up to 50% in first 30–60 min • Harthan AA et al. J Pediatr Pharmacol Ther 2014;19:288−95 (ex vivo) • Lemaitre F et al. Crit Care 2015;19:40 (ex vivo) Propofol 3.8–4.1 95–99 60 Losses 70% in 30 min, 89% 5 h and ≈100% in 24 h (PVC tubes, oxidation) • Lemaitre F et al. Crit Care 2015;19:40 (ex vivo) No ↓ half-life of oxygenator • Hohlfelder B et al. ASAIO J 2017;63:179−84. (in vivo, ECMO VV and VA. • Lamm W et al. Int J Artif Organs 2019;42:233−40. (in vivo, ECMO VV and VA) Dexmedetomidine 2.8–3.3 94 2 Losses 30–90% in 24 h • Wagner D et al. Perfusion 2013;28:40−6. (ex vivo) PVC tube? Oxygenator? • Park J et al. ASAIO J 2017;63:293−8. (in vitro) • Dallefeld SH et al. Perfusion 2020;35:209−16. (ex vivo) Ketamine 2.7–3.3 53 4 Moderate sequestration? No pharmacokinetic studies. Thiopental 2.8–3 80 1–1.5 Loss 88% in 24 h • Shekar K et al. Crit Care 2015;19:164. (ex vivo) Log P (octanol/water coefficient; determines degree of lipophilicity), increased lipophilicity and protein binding = greater adsorption potential.
Vd (volume of distribution), lesser Vd = greater pharmacokinetic impact of ECMO.
PVC (polyvinyl chloride); rpm (revolutions per minute); VV (veno-venous); VA (veno-arterial).
- 2-
Increased volume of distribution through the circuit, drug sequestration, initial fluid resuscitation and possible inflammatory reaction associated with the device.6,8,10
- 3-
Changes in clearance of drugs secondary to increased cardiac output or to the frequent coexistence of renal and/or liver failure.
There are still not enough pharmacokinetic studies to allow solid dosing recommendations to be made. Much of the available information comes from in vitro or ex vivo models or studies in pediatric populations, and is not always extrapolatable to adult critical patients. Table 1 shows the results obtained by some experimental studies, most of which involve circuits similar to those used nowadays. These studies have important limitations. In effect, most of them are of short duration (≤24 h), involve a single drug dose administered in a closed circuit, and the concentrations are subsequently measured at different timepoints. Furthermore, the patient-mediated metabolic or elimination effects are not taken into account. Little is known of the impact of maintaining continuous infusion upon drug concentration, extraction and saturation in the circuit, though the drug levels may be expected to increase. In turn, differences in the types of circuits and priming solutions used can also exert an influence.
Is there really an increase in sedoanalgesic requirements during ECMO?A study has reported that 60% of those surveyed consider higher doses to be needed.3 This has also been evidenced in patients with acute respiratory distress syndrome (ARDS) due to influenza A or SARS-CoV-2. It is true that since these are seriously ill patients, many with a high respiratory drive and frequent use of prone position or neuromuscular blockers (NMBs), higher doses may be needed to maintain protective MV — though other factors such as the age of the patient, the degree of inflammatory response or the appearance of tolerance phenomena also exert an influence.
Few studies have evaluated the sedoanalgesic needs of adults on ECMO, and the results are moreover contradictory. In most of them, sedoanalgesic management was left to the criterion of the clinician in charge, with no specific protocol, and often with insufficient monitoring. Some authors have reported an increase in the sedoanalgesic requirements.4,11,12 As an example, Shekar et al. described an average increase of 29 mg/day of morphine and a 10% increase in the daily dose of midazolam. However, in contrast to in vitro studies, no significant increase in fentanyl requirements was observed.11 Other authors have recorded no increase in requirements over time.5,13 These discrepancies may be due to differences in the indications of ECMO, patient severity and in the protocols or drugs used. The first studies were limited to patients with ARDS subjected to prolonged deep sedation.4,12 In contrast, later studies also included patients with ECMO as a bridging strategy for transplantation, using less benzodiazepines, and the aim was to provide mild sedation.5,13 Centers with greater experience tend to minimize deep sedation, seeking mild sedation as early as possible and with a lesser use of benzodiazepines.3
It is also not clear whether dose elevation is entirely attributable to ECMO-related factors. While ECMO may exert an influence, particularly when using certain drugs with concrete physicochemical properties, there probably are other more important factors. Indeed, on analyzing the factors associated with sedoanalgesic requirements, the variable ECMO showed no statistical significance,12 while in contrast an association was observed with patient severity,11 age12 or the administration of high opioid doses.12 Probably, the development of tolerance/deprivation is more important than ECMO as such.14,15 Other factors, such as respiratory disease or the recovery of organ function over time, may also exert an influence.
What sedoanalgesic strategy should be used during ECMO?The current guides do not establish precise recommendations during ECMO.1 The ELSO guidelines advise greater depth at the start, and the reduction of sedation to minimum levels once the patient has been stabilized (https://www.elso.org/ecmo-resources/elso-ecmo-guidelines.aspx). The guide on sedoanalgesia of the Pan-American and Iberian Federation of 2020 suggests the use of less lipophilic opioids (morphine) and the coadjuvant administration of ketamine.16
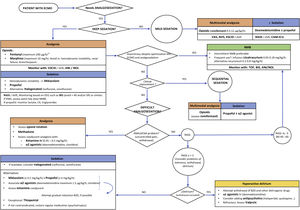
Although the literature remains scarce, a reasonable option or strategy would be that reflected in Fig. 1. In our opinion, the approach to sedoanalgesia should be similar to that used in other critical patients of equivalent severity, taking into account the possible effect of the device — though in view of the heterogeneity involved, caution is required in interpreting the results of the mentioned studies. Probably, the effect is more relevant at the start of therapy or on replacing the oxygenator (possible drug sequestration), as well as perhaps during weaning (possible reservoir effect) — without neglecting the potential saturation of the circuit during treatment. In order to achieve the desired sedoanalgesia, we might need greater doses at the start — though adjustment subsequently is required based on the response obtained. It is essential to individualize treatment, with an adequate selection of the drugs according to their characteristics and the desired sedoanalgesic depth, as well as to ensure optimum monitoring.
Proposed sedoanalgesia algorithm in patients subjected to ECMO.
VAS (visual analog scale), NVS (numeric visual scale), ESCID (Behavioural Indicators of Pain Scale), RASS (Richmond Agitation Sedation Scale), CAM-ICU (Confusion Assessment Method for the Intensive Care Unit), ANI® (analgesia nociception index), NOL® (nociception level index), EEG (electroencephalogram), BIS® (bispectral index), SR (EEG burst suppression rate), EMG (electromyogram), NMB (neuromuscular blocker), TOF (train-of-four), CK (creatine kinase), MV (mechanical ventilation), BZD (benzodiazepines).
The sedoanalgesia guidelines in the critically ill recommend the minimization of sedation and the maintenance of mild sedation.1 However, in patients subjected to ECMO, strict adherence to such recommendations is not always possible. Maintaining superficial sedation and avoiding benzodiazepines might not be feasible in the first stages in unstable patients involving VV-ECMO due to ARDS or VA-ECMO due to cardiogenic shock. In contrast, it may be particularly important when ECMO is indicated as a bridge measure to lung transplantation. Many patients will need an initial period of deep sedation and even NMBs to achieve adequate flows and optimum synchronization with MV. During this period, with adequate monitoring and adjusting the drugs to the objectives, it is possible to minimize the possible complications. Once the patient improves or stabilizes, we should switch early to a sequential/dynamic sedation strategy in order to avoid the prolongation of MV or Intensive Care Unit (ICU) stay, and reduce the risk of nosocomial infection and neuromuscular alterations. Some patients may present problems in reaching these objectives. In such cases, we must discard the presence of pain and also assess the possible appearance of tolerance, withdrawal and/or delirium.15 It is important to anticipate the aforementioned problems, adjusting the medication to the clinical effect, and doing so requires strict monitoring. This is made easier when sedation is mild or moderate, based on the use of scales for the assessment of pain (VAS, ESCID), sedation (RASS) and delirium (CAM-ICU). The problem lies in patients under deep sedation, particularly in the presence of neuromuscular block. Here such scales have limitations or are of little use. At present there are devices for the objective monitoring of pain, such as the ANI® (analgesia nociception index) or NOL® (nociception level index), though firm evidence on their usefulness is lacking, and studies confirming their validity during MV with low volumes and frequencies are still needed. Nevertheless, for the time being there are no other options.1 In deep sedation, we should use point-of-care electroencephalographic (EEG) devices to avoid EEG burst suppression phases.17 The appearance and duration of these phases are associated with an increased incidence of delirium and mortality. Monitoring of the EEG spectrogram may help to improve dosing and allow the early detection of neurological complications. During neuromuscular block, it is important to adjust the latter to the minimum level necessary, and thus avoid pharmacological denervation.
In conclusion, the aim is to secure patient comfort, facilitate adaptation to ECMO and MV, and minimize the possible adverse effects. In patients subjected to ECMO, sedoanalgesia may pose a challenge. In addition to the pharmacokinetic changes related to critical illness and the possible tolerance problems resulting from the prolonged use of opioids and sedatives, ECMO introduces other parameters (drug sequestration within the circuit, changes in volume of distribution, clearance alterations) that can condition the efficacy of drug treatment. The impact of these alterations is still not fully understood, and further prospective studies are needed to produce more evidence capable of guiding clinical practice. In the meantime, it seems reasonable to follow the same principles as in other critical patients of similar severity: individualization of treatment, adequate drug selection, correct monitoring (with caution especially at the start, replacement or end of ECMO), adequate analgesia, and the minimization of sedation.