To determinate the adherence and barriers of our early mobilization protocol in patients who had received mechanical ventilation >48h in routine daily practice through clinical information system during all Intensive Care Unit (ICU) stay.
DesignObservational and prospective cohort study.
SettingPolyvalent ICU over a three-year period (2017–2019).
PatientsAdult patients on mechanical ventilation >48h who met the inclusion criteria for the early mobilization protocol.
InterventionsNone.
Main variables of interestDemographics, adherence to the protocol and putative hidden adherence, total number of mobilizations, barriers, artificial airway/ventilatory support at each mobilization level and adverse events.
ResultsWe analyzed 3269 stay-days from 388 patients with median age of 63 (51–72) years, median APACHE II 23 (18–29) and median ICU stay of 10.1 (6.2–16.5) days. Adherence to the protocol was 56.6% (1850 stay-days), but patients were mobilized in only 32.2% (1472) of all stay-days. The putative hidden adherence was 15.6% (509 stay-days) which would increase adherence to 72.2%. The most common reasons for not mobilizing patients were failure to meeting the criteria for clinical stability in 241 (42%) stay-days and unavailability of physiotherapists in 190 (33%) stay-days. Adverse events occurred in only 6 (0.4%) stay-days.
ConclusionsData form Clinical Information System showed although adherence was high, patients were mobilized in only one-third of all stay-days. Knowing the specific reason why patient were not mobilized in each stay-day allow to develop concrete decisions to increase the number of mobilizations.
Determinar la adherencia y barreras del protocolo de movilización precoz en pacientes que recibieron ventilación mecánica >48 horas en la práctica diaria habitual a través del sistema de información clínica durante toda su estancia en Unidad de Cuidados Intensivos (UCI).
DiseñoEstudio observacional y prospectivo.
ÁmbitoUCI polivalente durante un periodo de tres años (2017-2019).
PacientesSe incluyeron pacientes adultos en ventilación mecánica > 48 horas que cumplieron los criterios de inclusión del protocolo.
IntervencionesNinguna.
Variables principalesSe aplicaron variables demográficas, adherencia y adherencia oculta, número total de movilizaciones, barreras, tipo vía aérea artificial/soporte ventilatorio en cada nivel de movilización y eventos adversos.
ResultadosAnalizamos 3.269 días de estancia de 388 pacientes con una mediana de edad de 63 (51-72) años, mediana de APACHE-II 23(18-29) y estancia en UCI mediana de 10,1 (6,2-16,5) días. La adherencia al protocolo fue del 56,6% (1.850 días de estancia), pero los pacientes se movilizaron solo el 32,2% (1.472) de todos los días de estancia. La adherencia oculta fue del 15,6% (509 días de estancia), aumentando la adherencia al 72,2%. Las causas más comunes para la no movilización fueron el incumplimiento de los criterios de estabilidad clínica en 241 (42%) días de estancia y la falta de disponibilidad de fisioterapeutas en 190 (33%) días de estancia. Los eventos adversos ocurrieron en solo 6 (0,4%) días de estancia.
ConclusionesAunque la adherencia fue alta, los pacientes se movilizaron en solo un tercio de todos los días de estancia. Conocer el motivo específico por el cual los pacientes no fueron movilizados permite desarrollar decisiones concretas para incrementar el número de movilizaciones.
Forced rest in intensive care units (ICU) often results in muscle weakness within days. The term ICU-acquired weakness refers to myopathy and/or polyneuropathy secondary to critical illness and immobilization among other factors.1,2 ICU-acquired weakness occurs in 25%–50% of ventilated patients.3 Bed rest is also associated with long-term complications, manifesting as post intensive care syndrom comprising physical, cognitive, and psychiatric sequelae that prevent patients from recovering their prior quality of life after discharge from the ICU.4
Although benefits of mobilization have been known for decades, early mobilization (EM) was first implemented in ICUs only 20 years ago.5,6 EM is safe and feasible,7,8 and it is associated with better outcomes, including improved functional status, shorter duration of mechanical ventilation (MV), lower incidence of delirium, increased muscle strength, and better quality of life.9–11
EM has recently been included in a package of measures, the ABCDEF care bundles. They are defined as a set of evidence-based interventions that are addressed to minimize the risks derived from sedation, delirium and immobilization, with the aim of potentially reducing the incidence of PICS.12,13
Advances in technology and medicine have increased the complexity of critical care medicine, making clinical information systems (CIS) useful to ensure safe, timely, and effective care. CIS include databases, allowing us to measure what we do, analyze adherence to recommendations based on scientific evidence, improve professional performance, and evaluate the impact of improvement strategies.14
Although some studies have evaluated the practice of EM in ICU,15,16 few have evaluated daily mobilization practices in mechanically ventilated patients,15 and most of these measured adherence at a single point in time.16 To our knowledge, no reports of longitudinal studies evaluating mobilization practices in MV patients in routine clinical practice have been published. The current study aimed to analyze EM in our ICU over a 3-year period. To this end, we used data from our CIS database to determine adherence to EM protocols, the incidence of adverse events, the role of ventilator support, and barriers.
ObjectivesThe primary endpoint was adherence to the EM protocol, defined as the percentage of stay-days in which the EM form extracted from the CIS contained information regarding the type of mobilization or justified reasons for not mobilizing the patient. Secondary endpoints were (a) total number mobilizations, defined as the percentage of stay-days in which patients were mobilized in relation to total stay-days; (b) putative hidden adherence, defined as the percentage of stay-days in which the mobilization form had not been completed, but patients did not meet clinical stability criteria for mobilization; (c) delay in beginning the EM protocol, defined as stay-days before begining the EM protocol in which the EM form was not completed and patients met de clinical stability criteria; (d) barriers; (e) levels of mobilization according to the ventilatory support, type of airway and level of sedation; (f) analysis of non-working days, and (g) adverse events during mobilization, defined as arrhythmias, falls, accidental removal of devices, hypertension (systolic blood pressure >200mmHg), hypotension (systolic blood pressure <80mmHg), hypoxemia (SatO2 <80%), and accidental extubation.7
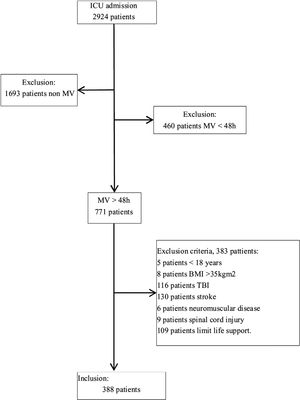
Material and methodsStudy populationThis observational study analyzed data collected prospectively at a 24-bed polyvalent ICU at a tertiary university hospital during a three-year period (January 2017 to December 2019). We included data from patients aged ≥18 years with Barthel Index (BI)>70 prior to admission to the ICU who required >48h MV. We excluded data from patients with spinal cord injuries, history of neuromuscular disease, body mass index (BMI)>45, unsafe airways, cardiac arrests, traumatic brain injuries, or strokes. We discarded stay-days that occurred after decisions to limit life support. All patients or their legal representatives provided written informed consent, and our center's research ethics committee approved the study protocol (CEIC Institut d’Investigació Sanitària Pere Virgili. Reference: 41/2016).
Protocol workflowA multidisciplinary team comprising intensivists, physiotherapists, rehabilitators, and nurses screened all candidates for EM daily (except on weekends and holidays) and began to implement the EM protocol as soon as the patient met the criteria for clinical stability, which were adapted from expert consensus and recommendations.17 The protocol comprised five levels (Appendix 1). Physiotherapists's assessments determined the initial level of EM for each patient and their readiness to progress to the next level. From Monday to Friday, the physiotherapist spent 4h a day. In in bed mobilizations, the sessions lasted approximately 20min
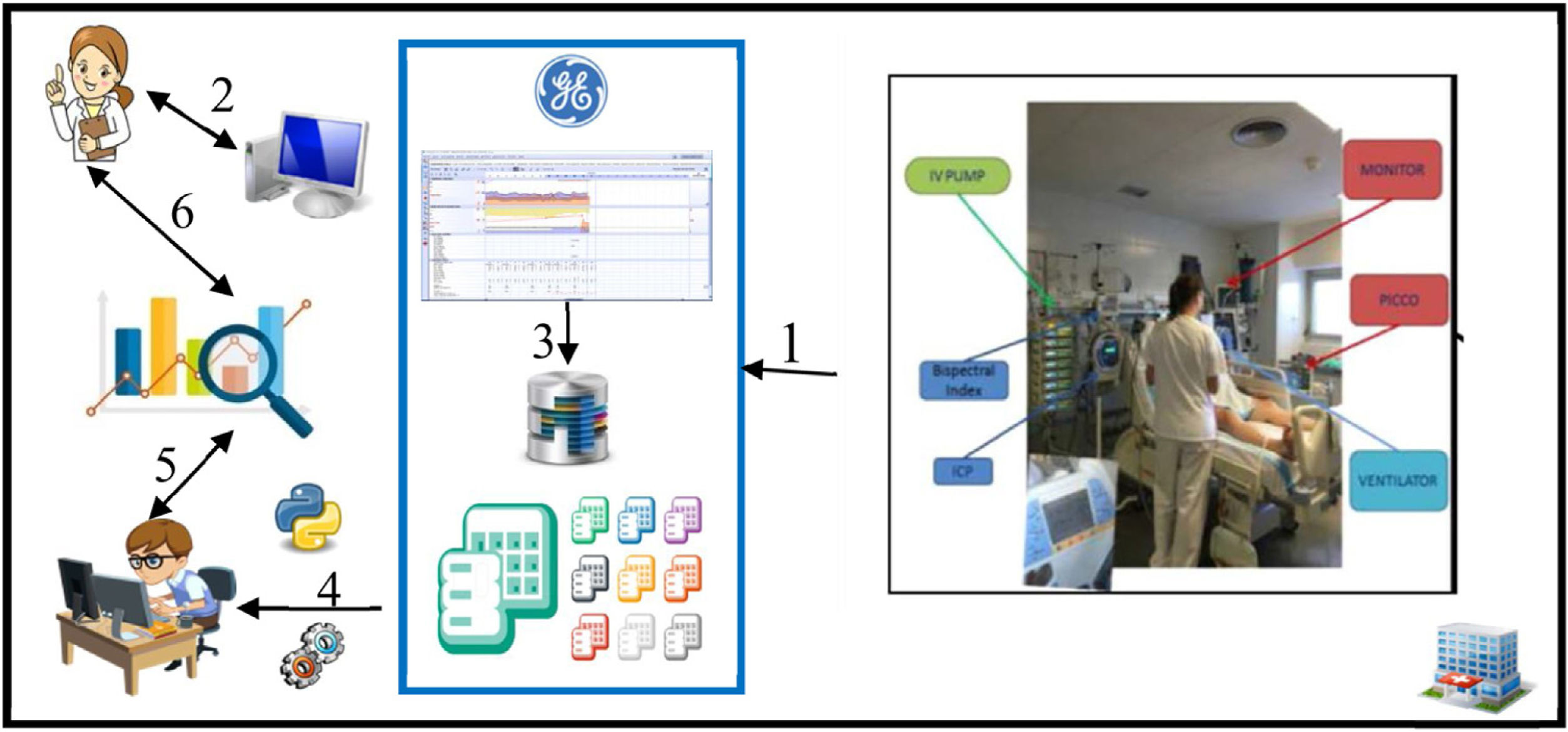
Data collection and representationAll variables were extracted from data routinely stored in our CIS (Centricity Critical Care®, GE Healthcare; Chicago, IL, USA) through automatic capture from connected devices, automatic entry from integrated information systems [laboratory results and data from our hospital's electronic health record (SAP®; Walldorf, Germany)], and manual entry (Appendix 2). Depending on their type and source, data are stored in different tables in the CIS database. Each table contains at least one key field that enables the information it contains to be connected with information in other tables; this relational schema makes it possible to integrate information from different data types and sources with extract, transform, and load (ETL) processes. We used Python 3.0 to execute the ETL process that generated our final dataset from the raw tables within our CIS database (Fig. 1).
Flow of data in the ICU. The arrows show the flow of data from acquisition to display and analysis. Data from bedside devices are sent to the CIS (arrow 1), where they are stored in the database, organized in tables (arrow 3). Clinicians interact with the CIS, manually entering data and retrieving data through their own computers (arrow 2). Data scientists use ETL processes to integrate different types of data from different sources (arrow 4) and work together with clinicians to analyze the data statistically to interpret the data (arrows 5 and 6).
The ETL process allowed us to automatically obtain a large dataset in which each row contains all the relevant data for each patient stay, including both static (patient-related) and dynamic (stay-related) data. Static data included age, sex, comorbidities, Acute Physiological and Chronic Health Evaluation (APACHE)II score at admission, Sequential Organ Failure Assessment (SOFA) score at admission, reason for admission, ICU length of stay (LOS), and hospital LOS. Dynamic data included data from the EM form stored in the CIS (Fig. 1), artificial-airway/ventilatory-support data, and all the data required to determine whether patients met the criteria for clinical stability on each day of the stay. To facilitate daily decisions related to the EM protocol, the ETL automatically determined whether these criteria were met based on the data recorded between 8 and 10am.
Statistical analysisTo determine adherence to the protocol and barriers, we performed a descriptive analysis of the data. Numerical variables are expressed as median and interquartile range, and categorical variables are expressed as frequencies and percentages. All data mining and processing were carried out using Python 3.0. The unit of analysis was ICU stay-day.
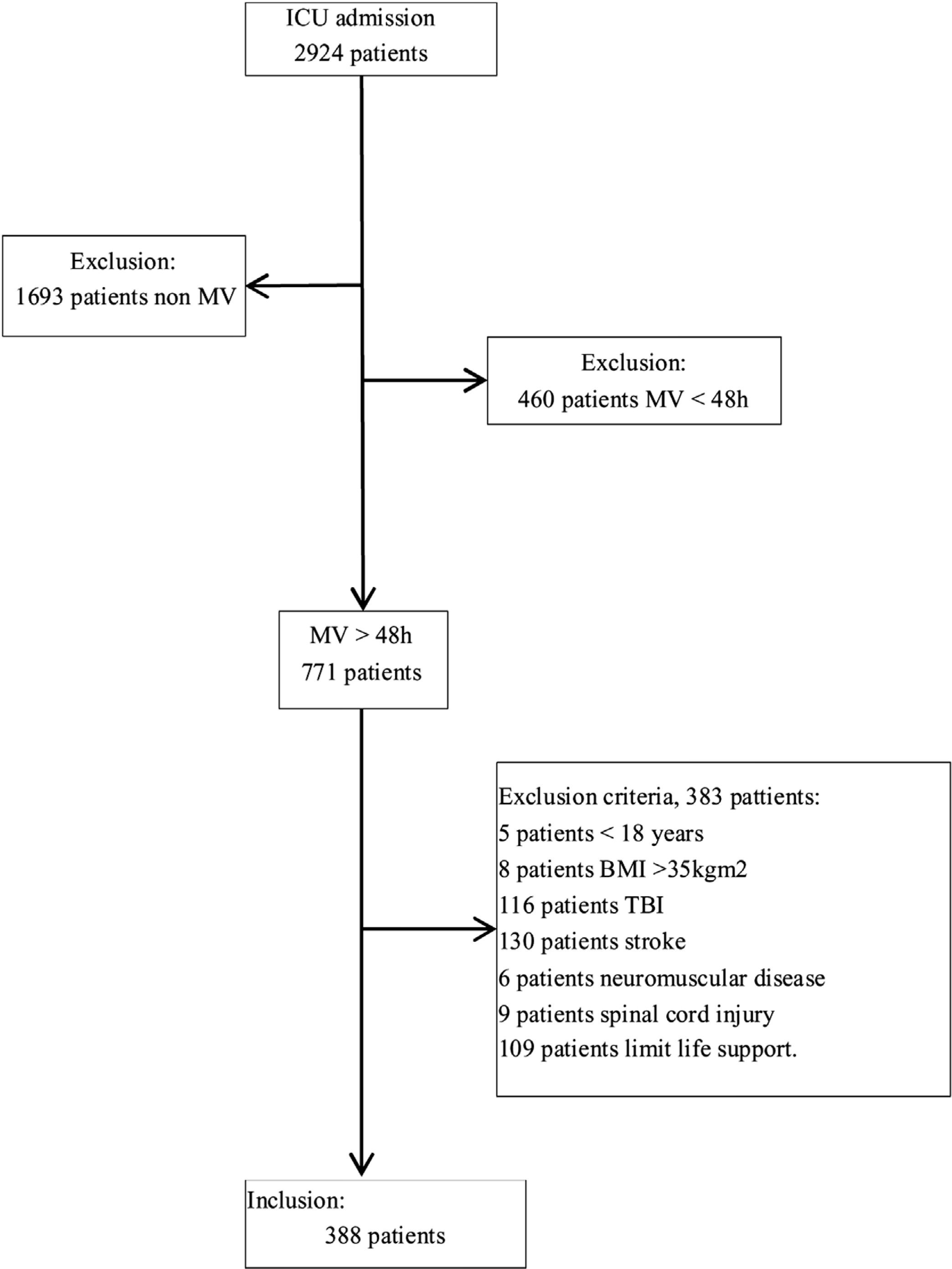
ResultsOf the 2924 patients admitted to the ICU during the study period, 1231 (42.1%) required MV; of these 771 (62.6%) received MV>48h and after excluded 383 (49.7%) for meeting the exclusion criteria, we included 388 (50.3%) patients (Fig. 2) (median age, 63 [51–72]y; 273 (70.4%) men). At admission, the median APACHE II score was 23 (18–29) and the median SOFA score was 3 (1–5.8). The most frequent reason for admission was respiratory failure 23.5%, followed by postsurgical care 19.8% and septic shock 18.3%.
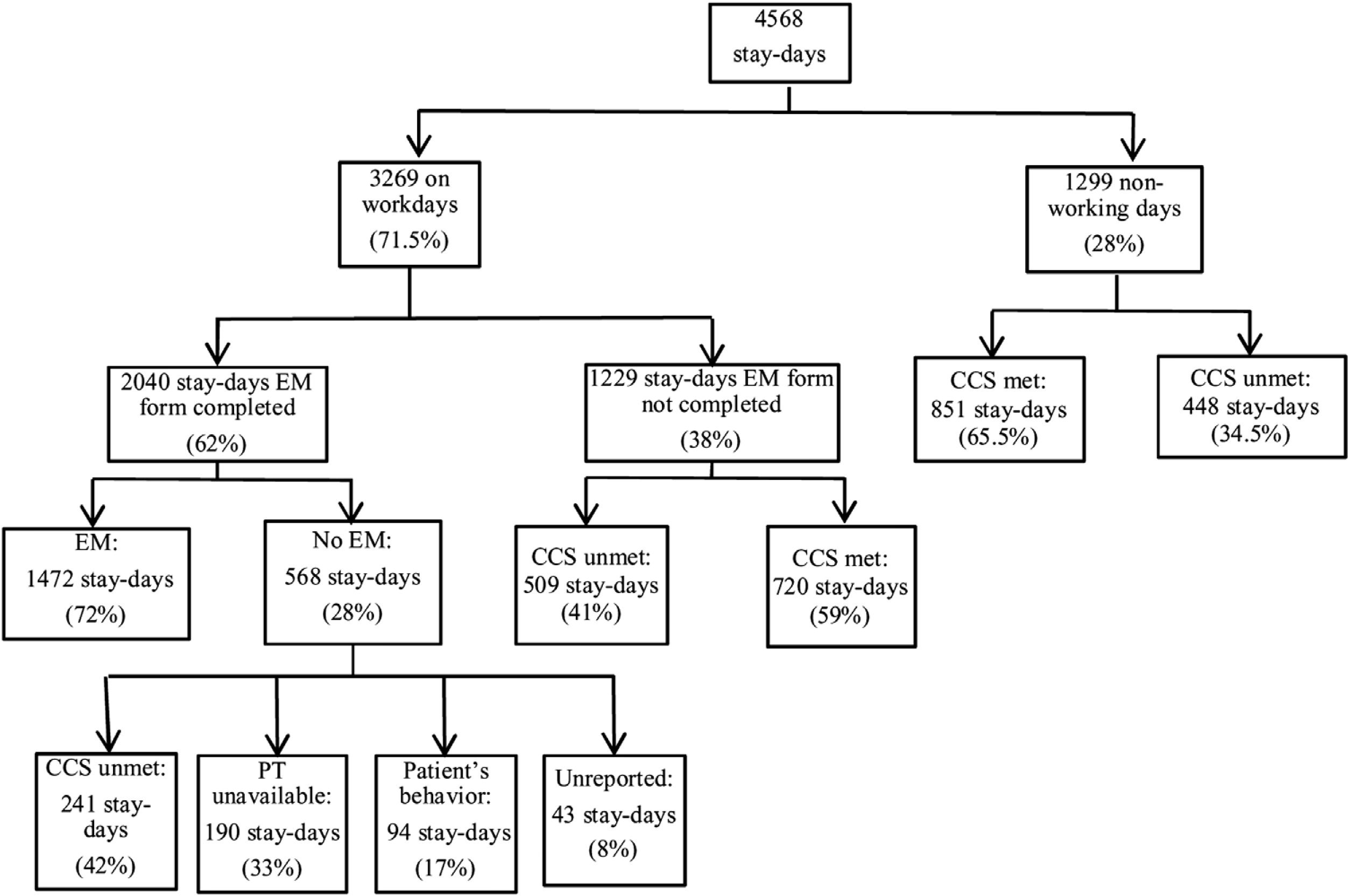
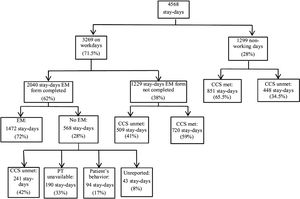
The ICU stays for these 388 patients comprised a total of 4568 stay-days; of these, 1299 fell on weekends or holidays when patients were not screened for EM. Thus, a total of 3269 stay-days were analyzed to obtain the adherence (Fig. 3). The EM form was completed in 2040 stay-days; of these, patients were mobilized in 1472 stay-days. Since the lack of a physiotherapist in 190 stay-days is a cause of non-adherence to the protocol, they were not taken into account within the 2040 stay-days, so the adherence was 1850 (56.6%) stay-days compared to the 3269 stay-days evaluated.
Secondary outcomesTotal number of mobilizationsWhen all 4568 stay-days were considered to calculate the total number of mobilizations patients underwent EM in only 1472 (32.2%) stay-days.
Putative hidden adherenceThe EM form was not completed in 1229 stay-days (Fig. 3). In 509 of these stay-days, patients did not meet the criteria for clinical stability and would have been ineligible for EM. Since failure to complete the form in these cases was certainly an oversight because clinicians were totally aware of patients’ ineligibility, generating hidden protocol adherence of 15.6%, which would increase adherence to 72.2%.
Delay in beginningIn the remaining 720 stay-days, patients meet the criteria for clinical stability and of these 285 stays-days were days of delay in the beginning of the protocol.
The median ICU length of stay in the patients who underwent EM was 10.1 (6.2–16.5) days. EM was initiated a median of 3 (1–5) days after admission to the ICU, and active mobilization (level II) was initiated a median of 6 (4–10) days after admission.
BarriersTo obtain the barriers we analyzed 568 stay-days in which the patients were not mobilized (Fig. 3). The reasons were: failure to meet the criteria for clinical stability in 241 (42.2%) stay-days, unavailability of physiotherapists in 190 (33.3%) stay-days, patient's behavior in 88 (17%) stay-days, unknown—i.e., the form was completed but mobilization did not take place and no reason was recorded in 43 (8%) stay-days. Failure to meet the criteria for clinical stability was due to hemodynamic factors in 121 (50.2%) stay-days, respiratory factors in 81 (33.6%) stay-days, neurologic factors in 27 (11.2%) stay-days, and infectious factors in 12 (5%) stay-days.
Levels of mobilization according to the ventilatory support, the type of airway and level of sedationFinally, we analyzed the levels of mobilization according to the ventilatory support and the type of airway (Table 1). Mobilizations took place in bed (Level I–II) in 972 (66%) stay-days and out of bed (Level III–V) in 500 (34%) stay-days. In mobilizations that took place in bed, patients were on invasive MV in 707 (48%) stay-days (with an endotracheal tube in place in 467 (66%) stay-days and with a tracheostomy tube in place in 240 (34%) stay-days, without ventilatory support in 179 (12.1%) stay-days, on high-flow nasal cannula in 53 (3.6%) stay-days, and noninvasive MV in 33 (2.3%) stay-days.
Representing the percentage of each O2 therapy – early mobilization level combination in the 1472 stay-days when early mobilizations took place.
| EM level | EM stay-days | Ventilatory support | ||||
|---|---|---|---|---|---|---|
| HFO | IMV | IMV-T | NIMV | No support | ||
| n=1472 (%) | n=93 (%) | n=498 (%) | n=314 (%) | n=61 (%) | n=506(%) | |
| In bed | 972 (66) | 53 (57) | 467 (94) | 240 (76) | 33 (54) | 179 (35) |
| Level 1 | 530 (36) | 7 (7) | 368 (74) | 131 (42) | 4 (6.5) | 23 (4) |
| Level 2 | 442 (30) | 46 (49) | 99 (20) | 109 (34) | 29 (47.5) | 156 (31) |
| Out bed | 500 (34) | 40 (43) | 31 (6) | 74 (24) | 28 (46) | 327 (65) |
| Level 3 | 103 (7) | 4 (4) | 25 (5) | 25 (8) | 14 (23) | 40 (8) |
| Level 4 | 206 (14) | 18 (20) | 4 (0.8) | 13 (4) | 14 (23) | 146 (29) |
| Level 5 | 191 (13) | 18 (20) | 2 (0.4) | 37 (12) | 0 (0) | 141 (28) |
HFO: high-flow nasal cannula; IMV: invasive mechanical ventilation; IMV-T: invasive mechanical ventilation and tracheostomy tube; NIMV: noninvasive mechanical ventilation.
In mobilizations that took place out of bed, patients were without ventilatory support in 327 (22.2%) stay-days, on invasive MV in 105 (7.1%) stay-days with an endotracheal tube in place in 31 (30%) stay-days and a tracheostomy tube in place in 74 (70%) stay-days, on high-flow nasal cannula in 40 (2.7%) stay-days, and on noninvasive MV in 28 (2%) stay-days. Of the 105 stays with artificial airway and connected to MV or mechanical ventilation-tracheostomy (MV-T), 50 stay-days sitting in bed (Level 3), 17 stays sitting out of bed (Level 4) and 39 stay-days standing out of bed and walking (Level 5).
In 530 stay-days where Level I mobilizations took place, 439 (82.7%) stay-days patients were sedated (RASS≤−1), 25 (4.8%) stay-days were agitated (RASS≥1), and 66 (12.5%) stay-days were awake and calm (RASS=0).
Analysis of non-working daysIn 1299 stay-days patients were not mobilized due to non-working days. Of these, in 851 (65.5%) stay-days patients met the clinical stability criteria and in 448 (34.5%) stay-days patients failed to meet the clinical stability criteria.
Advers eventsSix adverse events (0.4%) took place in the 1472 stay-days: three episodes of hypoxemia (quickly corrected with supplemental oxygen) and one each of orthostatic hypotension, hypertension, and arrhythmia.
DiscussionOur study shows that using real-world data stored in the CIS the EM took place in 56.6% stay-days, showing that there is much room for improvement. The main barrier was failure to meet the clinical stability criteria, followed by the unavailability of physiotherapists.
Dubb et al.18 identified 28 barriers to EM, concluding that successful implementation of EM protocols requires each ICU to identify barriers based on its patient mix, equipment, staff expertise, and local culture. Most studies report that patient-related factors such as hemodynamic instability, delirium, or sedation levels are major barriers to EM. In our study, the main barrier to EM was failure to meet the safety criteria, which was the reason mobilization was not performed in half of the cases; these findings corroborate those reported in other studies.7,15,19 Although consensus recommendations regarding the safety criteria for mobilizing mechanically ventilated patients can be used to guide rehabilitation in the ICU while minimizing the risk of adverse events,17 the absence of clinical practice guidelines suggests that cautious application of more flexible criteria guided by analyses of data from the CIS might enable more patients to benefit from EM.
EM in critically ill patients requires coordination, commitment, and physical effort by the multidisciplinary team. Physiotherapists play a key role in EM; however, our country's ICU culture assigns a limited role for physiotherapists compared to other countries.20 A recent study in our country found that 18.6% of ICU teams included no physiotherapists; 10.5% had a physiotherapist for less than 5h a week, 12.8% had one between 5 and 10h a week, 10.5% between 10 and 15h a week, 8.1% between 15 and 20h a week, and only 4.6% had one for more than 24h a week. In the remaining 34.9%, physiotherapists could be consulted through the rehabilitation service.21 Our protocol was designed considering that physiotherapists would be present for 6h a day on weekdays that were not on non-working-days. Our results show that in 851 non-working stay-days patients met the safety criteria but were not mobilized because physiotherapists were unavailable.
One way to mitigate the unavailability of physiotherapists is to involve nurses and family members in EM. Integrating EM training into nursing education can help nurses understand the risks and benefits of mobilization in critically ill patients; increasing nurses’ knowledge can improve outcomes through better management of personnel needed to complete transfers of patients being mobilized.22 Rukstele et al.23 found that the participation of family members in mobilization efforts increased adherence to recommendations for mobility in the ICU constantly over a 6-month period.
In our study, patients were still connected to invasive MV in 48% of mobilizations, and patients remained in bed in 66%. This is logical, considering that patients were in the subacute phase of their disease and were sedated or being weaned from sedation. RASS scores show that patients were sedated in 82.7% of the stay-days in which Level I early mobilizations took place. Reviewing sedation protocols with the aim of shortening the time under sedation, as recommended in recent guidelines,24 might also improve the rate of early mobilizations. Nonetheless, 34% of mobilizations in our study were done outside the bed; of these, 22.2% took place after patients no longer required ventilatory support, although patients remained connected to MV in 7.1% of all mobilizations, mostly through tracheostomy tubes (5% of all mobilizations). Recent point-prevalence studies have also found low rates of out-of-bed mobilizations. Although a German study reported that 24% of mobilizations took place outside bed, 50% of the patients in that study were on noninvasive MV. Another study, done in Australia and New Zealand, reported that none of the patients who required MV sat out of bed, stood, or walked, and only 12% sat over the side of the bed.5 A recent study in Brazil reported that only 3 of 158 mechanically ventilated patients stood, marched on spot, or walked during mobilizations.16 Although the rate of out-of-bed mobilizations in patients undergoing invasive MV in our study is low, it is to our knowledge the highest reported to date.
The lack of data in one-third of all stay-days reveals the need to establish mechanisms to audit routine data collection in our unit. In the stay-days in which the EM form was not completed, it would not have been possible to carry out the mobilization in 720 (59%) stay-days because patients failed to meet the clinical stability criteria; however, in the other 509 (41%) stay-days, patients met the criteria and should have been mobilized. Our group recently developed and validated a tool that can help us to improve data collection through real-time random safety audits, which have proven effective in detecting and correcting errors of omission in real time, thereby improving adherence to guidelines.25,26 Including the audit of the EM form in our routine real-time random safety audits will improve adherence to our EM protocol.
We found that EM started a median of 3 days after admission; this finding is similar to the time to mobilization reported by Basset et al.27 However, a recently published meta-analysis concluded that the time when patients are mobilized varies widely, ranging from the first to the eighth day after ICU admission.28 It is important to note that some studies define the starting point of EM as the initiation of passive mobilizations27 and others define it as the initiation of active mobilizations.29
The incidence of adverse events (0.4%) was even lower than those reported by Bourdin et al.8 (3%) and Bailey et al.7 (<1%), corroborating that EM is safe.
Our study has several limitations. This was a single-center, so caution is warranted in extrapolating our results and conclusions to other ICU. Our EM protocol was designed following the recommendations of experts and published studies, adapting them to the resources available at our institution. The lack of guidelines to unify protocols in terms of the criteria for clinical stability, types of mobilization, and duration of treatment makes comparisons with other studies difficult.
Our study analyzed data stored in the CIS, and the reliability of some of these data depends on the staff's commitment to collecting and entering them during routine daily practice. In this regard, it is important to highlight that the ETL enabled us to determine that patients did not meet the safety criteria for mobilization in 509 of 1229 stay-days for which no EM form was registered in the CIS (considered stay-days in which protocol was not adhered to). It is possible that in some of these cases the staff decided not to perform EM without completing the form in the CIS, and failure to register the decision could account for up to 15.6% of the cases that would be classified as failure to adhere to protocol.
ConclusionsOur study shows that it is possible to use data from the CIS to evaluate many aspects of an EM protocol during a long period of clinical practice. Although adherence to the protocol was high, patients were mobilized in only one-third of all stay-days. The information from this study points to ways we can improve EM in our ICU, including reviewing the sedation protocol, assessing the suitability of the criteria for clinical stability, and increasing the presence of physiotherapists or trying to involve family members. Importantly, the entire study required no extra efforts in data collection thanks to staff registering information in the CIS in their daily routine. The lack of data for one-third of all the stay-days pointed out the need to implement mechanisms (e.g., real-time random safety audits) to audit routine data collection in our ICU.
Author contributionsM.M., P.P. and J.G. designed the study, design of statistical analyses and data analysis. All authors contributed to critical examination of the paper for important intellectual content and approval of the final manuscript. All authors have read and agreed to the published version of the manuscript.
FundingThis study was supported by grants from the Fondo de Investigación Sanitaria (Carlos III Institute of Health, Spain, FIS grants, project PI20/01674). Agència de gestió d’Ajuts Universitaris i de Recerca 2017 SGR 00127. FEDER.
Conflicts of interestThe authors declare that they have no conflict of interest.
The authors would like to thank all the ICU physicians, nurses, nursing assistants, physiotherapist, rehabilitors and health care personnel for their commitment and contribution to the project. John Giba edited the manuscript.