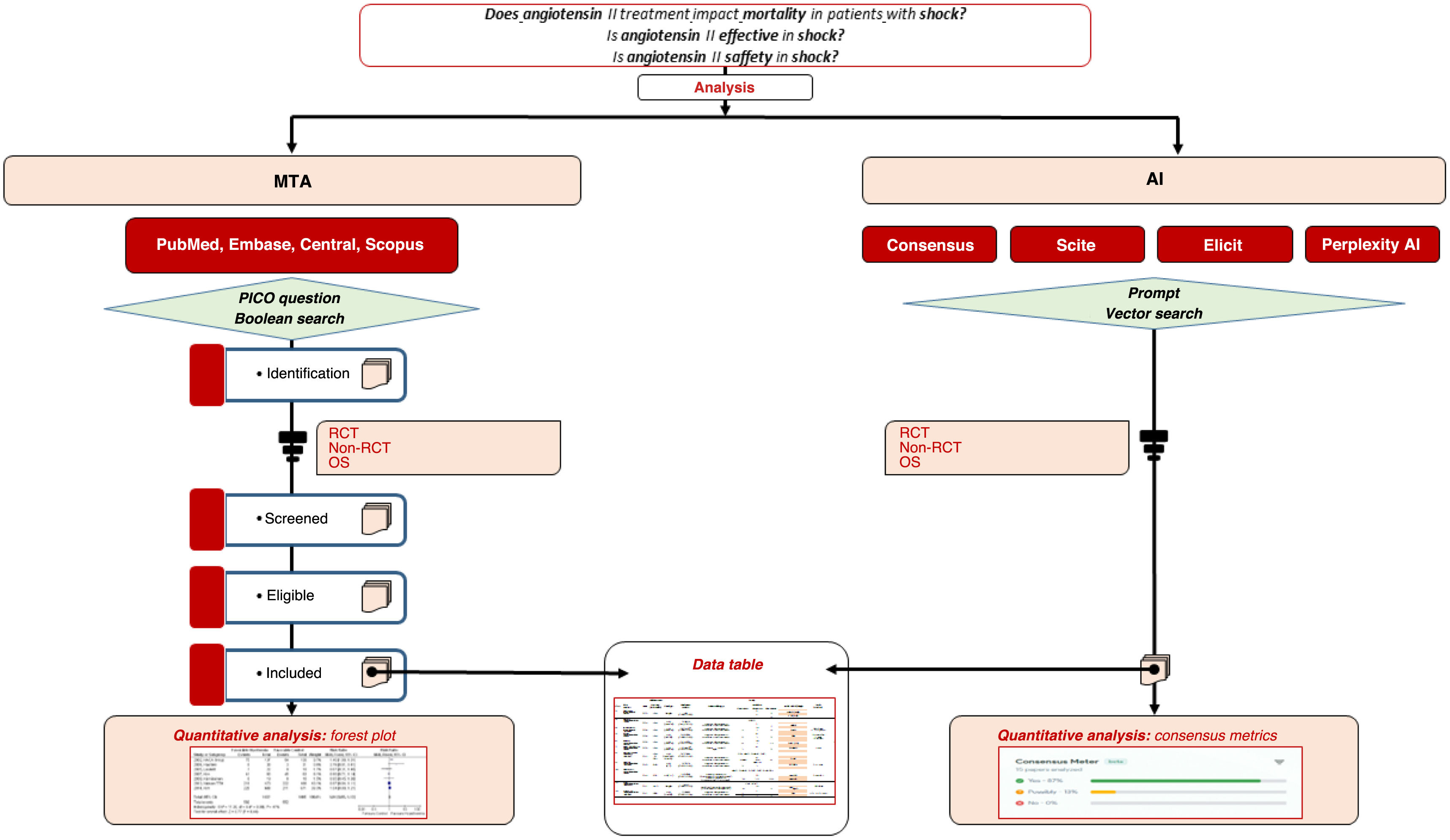
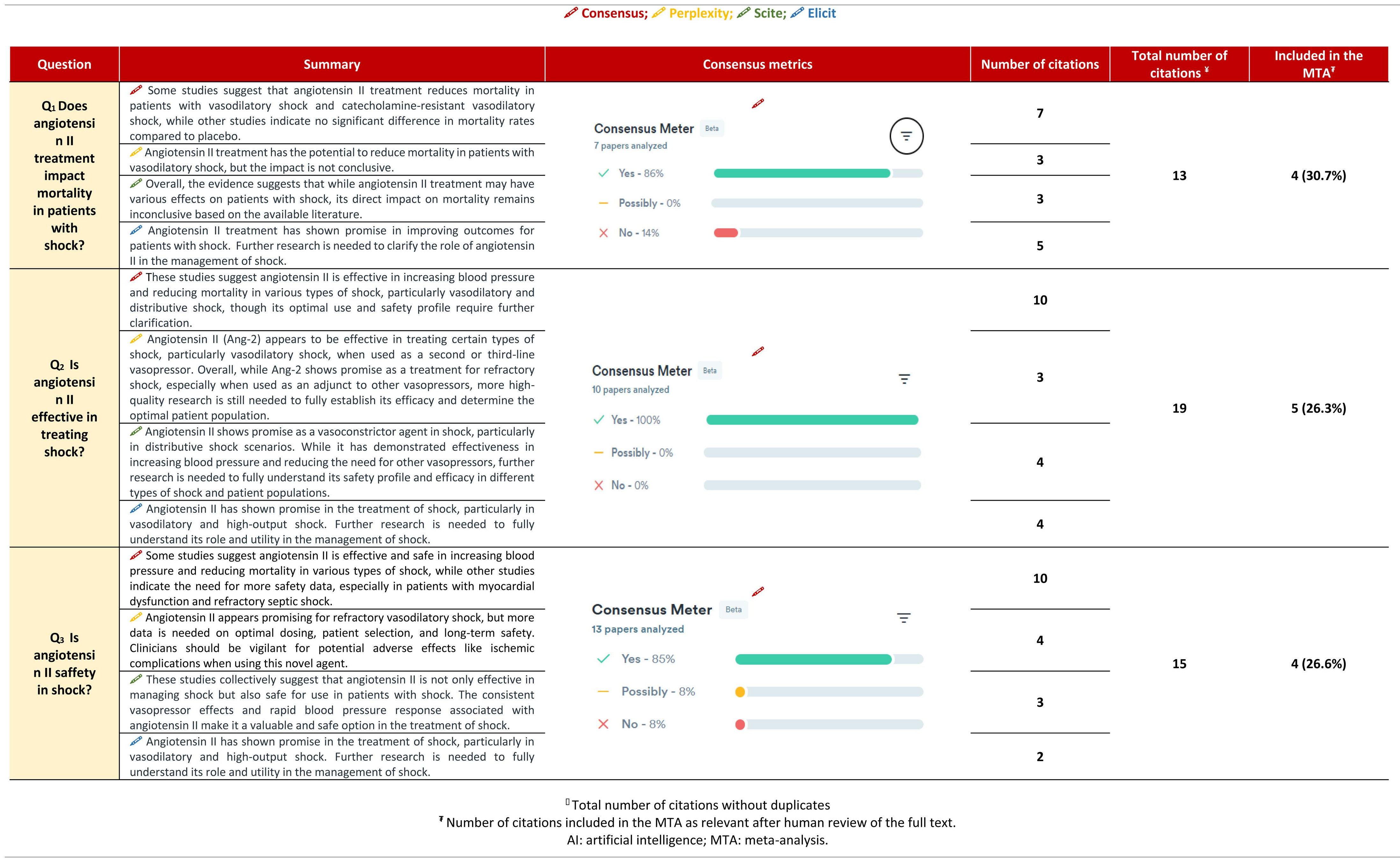
Angiotensin II (ATII) was approved for distributive shock in Spain (2023). The objective is to assess the experience with ATII by comparing a meta-analysis (MTA) and 4 Artificial Intelligence (AI) tools.
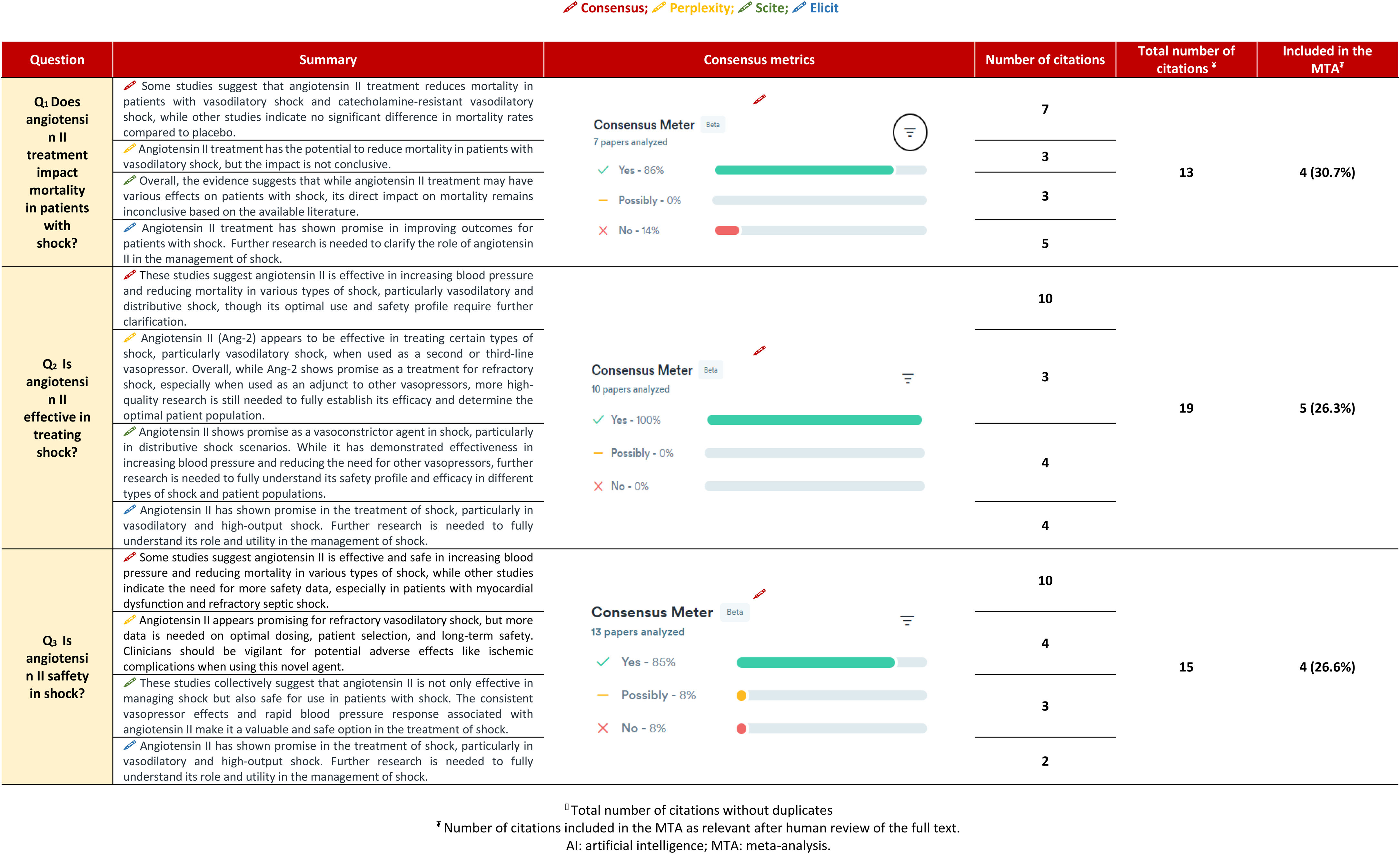
DesignA search was conducted in Pubmed®, Central®, Embase®, and Scopus®. Randomized clinical trials, non-randomized trials, and observational studies were included. The primary outcome was all-cause mortality. Odds ratios (OR) with 95% confidence intervals (CI) were pooled. Four AI tools were used: Consensus, Perplexity, Elicit, and Scite.
SettingIntensive care medicine.
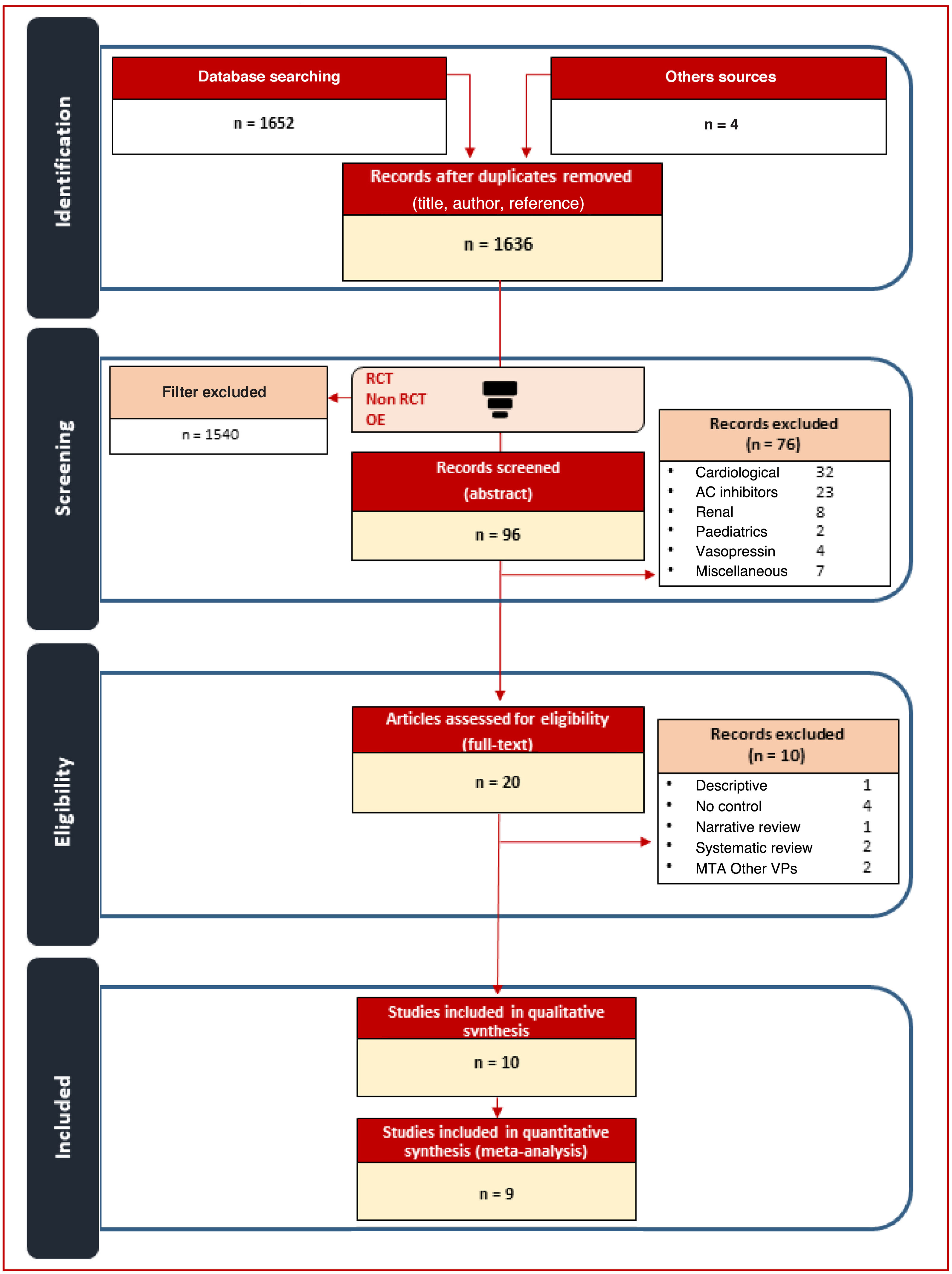
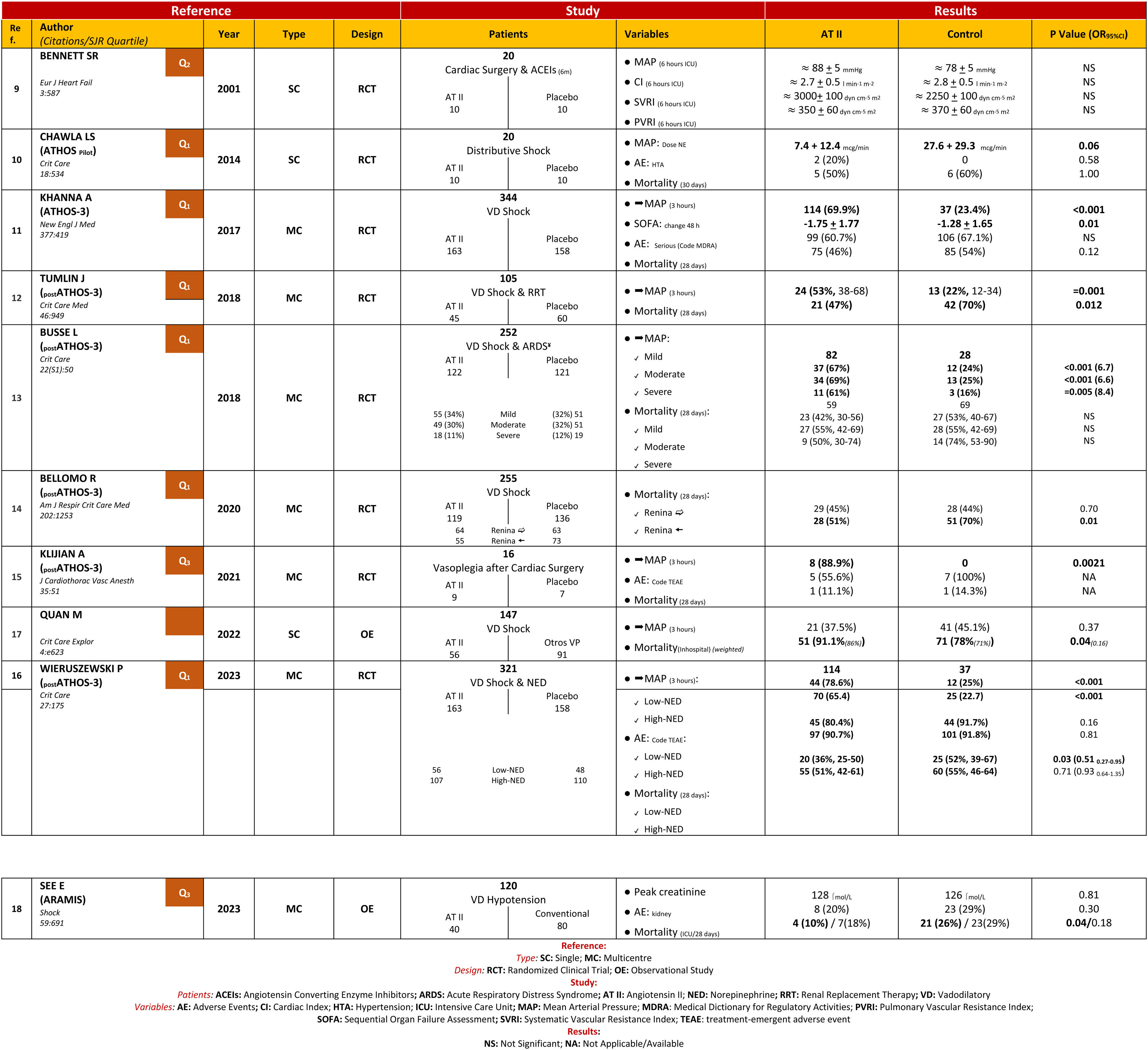
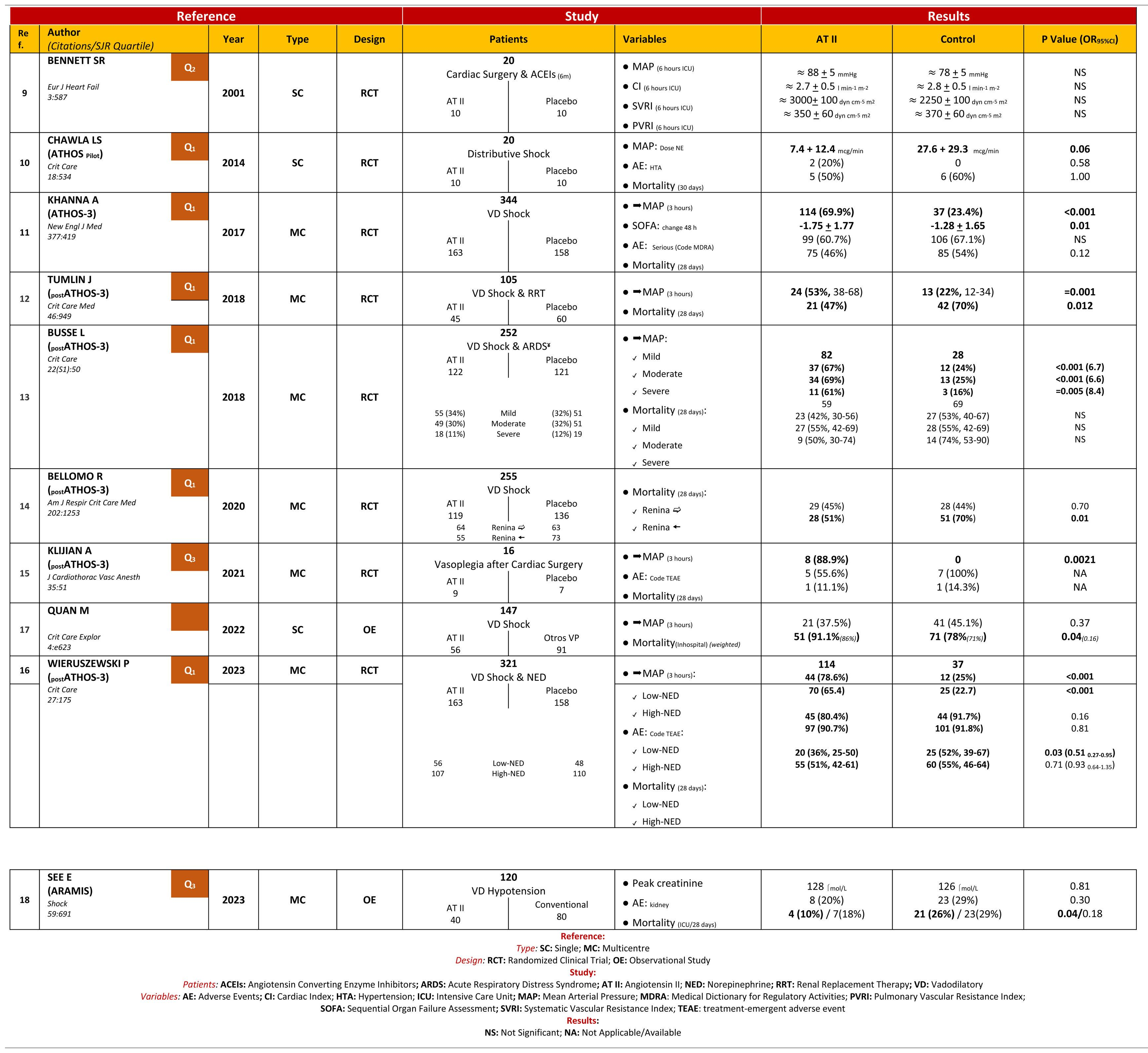
Patients or participantsOne thousand six hundred and thirty-six studies were identified, with 10 studies included in the MTA.
InterventionsNo interventions.
Main variables of interestMortality, efficacy, and safety.
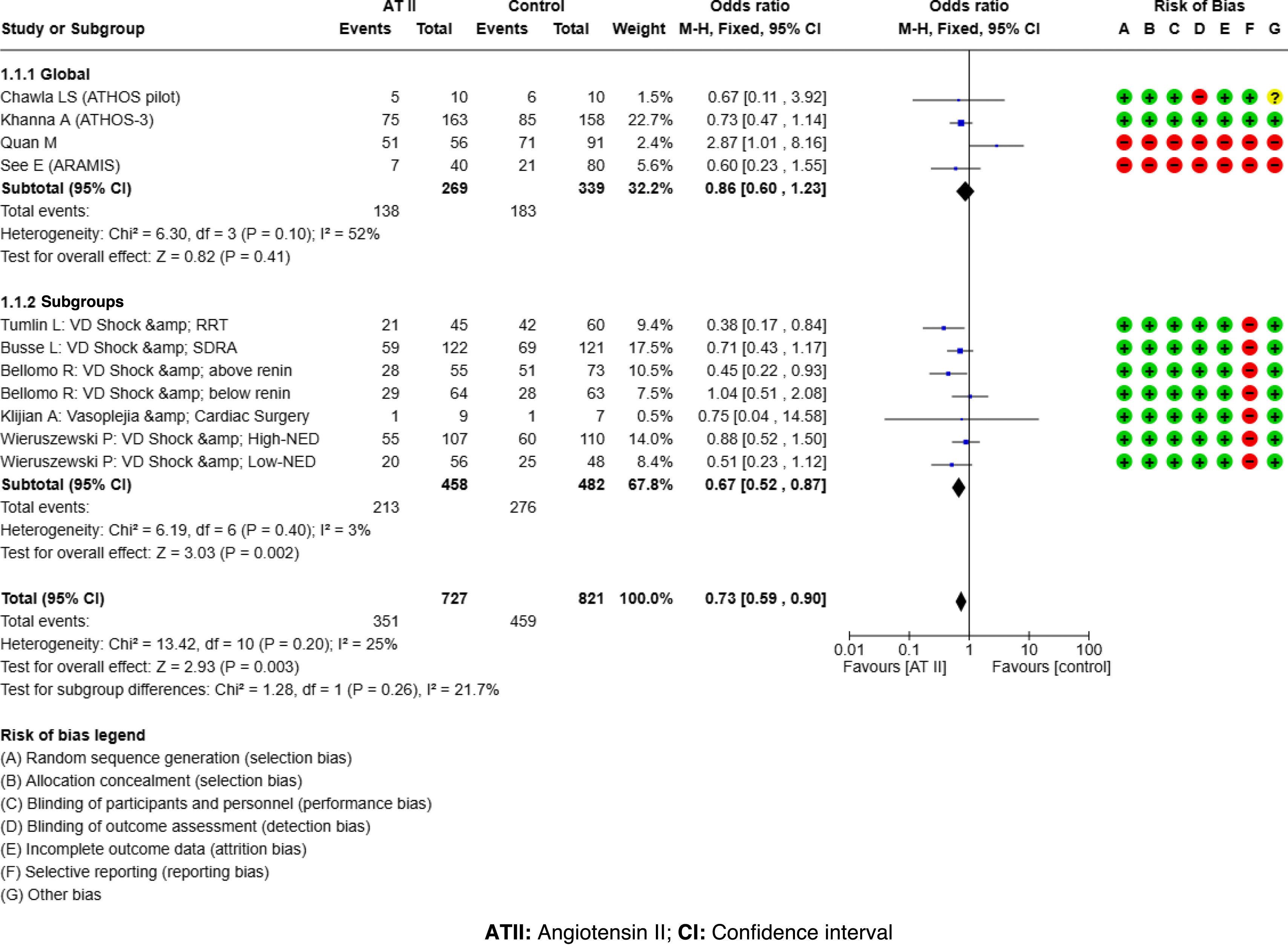
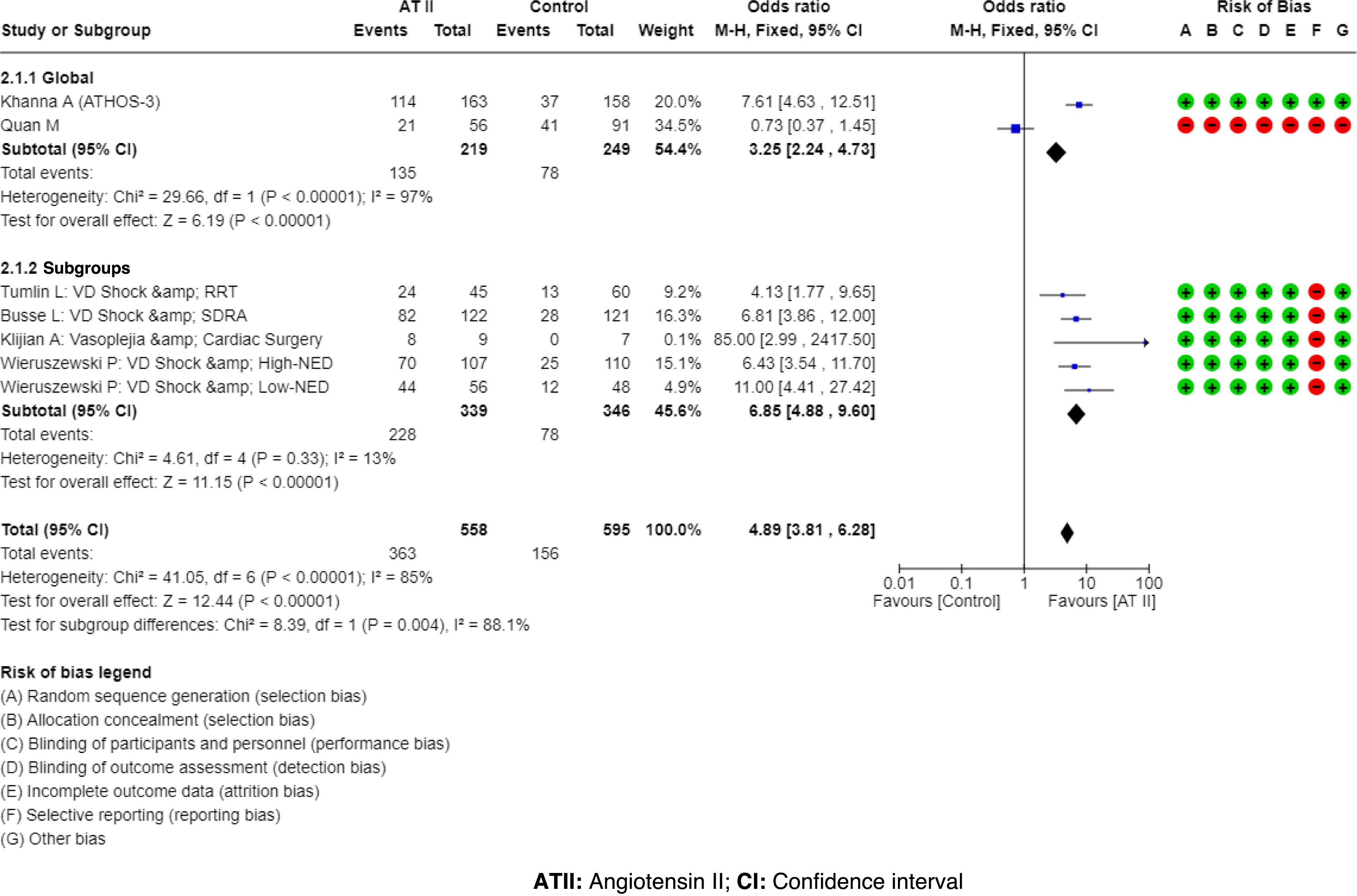
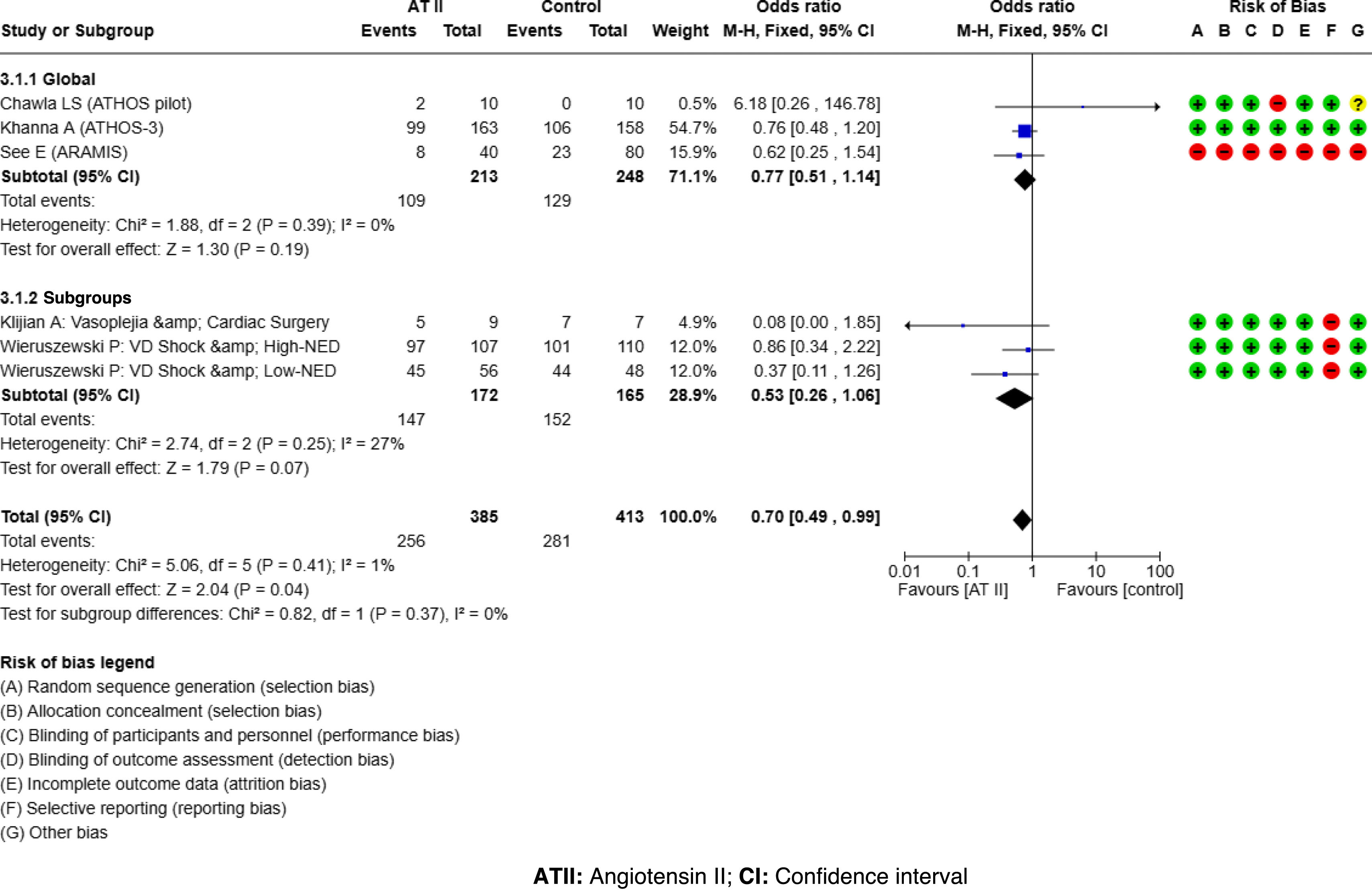
ResultsATII shows a trend towards mortality reduction when compared with controls, OR 0.86 (95% CI: 0.60–1.23); this reduction reaches significance in patient subgroups: High Renin Levels, OR 0.45 (95% CI: 0.22−0.93); shock with renal replacement therapy, OR 0.38 (95% CI: 0.17−0.84). ATII is very effective in increasing mean arterial pressure, OR 3.25 (95% CI: 2.24–4.73), without increasing events, OR 0.77 (95% CI: 0.51–1.14). The AI reached the same conclusions, but only 25%–30% of the studies were included in the MTA.
ConclusionsATII effectively increases blood pressure without side effects and without altering mortality. AI can assist in evaluating clinical evidence.
La angiotensina II (ATII) ha sido aprobada para el shock distributivo en España (2023). El objetivo es valorar la experiencia de la ATII comparando: un metanálisis (MTA) y 4 herramientas de inteligencia artificial (IA).
DiseñoSe buscó en Pubmed®, Central®, Embase® y Scopus®. Se incluyeron ensayos clínicos aleatorizados, no aleatorios y estudios observacionales. El resultado principal fue la mortalidad por cualquier causa. Se agruparon las odds ratio (OR) con intervalos de confianza (IC) del 95%. Se usaron 4 herramientas de IA: Consensus, Perplexity, Elicit y Scite.
ÁmbitoMedicina intensiva. Validación de herramientas de IA.
Pacientes o participantesMil seiscientos treinta y seis estudios, incluyendo en el MTA 10 estudios.
IntervencionesNo realizadas.
Variables de interés principalesMortalidad, eficacia y seguridad.
ResultadosLa ATII presenta una tendencia a disminuir la mortalidad respecto al control, OR 0.86 (IC del 95%: 0.60–1.23); en subgrupos de pacientes esta disminución resulta significativa: Niveles de Renina Alta, OR 0.45 (IC del 95%: 0.22−0.93); shock con reemplazo renal, OR 0.38 (IC del 95%: 0.17−0.84). La ATII es muy efectiva aumentando la presión arterial media, OR 3.25 (IC del 95%: 2.24–4.73), sin incrementar eventos, OR 0.77 (IC del 95%: 0.51–1.14). La IA llega a las mismas conclusiones, pero solo el 25%–30% de los estudios fueron incluidos en el MTA.
ConclusionesLa ATII aumenta la tensión de forma efectiva, sin efectos secundarios y sin modificar la mortalidad. La IA puede ayudar a la evidencia clínica.
Article
Go to the members area of the website of the SEMICYUC (www.semicyuc.org )and click the link to the magazine.