Infection due to SARS-CoV-2 produces pneumonia and acute respiratory distress syndrome in the context of a disease known as COVID-19.1 In only a few months, the virus spread throughout the world, infecting millions of people and causing hundreds of thousands of deaths.2 Contagion takes place through respiratory droplets (>5μm) and contact with contaminated objects (fomites).3,4 Recently, the World Health Organization (WHO) has acknowledged that airborne transmission through aerosols (<5μm) that remain in the air for hours is also a possible contagion route, due to the inhalation of viral particles that are deposited within the distal airway.5–7 Airborne transmission is of great relevance to public health and the protection of healthcare professionals, and in this regard the measures of caution need to be modified to avoid contagion–incrementing the required 2m safety distance, particularly in closed areas or spaces.
Intensive Care Units (ICUs) have periodic air renewal mechanisms, and during the pandemic, many Units were equipped with negative pressure systems. However, other places such as homes, restaurants, public transport or even hospital wards have no such safe ventilation systems. Some studies have reported that SARS-CoV-2 is able to remain in the air generated by aerosols for up to three hours,7 with demonstration of the presence of the viral genome in the air and filters of hospitals.
In Spain, the SARS-CoV-2 contagion rate among healthcare professionals is the highest in the world, followed by Italy (10%) and China (3,8%).8 According to the Spanish Ministry of Health, on 9 July 2020, the number of infected healthcare professionals totaled 52,643, representing over 22% of all cases in the country.9 Those working in the ICU are at a high risk of contagion due to the great environmental exposure to SARS-CoV-2 and the use of techniques that generate aerosols.5,10,11 Thus, one of our major concerns was to improve the safety measures and minimize the risk of contagion among the healthcare professionals. In this context, we decided to analyze the presence of SARS-CoV-2 in the air of two ICUs and in the pneumology ward dedicated to the treatment of patients with COVID-19. The study was carried out in late May 2020 in 5 different boxes of the ICU (Table 1), placing the extraction equipment on the floor, close to the head of the patient and as far as possible from the air outlet, collecting the air samples during a time of 2–4h. The entire ICU was equipped with negative air pressure (−10Pa), and the air was renewed at a rate of 15–20 cycles/h. The hospital ward had no such air renewal system. The air volume in the ICU boxes is variable, since their dimensions differ–though the average is 51m3, i.e., 51,000l, and the hospital ward rooms have a volume of 54.4m3. The air samples were obtained using two different methods: (1) SAS Bioser Mod. Microbio 0111302 sampling equipment with an air flow of 500l/300s and a Rodac plate measuring 55mm in diameter from which samples were subsequently obtained with pre-humidified swabs. With this system the estimated volume of air passing through the plate in one hour is 5,967l; and (2) A filtration ramp with a polyethersulfone membrane filter (FILTER-LAB®) of pore size 0.1μm and measuring 47mm in diameter, connected to the hospital vacuum system by means of a 60kPa vacuometer. There is a small variability in vacuum of the different ICU boxes, ranging between 55–60kPa; as a result, calculation of the air passing through the plate is only an estimate. Considering 55kPa, tubing measuring 8mm in diameter, and the different loading losses, the calculated sampled air volume is 2,160l/h. Thus, with the studied time period of 2–4h and with the estimated flow rate, we analyzed 4,320l in two hours and 8,640 in four hours–this in most cases representing 16.9% of the air in the ICU box.
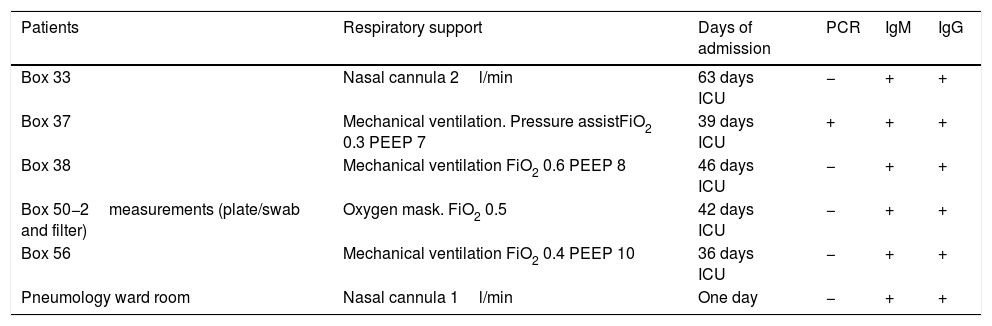
Respiratory support, days of admission, and microbiological characteristics of the patients with COVID-19 in boxes where the air was analyzed.
| Patients | Respiratory support | Days of admission | PCR | IgM | IgG |
|---|---|---|---|---|---|
| Box 33 | Nasal cannula 2l/min | 63 days ICU | − | + | + |
| Box 37 | Mechanical ventilation. Pressure assistFiO2 0.3 PEEP 7 | 39 days ICU | + | + | + |
| Box 38 | Mechanical ventilation FiO2 0.6 PEEP 8 | 46 days ICU | − | + | + |
| Box 50−2measurements (plate/swab and filter) | Oxygen mask. FiO2 0.5 | 42 days ICU | − | + | + |
| Box 56 | Mechanical ventilation FiO2 0.4 PEEP 10 | 36 days ICU | − | + | + |
| Pneumology ward room | Nasal cannula 1l/min | One day | − | + | + |
FiO2: fraction of inspired oxygen; PCR: polymerase chain reaction; PEEP: positive end-expiratory pressure; ICU: Intensive Care Unit.
The presence of SARS-CoV-2 was analyzed by detecting the viral genome using multiple quantitative RT-PCR. The nucleic acids were purified by the MagNa Pure 96 System (Roche, Geneva, Switzerland) from the swab transport medium or the eluate obtained after incubating the filters for half an hour at 37°C. The extracts were subjected to an amplification reaction supplemented with a mixture of TaqMan® MGB primers and probes with two targets: the ORF1ab and N genes. The amplifications and their posterior analysis were performed using the Applied Biosystems® 7500 Real-time PCR System (ABI, Foster City, CA, USA).
A total of 7 air samples were obtained (Table 2). In no case did quantitative RT-PCR testing detect the SARS-CoV-2 genome in the samples obtained with the two described methods. In the present study we therefore could not demonstrate the presence of SARS-CoV-2 in the air of either the ICU or the hospital ward. These findings are consistent with those of authors such as Cheng et al.,12 who did not detect coronavirus in 8 samples of air collected at 10cm from the chin of a patient with COVID-19. However, other studies have detected viral RNA in between 35–68% of the air samples analyzed.13–15 In the study published by Guo et al.,14 the aerosols reached a distance of 4m and proved positive in 40.6% of the air samples of the box in which the patient was located, and in 12.5% of the samples in the working areas. The ICU described by Guo et al. was equipped with air renewal at a rate of 16 cycles per hour, though no mention was made of whether the Unit was under negative pressure or not. Santarpia et al.15, in an isolation unit for patients in quarantine and in a hospitalization area of the University Medical Center of Nebraska, measured the presence of SARS-CoV-2 in the air of the room, and recorded a 63.2% positivity rate with a mean concentration of 2.86 copies/l of air. The air samples taken from the corridors in turn yielded a positivity rate of 66.7% with a mean concentration of 2.59 copies/l of air. Posteriorly, and in order to calculate infective capacity, Vero E6 cells were cultured with these samples, though the tests indicated no viral replication. The finding of SARS-CoV-2 RNA does not report on the presence of viable viruses and thus of their infective capacity, though contamination of the air would indicate that aerosol transmission of SARS-CoV-2 is plausible. The mentioned study did not specify air renewal (cycles per hour) or whether there was negative pressure in the rooms. In our ICU, all the boxes were equipped with negative air pressure (−10Pa), and the air was renewed at a rate of 15–20 cycles/h, which could explain the absence of SARS-CoV-2 RNA in our study.
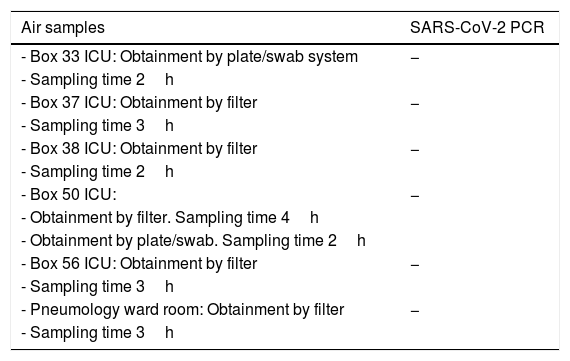
Air samples collected, obtainment method and SARS-CoV-2 PCR test results.
| Air samples | SARS-CoV-2 PCR |
|---|---|
| - Box 33 ICU: Obtainment by plate/swab system | − |
| - Sampling time 2h | |
| - Box 37 ICU: Obtainment by filter | − |
| - Sampling time 3h | |
| - Box 38 ICU: Obtainment by filter | − |
| - Sampling time 2h | |
| - Box 50 ICU: | − |
| - Obtainment by filter. Sampling time 4h | |
| - Obtainment by plate/swab. Sampling time 2h | |
| - Box 56 ICU: Obtainment by filter | − |
| - Sampling time 3h | |
| - Pneumology ward room: Obtainment by filter | − |
| - Sampling time 3h |
PCR: polymerase chain reaction; SARS-CoV-2: severe acute respiratory syndrome coronavirus-2; ICU: Intensive Care Unit.
Following lockdown, a total of 237 ICU workers–including the cleaning staff–underwent PCR testing for SARS-CoV-2 and serological testing with the ELISA technique. All these tests proved negative.
With regard to the limitations of our study, mention must be made of the sample number, the fact that the screened volume of air represented only a part of the total volume, and the fact that air renewal and negative pressure in the ICU facilitate the elimination of SARS-CoV-2. Nevertheless, it is interesting to note that to our knowledge, this is the first study on the presence of coronavirus in the air of an ICU in Spain, and that few studies of this kind can be found in the literature.
The safety of healthcare professionals must be viewed as a priority concern in order to avoid collapse of the health systems and impede disease transmission from the hospitals to the rest of the community. In this regard it is particularly important to control the presence of SARS-CoV-2 on both the exposed environmental surfaces of the ICU and in the air, with a view to minimizing contagion risk, and to adopt strict safety measures during all maneuvers and treatments capable of generating aerosols in the ICU.
AuthorshipDolores Escudero designed the study and wrote the manuscript.
José Antonio Boga, Gabriel Martín and Juan A. Barrera participated in preparation and correction of the manuscript.
Salvador Balboa and Silvia Viñas performed collection of the samples and reviewed the manuscript.
Financial supportThis study has received no financial support.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We wish to express our support of the patients who have suffered COVID-19 and our appreciation of their families for their courage in facing the disease under extreme conditions.
Thanks are due to Dr. Sergio Pérez-Holanda and to Mr. Josu Jiménez-Idoeta of Hospital Universitario Central de Asturias, for their permanent support in all the ICU projects.
Please cite this article as: Escudero D, Barrera JA, Balboa S, Viñas S, Martín G, Boga JA. Análisis de SARS-CoV-2 en el aire de una UCI dedicada a pacientes covid-19. Med Intensiva. 2021;45:247–250.