Abnormal endotoxin activity in critically ill patients has been described in the absence of Gram-negative bacterial (GNB) infection. As disease severity seems to be crucial in the detection of this phenomenon, we decided to assess and compare endotoxin exposure in those patients representing the critical situation: septic shock and cardiogenic shock.
DesignProspective, observational non intervention study.
SettingCritical Care Department of a University tertiary hospital.
PatientsCardiogenic shock (CS) and septic shock (SS) patients.
InterventionsNone.
Measurements and main resultsFollow-up was performed for the first three days. Inflammatory biomarkers (C-reactive protein, procalcitonin and interleuquin-6) and IgM antiendotoxin-core antibodies titter (IgM EndoCAb) were daily analyzed. Sixty-two patients were included; twenty-five patients with SS and thirty-seven with CS. Microbial etiology was established in 23 SS patients (92%) and GNB were present in 13 cases (52%). Although infection was suspected and even treated in 30 CS patients (81%), any episode could be finally confirmed.
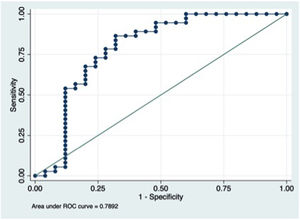
EndoCAb consumption was more intense in SS patients, although twenty-two CS patients (59.5%) had IgM anti-endotoxin value below 10th percentile range for healthy people. No statistically significant difference in endotoxin exposure was detected between Gram-positive and Gram-negative infections in the SS group. Endotoxin exposure ability to distinguish between SS and CS was moderate (AUC 0.7892, 95% IC: 0.6564–0.9218).
ConclusionsIn the severely ill patient some mechanisms take place allowing endotoxin incursion and therefore blurring the limits of diseases pathophysiology. Our work representatively shows how exposure to endotoxin was not fully capable of distinguishing between CS and SS.
En el paciente crítico se ha descrito una actividad incrementada de la endotoxina no asociada a infección por bacterias gramnegativas (BGN). La gravedad de la enfermedad influye en este fenómeno, por ello realizamos este estudio en el paciente crítico por antonomasia: shock séptico y cardiogénico.
DiseñoEstudio prospectivo, observacional, sin intervención.
Lugar de estudioUnidad de Cuidados Intensivos.
PacientesPacientes en shock cardiogénico (SC) o séptico (SS).
IntervenciónNinguna.
Determinaciones y principales resultadosSeguimiento durante los 3 primeros días. Proteína C reactiva, procalcitonina e interleucina-6, y el título de anticuerpos IgM anti-edotoxina (IgM EndoCAb) se analizaron diariamente. Se incluyó a 62 pacientes; 25 con SS y 37 con SC. La etiología fue identificada en 23 pacientes con SS (92%), los BGN estuvieron presentes en 13 casos (52%). Se sospechó e incluso trató la infección en 30 pacientes con SC, pero en ningún caso se pudo confirmar. El consumo de EndoCAb fue más intenso en los pacientes con SS, pero 22 pacientes con SC (59,5%) tuvieron unos valores por debajo del percentil 10. Los niveles de EndoCAb no fueron significativamente diferentes entre las infecciones por BGN y cocos grampositivos. La capacidad de EndoCab para diferenciar entre SC y SS resultó ser moderada (AUC 0,7892; IC del 95%, 0,6564-0,9218).
ConclusionesEn el paciente crítico es frecuente que la endotoxina provoque una respuesta inflamatoria y la sumación de distintos mecanismos fisiopatológicos. En este sentido, nuestro trabajo pone de manifiesto que la determinación de exposición a endotoxina no es totalmente capaz de distinguir entre los pacientes con SC y SS.
Cardiovascular failure in critically ill patients is mainly due to heart breakdown (cardiogenic shock, CS) or to bacterial sepsis (septic shock, SS). However, the limits between these two etiological and physiopathological entities are often blurred. Myocardial dysfunction caused by the inflammatory response to sepsis is a well-recognized complication and increases notably the difficulty in the management of these patients.1 On the other hand, CS patients often develop inflammatory signs and symptoms that make clinicians hesitate about the possibility of an infectious complication. In fact; fever, leukocytosis, a decrease in systemic vascular resistances and even an increase in the serum levels of infectious biomarkers are common findings in CS patients.2 Bacterial translocation and mainly endotoxinemia (bacterial lypopolisacharid (LPS) presence in blood) has been pointed out by some authors as the possible explanation for this situation.3
LPS is responsible for triggering inflammatory response in Gram-negative bacterial infections.4 However, abnormal endotoxin activity has been detected in critically ill patients in absence of bacterial isolation.5 An increase in intestinal permeability due to mucosal damage has been identified as the source of endotoxin in chemotherapy mucositis or in severe pancreatitis.6 Even more, intestinal hypoperfusion and bacterial translocation was demonstrated in chronic cardiac failure patients and was associated with a worse prognosis.7 In CS patients intestinal hypoperfusion can be aggravated by the use of vasoconstrictive drugs promoting a mucosal disruption and therefore allowing the entry of bacteria or their products.8 In fact, our group recently demonstrated endotoxin exposure in CS patients by means of an excessive antiendotoxin antibodies consumption.9
Inflammatory response has been previously compared in CS and SS in one study and the detection of elevated procalcitonin (PCT) serum levels in CS patients were theoretically attributed to bacterial translocation.3 In this study we decided to assess inflammatory response and endotoxin exposure (by means of anti-endotoxin antibodies titration) in both CS and SS patients. Our goals were to characterize and compare inflammatory response in both situations and to analyze the relationship with endotoxin exposure and patients prognosis.
MethodsStudy design and inclusion criteriaProspective observational study performed in a 24-bed medical ICU of a 1200-bed university hospital. We included and followed up all consecutive patients with cardiogenic shock and septic shock from December 2018 to June 2019. During the first three days after shock onset serum samples were collected to analyze inflammatory biomarkers (C-reactive protein (CRP), procalcitonin (PCT) and interleuquin-6 (IL-6)) and IgM antiendotoxin-core antibodies (IgM EndoCAb). The Institutional Review Board approved the study and informed consent was obtained from the patients’ relatives.
Data collection protocolThe following data were collected on study inclusion: sex, co-morbidities, severity scores prior to intubation (including Acute Physiology and Chronic Health Evaluation Score-II (APACHE-II),10 Sequential Organ Failure Assessment Score (SOFA)11 and the reason for ICU admission. Clinical, analytical, hemodynamic data and therapeutic measures along ICU stay were recorded. Nosocomial infections were pursuit by means of daily evaluation of signs and symptoms and microbiological assessment in case of clinical suspect.
DefinitionsCardiogenic shock: Patients had to fulfill all this criteria: (a) Persistent hypotension (systolic blood pressure<80–90mm Hg or mean arterial pressure 30mm Hg lower than baseline); (b) Severe reduction in cardiac index (<1.8Lmin−1m−2 without support or <2.0–2.2Lmin−1m−2 with support) or severe left ventricular systolic dysfunction detected by echocardiography and adequate or elevated filling pressure assessed by pulmonary artery catheterization or by Doppler echocardiography and (c) Tissue hypoperfusion manifested by cool extremities, decreased urine output, and/or alteration in mental status.12
Septic shock: SS is defined as a subset of sepsis in which underlying circulatory and cellular metabolism abnormalities are profound enough to substantially increase mortality. Three variables must be identified: hypotension defined as mean arterial pressure less than 65mmHg, serum lactate level more than 2mmol/l and a sustained need for vasopressor therapy.13
Diagnosis of infection: Infection diagnosis was established according to the CDC criteria. In summary infection diagnosis requires the presence of some clinical criteria followed by a microbiological confirmation.14 For ventilator-associated pneumonia (VAP) two or more of the following should be present: temperature greater than 38°C, leukocytosis greater than 12,000/mm3 or leucopenia lower than 4000/mm3, or purulent respiratory secretions; plus a new or progressive pulmonary infiltrate on chest X-ray. VAP confirmation was defined by the quantitative culture of tracheobronquial aspirate≥105cfu/ml2, bronchoalveolar lavage with ≥104cfu/ml or mini bronchoalveolar lavage≥103cfu/ml.15
Study of inflammatory markersBlood samples were centrifuged (1500rpm, 10min) and serum was frozen at −80°C. PCT was measured by TRACE (Time-Resolved Amplified Crypate Emission) technology in a Kyptor analyzer (Brahms Diagnostica, Berlin, Germany). Measurement of CRP was performed with an immunoturbidimetric method using a commercial kit (Tina-quant CRP, Roche Diagnostics, Mannheim, Germany). IL6 was measured by electrochemiluminescence (ECL) in the Cobas 6000 (E-170), Roche Diagnostics.
Study of antiendotoxin antibodiesCommercially available enzyme-linked immunosorbent assays were used according to the manufacturers’ protocols for measuring EndoCAb IgM (Hycult Biotech, The Netherlands). The EndoCAb ELISA was originally devised to screen blood donor plasma for high-titer antibodies to endotoxin core, which are cross-reactive with endotoxins of a number of Gram-negative bacterial species, and was then applied to a variety of clinical studies. The EndoCAb® standard median-units IgM (MU) are arbitrary and are based on medians of ranges for 1000 healthy adults; 10–90th percentile range have been established at 35–250MU/ml and mean normal value at 100MU/ml.5 According to manufactures’ instructions the kit has a detection level of 0.05MU/ml.
Statistical analysisQuantitative variables were presented as median with p25 and p75 quartiles. Continuous variables were compared using the Student t test for normally distributed variables and the Mann–Whitney U test for non-normally distributed variables. Categorical variables were compared using the Chi-square and the Fisher's exact tests when appropriate. The threshold for statistical tests significance was set at 5%. We use ROC curve to determinate the discriminative capacity of antibodies. The collected data were entered and analyzed in SPSS 15.0 software (SPSS, Chicago, IL).
ResultsDescription of the populationSixty-two consecutive patients were included in the study; twenty-five patients with SS and thirty-seven with CS. Most of the patients were male (n 43; 83%) and mean age was 59.6 [52.5–68] years. APACHE II score was 18 [11–24]. Demographic, clinical, hemodynamic and therapeutic maneuvers from both groups are depicted in Table 1.
Demographic and clinical characteristics of SC and SS patients at admission.
| Global(n 62) | Septic shock(n 25) | Cardiogenic shock(n 37) | p | |
|---|---|---|---|---|
| Male n (%) | 36 (69%) | 16 (64%) | 27 (73%) | 0.45 |
| Age | 59.6 [52.5–68] | 65 [54–74.5] | 58 [50–64] | 0.06 |
| APACHE II | 18 [11–24] | 24[20–28] | 13 [8–17] | 0.01 |
| Smoking n (%) | 18 (35.5%) | 5 (20%) | 17 (45.9%) | 0.03 |
| Alcohol n (%) | 5 (9.7%) | 4 (16%) | 20 (5.4%) | 0.18 |
| Diabetes mellitus n (%) | 15 (29%) | 7 (28%) | 11 (29.7%) | 0.83 |
| Hypertension n (%) | 20 (38.7%) | 11 (44%) | 13 (35.1%) | 0.53 |
| COPDan (%) | 11 (21%) | 9 (36%) | 4 (10.8%) | 0.02 |
| CHFbn (%) | 8 (21.6%) | 0 | 8 (21.6%) | 0.47 |
| Lactate (mmol/l) | 3.8 [2.3–6.2] | 4.6 [2.4–8.3] | 3.5 [2.1–6.2] | 0.22 |
| Creatinine (mg/dl) | 1.5 [1–2.5] | 2 [1.48–2.8] | 1.25 [1–2.1] | 0.01 |
| Urea (mg/dl) | 76.5 [52–111] | 106 [58–147] | 70 [46–91] | 0.09 |
| AST (UI/ml) | 146 [51–754] | 117 [42–333] | 367 [70–937] | 0.08 |
| ALT (UI/ml) | 96 [43–236] | 67 [35–166] | 107 [50–278] | 0.36 |
| Bilirrubine (mg/dl) | 1.1 [0.7–2.3] | 1.1 [0.6–2.5] | 1.1 [0.7–1.95] | 0.84 |
Results are expressed as median with p 25 and p 75.
AMI: Acute myocardial infarction; COPD: Chronic obstructive pulmonary disease; CHF: Chronic heart failure; CRRT: Continuous renal replacement therapy; ECMO: Extracorporeal membrane oxygenation; IABP: Intra aortic balloon pump; NYHA: New York heart Association.
In SS population, the most frequent infectious foci were urinary (n 8; 32%), abdominal (n 8; 32%), respiratory (n 6; 24%), soft tissue infection (n 2; 8%) and bacteremia (n 1; 4%). Microbial etiology was established in 23 patients (92%). Gram-negative bacteria (GNB) in 13 cases (52%); Escherichia coli (n 6; 46%), Pseudomonas aeruginosa (n 5; 56.5%) and Klebsiella pneumoniae (n 2; 15%). Gram-positive bacteria (GPB) in 10 cases (43.4%); Staphylococcus aureus (n 3; 30%), Enterococcus faecium (n 3; 30%), Streptococcus pneumonia (n 2; 20%), Staphylococcus epidermidis (n 1; 10%) and Streptococcus pyogenes (n 1; 10%). Any SS patient developed sepsis-associated myocardial dysfunction.
CS etiology was acute myocardial infarction in 28 cases (75.7%), followed by decompensated chronic heart failure in 8 patients (21.65%) and heart transplant rejection in one patient (2.65%). There was a clinical suspicion of infection and therefore antibiotic treatment onset and performance of microbiological study (blood cultures, respiratory sample culture or/and urine culture) in 30 CS patients (81%). Twenty-two patients (60%) had body temperature>38.3°C or <35°C; and twenty-three patients (62%) had a leukocyte count>14,000/mm3 or <4000/mm3. No infection was finally diagnosed.
Supportive interventions are depicted in Table 2.
Clinical, supportive and outcome parameters in patient follow-up.
| Global(n 62) | Septic shock(n 25) | Cardiogenic shock(n 37) | p | |
|---|---|---|---|---|
| Axilar temperature (°C) | ||||
| max | 37.8 [37.1–38.2] | 37.7[37–38.7] | 38 [37.2–38.1] | 0.84 |
| min | 36 [35–36.4] | 35.5[35–36] | 36 [35.6–36.5] | 0.02 |
| Leukocyte count | ||||
| max | 17,800 [12,600–25,225] | 25,300 [16,400–32,750] | 14,800 [10,100–18,400] | 0.01 |
| min | 8400 [5225–10925] | 6400 [3750–9850] | 9300 [5700–11200] | 0.03 |
| NIMV n (%) | 17 (32.3%) | 3(12%) | 17 (45.9%) | 0.01 |
| IMV n (%) | 36 (58%) | 7 (28%) | 29 (78%) | 0.768 |
| NE n (%) | 43 (82.3%) | 24 (96%) | 27 (73%) | 0.02 |
| Max dose of NE (mcg/kg/min) | 1.1 [0.56–1.8] | 1.1 [0.51–1.8] | 1.09 [0.7–1.8] | 0.93 |
| CRRT n (%) | 9 (14.5%) | 5 (20%) | 4 (11%) | 0.8 |
| ICU mortality n (%) | 20 (38.4%) | 6 (24%) | 14 (38%) | 0.3 |
| Levosimendan n (%) | 15 (40%) | 0 | 15 (40%) | 0.434 |
| Dobutamine n (%) | 33 (89%) | 0 | 33 (89%) | 0.563 |
| ECMO n (%) | 15 (40%) | 0 | 15 (40%) | 0.673 |
| IABP n (%) | 17 (46%) | 0 | 17 (46%) | 0.635 |
| Heart transplantation n (%) | 11 (20%) | 0 | 11(20%) | 0.289 |
Results are expressed as median with p 25 and p 75.
CRRT: Continuous renal replacement therapy; ECMO: Extracorporeal membrane oxygenation; IABP: Intra aortic balloon pump; IMV: Invasive mechanical ventilation; NE: Norepinephrine; NIMV: Non-invasive mechanical ventilation.
All patients had serum PCT levels above normal values (0.5ng/ml). PCT was clearly higher in SS patients meanwhile CRP was indistinguishable between the two groups. In CS 51.4% of the cases (n=19) had at least one PCT value above 2ng/ml, and 10 patients (27%) had at least one PCT value above 5ng/ml. IL-6 was higher but not constantly in SS patients (Table 3).
Antiendotoxin antibodies and inflammatory biomarkers in septic and cardiogenic shock patients.
| Global | Septic shock | Cardiogenic shock | p | |
|---|---|---|---|---|
| (n 62) | (n 25) | (n 37) | ||
| EndoCAb (MU/ml) day 1 | 15.6 [8.5–35.8] | 7.8 [3.2–15.2] | 23.47 [13–46] | <0.01 |
| EndoCAb (MU/ml) day 2 | 17.5 [5.5–30.2] | 8.5 [3–17.6] | 26.1 [16–42.3] | <0.01 |
| EndoCAb (MU/ml) day 3 | 16.04 [4.5–39.4] | 6.8 [2.2–16.9] | 27.6 [13–39.6] | <0.01 |
| PCT (ng/ml) day 1 | 4.8 [0.7–22.5] | 24 [8.76–53.8] | 1.2 [0.35–5.7] | <0.01 |
| PCT (ng/ml) day 2 | 4.1 [0.7–23.4] | 21.04 [11.9–58.4] | 1.87 [0.3–4.6] | <0.01 |
| PCT (ng/ml) day 3 | 3.8 [1–10.7] | 6.1 [4.2–15] | 1.78 [0.7–3.] | 0.03 |
| PCR (mg/l) day 1 | 133 [41.5–257.4] | 208 [46–315.4] | 121.3 [28–238] | 0.11 |
| PCR (mg/l) day 2 | 191 [87.8–274.6] | 190.3 [87.6–397.7] | 201.5 [88–250] | 0.45 |
| PCR (mg/l) day3 | 147 [85.3–272.4] | 117 [61.5–257.4] | 149 [111–297] | 0.12 |
| IL-6 (pg/ml) day 1 | 279 [79–1703] | 1169 [201–4229] | 150 [69–488.5] | 0.01 |
| IL-6 (pg/ml) day 2 | 182 [76–337] | 127.4 [41–261] | 224 [84.4–408] | 0.33 |
| IL-6 (pg/ml) day 3 | 114 [38.4–229.3] | 53.08 [23.5–161] | 150 [72–297.4] | <0.01 |
Results are expressed as median with p 25 and p 75.
All patients had antiendotoxin antibodies bellow the normal median value (100MU/ml). EndoCAb consumption was more intense in SS patients (Table 3), although twenty-two CS patients (59.5%) had IgM anti-endotoxin value bellow 10th percentile range for healthy people (Table 3). SS patients in whom a GNB was isolated presented less EndoCAb (Fig. 1) in the three days in which it was determined when compared with SS due to GPB (6.9MU/ml vs 10.9MU/ml, 8.5MU/ml vs 8.7MU/ml, 6.6MU/ml vs 8.1MU/ml), without reaching statistical significance (p=0.13, p=0.16 and p=0.23, respectively).
EndoCAb capability to distinguish between SS and CS patients was evaluated by means of a ROC curve. First day measurement showed an AUC 0.78, 95% IC: 0.65–0.92 (Fig. 1); the optimal cut-off point was 79.07MU/ml (sensitivity 52% (44–63%), specificity 94.6% (82–98%)).
Patient outcomesMortality rate was 32.2% (n=20); six SS patients (24%) and fourteen CS patients (38%) died at 30-days follow-up (Table 2). Inflammatory biomarkers and EndoCAb levels showed no differences between deceased and survivors. Deceased CS patients had higher PCT and IL6 levels than those CS patients who survived (p<0.05). Although EndoCAb levels were lower in deceased SS patients they didn’t reach statistical significance along the three days follow-up.
DiscussionOur study has been able to demonstrate a universal endotoxin exposure in cardiovascular failure patients independently of its initial etiology. Although IgM EndoCAb consumption was more intense in SS patients, this phenomenon was equal in Gram-negative or Gram-positive infection. Moreover EndoCAb levels were not fully capable of distinguishing between SS and CS patients.
Endotoxin, also known as LPS, is the main membrane component of GNB. It plays as a potent activator of the host innate immune system triggering the biochemical cascade of acute systemic inflammation and causing the sepsis and septic shock syndrome.3,4 However, the clinical significance of endotoxin detection in blood as both a diagnostic and a prognostic test has remained unclear, despite over 100 studies published in the last 4 decades. Presumably those studies based in the Lymulus amebocyte lysis assay (developed for safety controls in the food industry) could have some results compromised by the endotoxin binding to its natural antibodies in the human model.16 But again, more precise methods to identify endotoxin exposure, such as endotoxin activity assay (EA) or the quantification of endotoxin antibodies, have not been consistent with the microbiological diagnosis of Gram-negative bacterial sepsis.
LPS is not a membrane component for Gram-positive bacteria. However, 70% (n 33) of Gram-positive bacterial sepsis included in the MEDIC study presented with a high or intermediate endotoxin activity.17 More recently Sekino et al. found high EA levels in one third of a cohort of critically ill patients with septic shock due to Gram-positive bacteria.18 In our study, endotoxin antibodies consumption was present in both groups of bacteria, and although endotoxin exposure was higher along the three days follow-up for Gram-negative septic shock, this was statistically non-significant. Bacterial translocation due to enterocyte ischemia in shock patients has been pointed out as the responsible pathogenic mechanism for these findings. Although this is the most reliable explanation, Sekino et al. failed to demonstrate a positive correlation between EA and plasma intestinal acid binding protein (I-FABP; a well-known marker of enterocyte injury).18 Nevertheless we should pointed out that some authors have detected a lag between these two variables, with the maximum I-FABP peak on ICU admission whereas the level of circulating endotoxin was maximal at day three.19
Endotoxin exposure has also been demonstrated in cardiovascular collapse without an infectious etiology.9 Intestinal mucosa disruption due to ischemia is a known condition in chronic heart failure patients and it has been linked to malabsorption and malnutrition, an inflammatory status and also to a worse prognosis.8 Logically it seems right to consider the same intestinal suffering in acute ischemia due to cardiac failure, or GNB septic shock, allowing the intestinal translocation of bacteria or their components, i.e. LPS. This gut involvement is the key piece for the blurring of the limits between entities such as SS and CS or GNB or GPB infection.
Our study has several limitations. Despite our sample size overcomes the number of patients studied in most researches concerning inflammatory biomarkers in shock, a higher number of patients could have allowed us to achieve stronger conclusions. Second, although our hypothetical explanation about LPS exposition in the absence of GNB infection is the existence of intestinal translocation, we did not study any marker of intestinal mucosa damage or dysfunction. Finally we only follow-up our patients for three days and probably the blurring between our studied groups will increase in the following days.
According to our results, and also in line with the MEDIC study findings, LPS pathophysiologic participation in the critically ill patient seems to be beyond GNB infection. Given the expected intestinal mucosa ischemic injury in cardiovascular failure and the high burden of bacteria present in the human intestinal tract, LPS exposition and therefore IgM EndoCAb consumption in these patients seems perfectly reasonable. Understanding the complex pathophysiology of the patient in circulatory collapse and the potential involvement of endotoxin is relevant to optimize the management of these patients, and potentially to apply future therapeutic measures that intervene on the origin or action of endotoxin.
Author's contributionAll authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
FundingAuthors declare no funding concerning this manuscript.
Conflict of interestNone.