Evaluation of glucometrics in the first week of ICU stay and its association with outcomes.
DesignProspective observational study.
SettingMixed ICU of teaching hospital.
PatientsAdults initiated on insulin infusion for 2 consecutive blood glucose (BG) readings ≥180mg/dL.
Main variables of interestGlucometrics calculated from the BG of first week of admission: hyperglycemia (BG>180mg/dL) and hypoglycemia (BG<70mg/dL) episodes; median, standard deviation (SD) and coefficient of variation (CV) of BG, glycemic lability index (GLI), time in target BG range (TIR). Factors influencing glucometrics and the association of glucometrics to patient outcomes analyzed.
ResultsA total of 5762 BG measurements in 100 patients of median age 55 years included. Glucometrics: hyperglycemia: 2253 (39%), hypoglycemia: 28 (0.48%), median BG: 169mg/dL (162–178.75), SD 31mg/dL (26–38.75), CV 18.6% (17.1–22.5), GLI: 718.5 [(mg/dL)2/h]/week (540.5–1131.5) and TIR 57% (50–67).
Diabetes and higher APACHE II score were associated with higher SD and CV, and lower TIR. On multivariate regression, diabetes (p=0.009) and APACHE II score (p=0.016) were independently associated with higher SD.
Higher SD and CV were associated with less vasopressor-free days; lower TIR with more blood-stream infections (BSI). Patients with higher SD, CV and GLI had a higher 28-day mortality. On multivariate analysis, GLI alone was associated with a higher mortality (OR 2.99, p=0.04).
ConclusionsGlycemic lability in the first week in ICU patients receiving insulin infusion is associated with higher mortality. Lower TIR is associated with more blood stream infections.
Evaluación de la glucometría en la primera semana de estancia en la UCI y su asociación con los resultados.
DiseñoEstudio observacional prospectivo.
ÁmbitoUCI mixta de hospital docente.
PacientesAdultos que iniciaron una infusión de insulina para dos lecturas consecutivas de glucosa en sangre (GS) ≥180mg/dl.
Principales variables de interésGlucometría calculada a partir de la GS de la primera semana de ingreso: episodios de hiperglucemia (GS >180mg/dl) e hipoglucemia (GS <70mg/dl); mediana, desviación estándar (DE) y coeficiente de variación (CV) de GS, índice de labilidad glucémica (ILG), tiempo en el rango objetivo de GS (TIR).
ResultadosSe incluyeron un total de 5.762 GS en 100 pacientes con una mediana de edad de 55años. Glucometría: hiperglucemia: 2.253 (39%), hipoglucemia: 28 (0,48%), mediana GS: 169mg/dl, DE 31mg/dl, CV 18,6%, ILG: 718,5 [(mg/dl)2/h]/semana, TIR 57%.
La diabetes y una puntuación APACHEII más alta se asociaron con una DE y un CV más altos y una TIR más baja. En la regresión multivariada, la diabetes (p=0,009) y la puntuación APACHEII (p=0,016) se asociaron de forma independiente con una DE más alta.
La DE y el CV más altos se asociaron con menos días sin vasopresores; menor TIR, con más infecciones del torrente sanguíneo (ITS). En el análisis multivariado, el ILG solo se asoció con una mayor mortalidad (OR: 2,99, p=0,04).
ConclusionesLa labilidad glucémica en la primera semana en pacientes de UCI que reciben infusión de insulina se asocia con mayor mortalidad. Una TIR más baja se asocia con más ITS.
Therapies to control the acute alterations in blood glucose (BG) levels have been the focus of research since Van den Berghe et al. demonstrated in 2001 that intensive insulin therapy (insulin infusion to keep the BG tightly controlled between 80 and 110mg/dL) could significantly lower morbidity and mortality.1 Unfortunately, subsequent studies did not yield similar outcomes.2,3 Glucometrics is defined as the “systematic analysis of inpatient BG data” and various measures have been studied.4
Hyperglycemia, is seen in up to 20–52% of all ICU patients and is associated with increase in morbidity and mortality in the critically ill.5–7 Similarly, hypoglycemia is also harmful by a number of different mechanisms.8,9 Glycemic variability (GV) was recognized later as a dimension of glycemic control of importance among critically ill patients.10–13 Another unifying domain of glycemic control, time in target range (TIR), has emerged and integrates hyperglycemia, hypoglycemia and GV.14,15 It is noteworthy that several studies have shown that while GV is higher in diabetics, this increased variability does not portend a worse outcome, leading some authors to coin the term ‘diabetes paradox’.13,16,17
Strategies like continuous glucose monitoring (CGM) or closed loop glycemic control help to keep glucose levels in target range for a greater proportion of time aided by computer-assisted algorithms and reduce complications, especially hypoglycemia.18–21 Such techniques and individualized approaches were assessed in a few recent trials, none showed proven benefit.19–22
Specifically, no study explores the effects of glucometrics in the acute phase (first week) of critical illness on patient outcomes. We hypothesized that glucometrics is worse during the acute phase week of critical illness and this adversely affects outcome; hence we designed this study to assess the glucometrics in the first week of ICU stay and to correlate it with patient outcomes, including 28 day mortality.
MethodsPatient selection and study designThis prospective, single center, cohort study was approved by the ethics committee of the Institute (Institutional ethics committee code: 2017-36-DM-96) and conducted in the 20-bed mixed ICU between May 2017 to June 2018. Consecutive patients were included after informed consent from the patient's family when these criteria were fulfilled: (1) age>18 years, (2) expected to stay more than 5 days and (3) requiring insulin for glycemic control. Insulin infusion was started for two consecutive blood glucose (BG) levels at or above 180mg/dL and titrated to keep the glucose between 120 and 160mg/dL. BG levels were measured using a standard hospital glucose meter (Freestyle Optium H, Abbott Ltd®) using arterial blood. Continuous insulin infusion was titrated according to the modified Yale (MY) insulin infusion protocol23; the usage of the protocol was guided by a phone-based application called Insulin IP Calc.24 Patients were excluded when: (1) consent was not provided, or (2) pregnant.
Study definitions and formulae used- 1.
Hyperglycemia–BG>180mg/dL.
- 2.
Hypoglycemia–BG<70mg/dL.
- 3.
Time in target range (TIR) – the percentage of time that the patient's BG values are in the glycemic target range, i.e., between 100 and 180mg/dL.
- 4.
Glycemic lability index (GLI) was measured by the formula GLI=([mg/dL]2/h)/week.
The difference between two consecutive BG readings was calculated and squared (mg/dL)2 and this value was divided by the time interval in hours between these two readings. The mean of all such values obtained for the first week of admission was calculated to compute the GLI for that patient.4,25,26 In a similar manner, lability index (LI) was calculated for the first day. An example has been worked out in the Supplementary material.
Data collectionAdmission demographic, clinical, laboratory data along with ICU prognostication scores, i.e., Acute Physiologic and Chronic Health Evaluation (APACHE) II score and Sequential Organ Failure Assessment (SOFA) score were recorded. BG values and measurement intervals were collected prospectively from active hospital charts from the start of insulin infusion till 7 days after inclusion. All patients were followed up until ICU discharge or D28, whichever was later. Length of stay, organ support, blood stream infections and mortality during ICU stay were noted.
OutcomesThe following were the outcome measures: (1) glucometrics of the first week of ICU stay, i.e., (a) episodes of hyperglycemia and hypoglycemia, (b) mean and median BG, (c) standard deviation of the mean BG (SD), (d) coefficient of variation of the glucose values around the mean (CV), (e) GLI, and (f) TIR. (2) Patient ICU outcomes, i.e., (a) length of ICU stay, (b) mortality at 28 days, (c) organ supports in the first 28 days, and (c) blood stream infections (BSI).
Sample sizeReviewing literature, GLI was used as the primary measure of GV and ICU mortality as the primary outcome. At a minimum two-sided 95% confidence interval (CI), and 95% power, to detect an estimated odds ratio (OR) of 2.5 for GLI to predict mortality in the study patients, with an assumed mortality of 33%, the minimum required sample size was calculated as 85 including 28 (33%) cases of non survivors. Sample size was estimated using software Power analysis and sample size version-16 (PASS-16, NCSS, LLC, USA). In this study, we included 100 patients including 33 non-survivors with an observed OR of 2.99, which achieved 99.57% power for the study.
Statistical analysisContinuous variables are described as median (25th–75th percentile; i.e., IQR), and categorical variables as number (%). Mann–Whitney U test was used for comparison of continuous variables and the Pearson Chi square test or Fisher's exact test for categorical variables.
GlucometricsThe number of episodes of hyperglycemia and hypoglycemia were noted. Median and mean BG was calculated for each patient and glycemic variability was calculated as SD, CV and GLI of mean BG. GLI was measured by the formula GLI=[mg/dL]2/h/week, as explained above. TIR 100–180mg/dL was calculated from the data collected using the recorded values, without any data extrapolation. These were compared in diabetics and non-diabetics. Binary logistic regression was performed to determine risk factors associated with worse glucometrics for SD, CV, GLI and TIR.
The measures of glucometrics were analyzed for association with patient outcomes. Factors found significant on univariate analysis were analyzed further using multivariable logistic regression. The goodness-of-fit was assessed with the Hosmer–Lemeshow test. Statistical analysis was done using the SPSS-20 software, p<0.05 was considered significant.
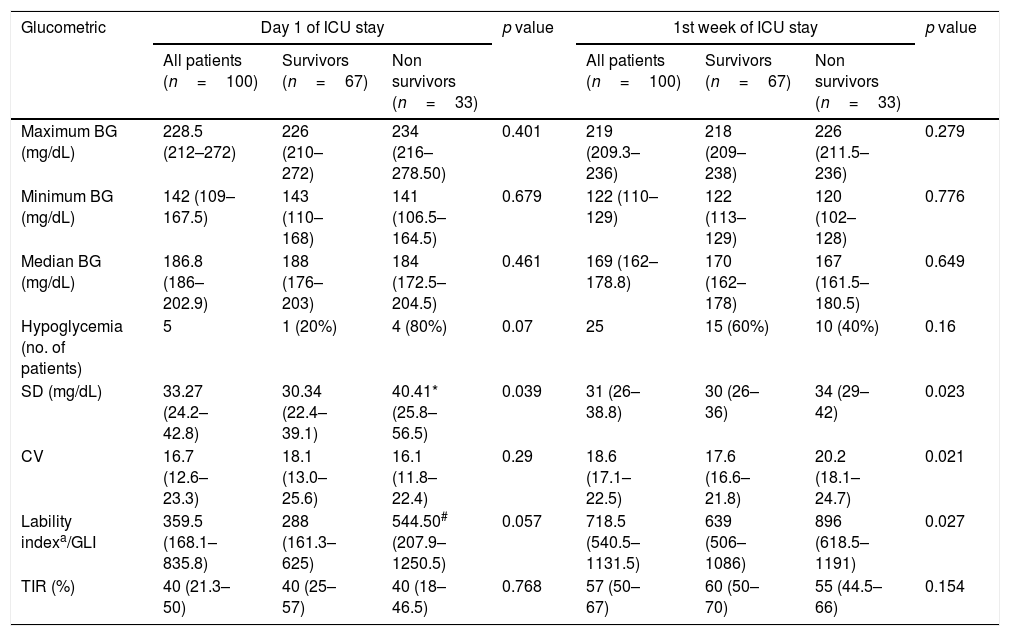
ResultsDuring the study period, 286 patients were screened; 100 patients met the inclusion criteria and were included (Figure S1, supplementary material). The median age was 55 years (IQR 41.25–65); 54% were male. The median APACHE II score was 18.5 (16–22) and median SOFA score 8 (6–10.25). Table 1 gives the baseline characteristics, the admitting diagnosis, the various ICU therapies and the ICU outcomes.
Baseline characteristics of the patients, organ support and outcomes.
| Baseline characteristics (n=100) | Values |
|---|---|
| Age (years) | 55 (41.25–65) |
| Sex (male:female), n | 54:46 |
| APACHE II | 18.5 (16–22) |
| SOFA | 8 (6–10.25) |
| Atleast one comorbidity, n and % | 81% |
| Diabetes | 42% |
| Hypertension | 43% |
| HbA1C (%) (n=66) | 6 (6–7) |
| Patients admitted with sepsis, n and % | 92% |
| Admission diagnosis, n and % | |
| Pneumonia (CAP/VAP/AECOPD) | 37% |
| Neurological conditions (CVA/SAH/SDH/Meningitis) | 18% |
| Severe acute pancreatitis | 10% |
| Other intra abdominal conditions | 5% |
| Tropical illness | 10% |
| Post operative | 5% |
| Other infections (cellulitis/urosepsis) | 6% |
| Miscellaneous | 9% |
| Organ failures, naand % | |
| Shock | 80% |
| Respiratory failure | 76% |
| Acute kidney injury | 52% |
| Neurological impairment | 41% |
| Hematological derangement | 14% |
| Therapies given in the ICU | |
| No. of ventilation days (n=91) | 12 days (6–21) |
| No. of vasopressor days (n=96) | 7 (4–12) |
| Patients requiring RRT, n and % | 33% |
| Median sessions of RRT | 4 (2–6) |
| No. of antibiotic days | 15.5 (10–24.25) |
| Patients requiring transfusions, n and % | 76% |
| No. of transfusions | 2 (1–4) |
| Outcomes | |
| BSI acquired during ICU stay, n and % | 34% |
| Length of stay in ICU (days) | 19 (11–32.5) |
| Length of stay in hospital (days) | 23 (15.75–40.25) |
| Mortality, n and % | 33% |
Values are in median (interquartile range) unless specified. APACHE II: Acute Physiologic and Chronic Health Evaluation II score; SOFA: Sequential Organ Failure Assessment; HbA1c: glycosylated hemoglobin; CAP: community acquired pneumonia; VAP: ventilator associated pneumonia; AECOPD: acute exacerbation of chronic obstructive airway disease; CVA: cerebrovascular accident; SAH: subarachnoid hemorrhage; SDH: subdural hematoma; RRT: renal replacement therapy; BSI: blood stream infection.
A total of 5762 BG measurements were included with an average of 8.2 readings per patient per day. The median BG at admission was 205.5mg/dL (193.5–225). There were 2253 episodes of hyperglycemia (39%); the maximum number was on day 1 (338/556; 61%) and minimum was on day 7 (217/775; 28%) (Figure S2). These episodes were significantly higher on day 1 as compared to day 2 (338/556, 61% vs. 349/905, 39%; p<0.0001), remaining steady subsequently.
Hypoglycemia was seen in 28 out of 5762 measurements (0.48%); single episode in 23 patients, 2 episodes and 3 episodes in one patient each. None of the 25 patients had severe hypoglycemia (<40mg/dL); the lowest reading was 50mg/dL.
The median BG for the study cohort was 169mg/dL (162–178.75) and the mean 174.30mg/dL. The SD of the BG readings for the entire week was 31mg/dL (26–38.75), the CV was 16.7% (12.6–23.3), the GLI was 718.50 (mg/dL)2/h/week (540.50–1131.50) and the median TIR was 57% (50%–67%).
Similar to the pattern seen in hyperglycemia, median TIR was significantly lower on day 1 (40%) as compared to day 2 (61%) (p=0.003) and remained between 56 and 62% for the rest of the days (Figure S2).
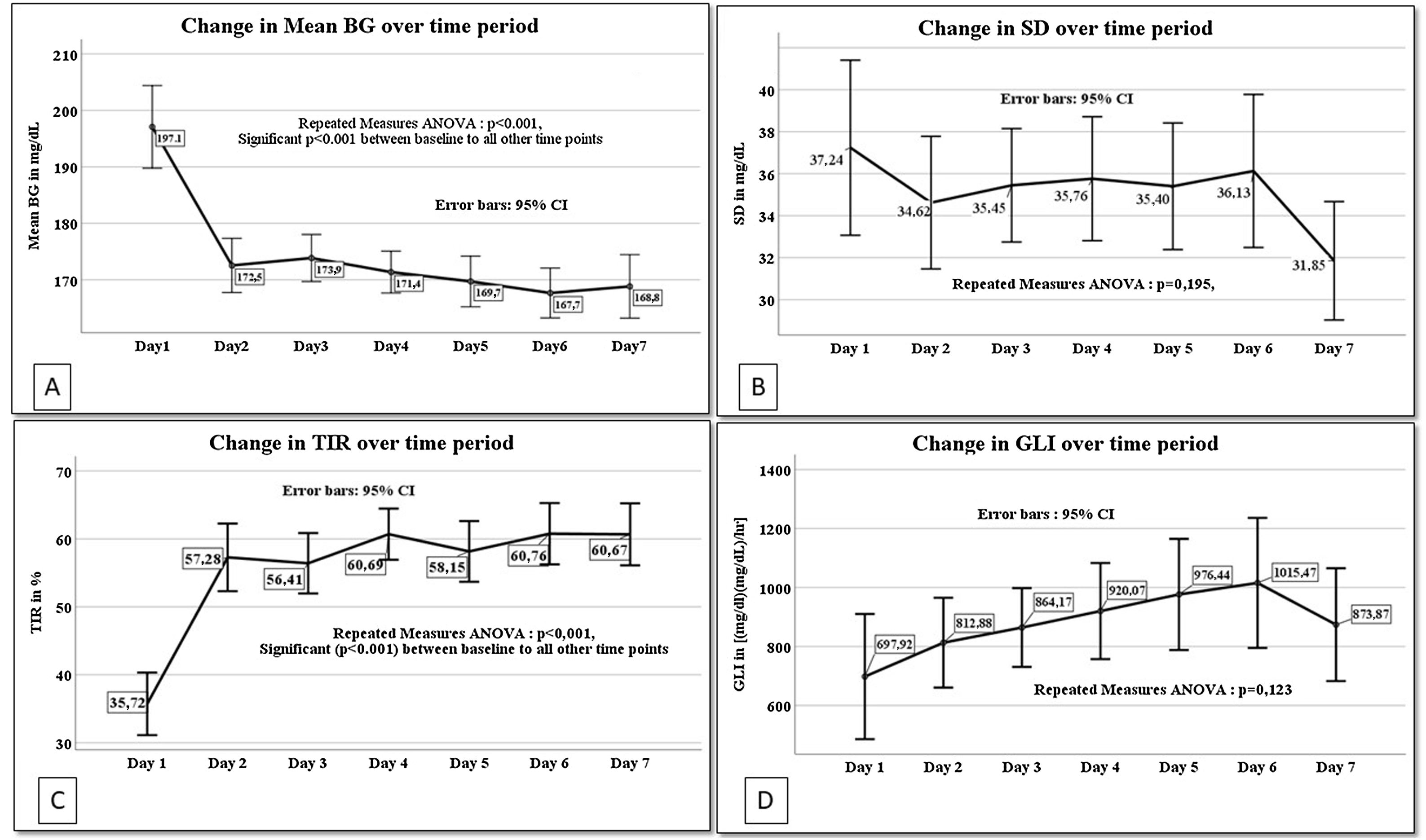
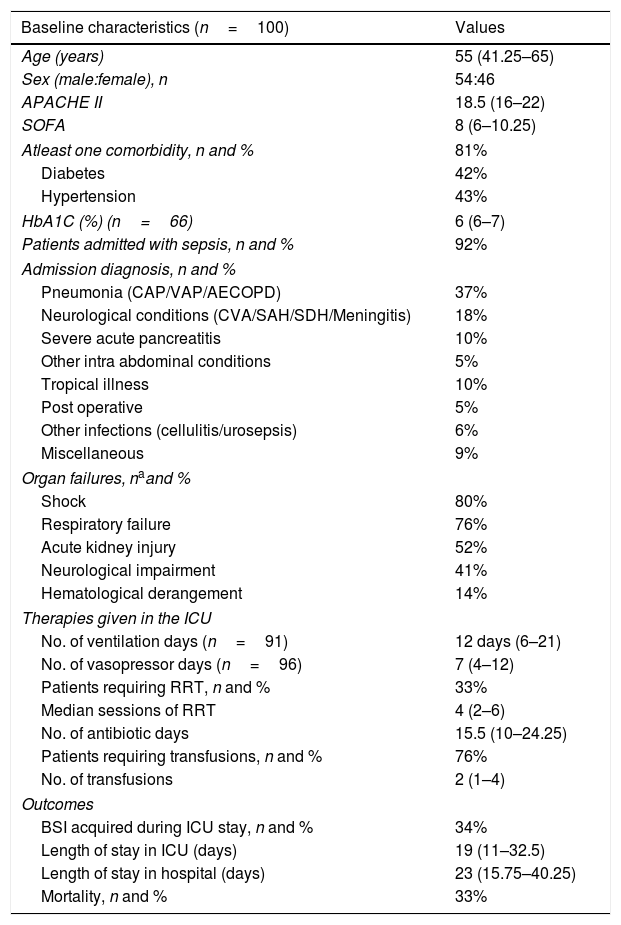
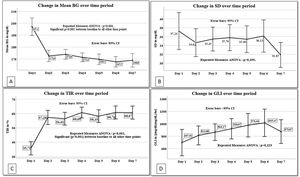
General linear regression model for repeated measures was done for the glucometrics’ values of days 1–7 (Fig. 1). Mean BG, SD, GLI and TIR of each patient for the different days was analyzed. Day 1 values are significantly different from each of the other day's values for mean BG and TIR (repeated measures ANOVA p<0.001 for both).
Linear regression for repeated measures from day 1 to day 7 for A. Mean blood glucose (mean BG), B. Standard deviation of mean blood glucose (SD), C. Time in blood glucose range (TIR) and D. Glycemic lability index (GLI). BG: blood glucose, SD: standard deviation of mean blood glucose, TIR: time in blood glucose range, GLI: glycemic lability index for each day.
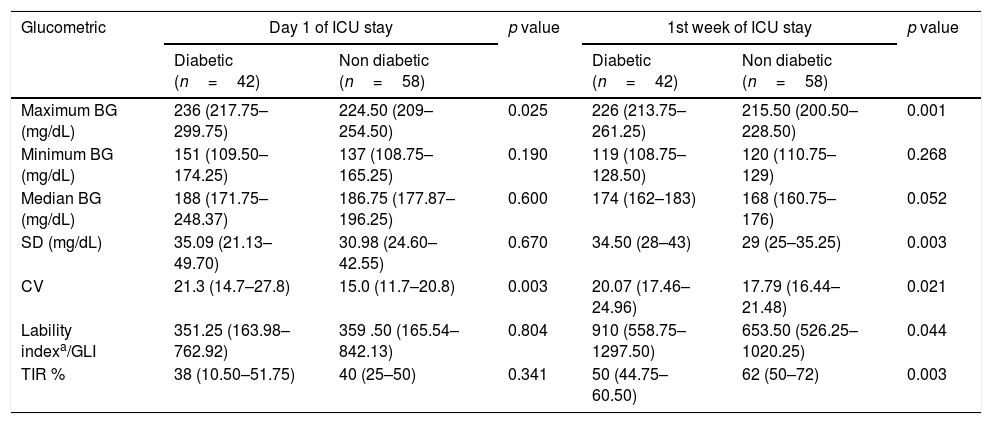
During the study period, 33 (33%) patients died. Table 2 compares the glucometrics among survivors and non survivors on the first day and the first week of ICU stay.
Glucometrics of Day 1 and for the 1st week of ICU stay based on survival status.
| Glucometric | Day 1 of ICU stay | p value | 1st week of ICU stay | p value | ||||
|---|---|---|---|---|---|---|---|---|
| All patients (n=100) | Survivors (n=67) | Non survivors (n=33) | All patients (n=100) | Survivors (n=67) | Non survivors (n=33) | |||
| Maximum BG (mg/dL) | 228.5 (212–272) | 226 (210–272) | 234 (216–278.50) | 0.401 | 219 (209.3–236) | 218 (209–238) | 226 (211.5–236) | 0.279 |
| Minimum BG (mg/dL) | 142 (109–167.5) | 143 (110–168) | 141 (106.5–164.5) | 0.679 | 122 (110–129) | 122 (113–129) | 120 (102–128) | 0.776 |
| Median BG (mg/dL) | 186.8 (186–202.9) | 188 (176–203) | 184 (172.5–204.5) | 0.461 | 169 (162–178.8) | 170 (162–178) | 167 (161.5–180.5) | 0.649 |
| Hypoglycemia (no. of patients) | 5 | 1 (20%) | 4 (80%) | 0.07 | 25 | 15 (60%) | 10 (40%) | 0.16 |
| SD (mg/dL) | 33.27 (24.2–42.8) | 30.34 (22.4–39.1) | 40.41* (25.8–56.5) | 0.039 | 31 (26–38.8) | 30 (26–36) | 34 (29–42) | 0.023 |
| CV | 16.7 (12.6–23.3) | 18.1 (13.0–25.6) | 16.1 (11.8–22.4) | 0.29 | 18.6 (17.1–22.5) | 17.6 (16.6–21.8) | 20.2 (18.1–24.7) | 0.021 |
| Lability indexa/GLI | 359.5 (168.1–835.8) | 288 (161.3–625) | 544.50# (207.9–1250.5) | 0.057 | 718.5 (540.5–1131.5) | 639 (506–1086) | 896 (618.5–1191) | 0.027 |
| TIR (%) | 40 (21.3–50) | 40 (25–57) | 40 (18–46.5) | 0.768 | 57 (50–67) | 60 (50–70) | 55 (44.5–66) | 0.154 |
All values are shown in median (interquartile range). BG: blood glucose; SD: standard deviation; CV: coefficient of variation; GLI: glycemic lability index; TIR: time in range.
Ten patients with hypoglycemia died (10/25; 40%). This difference in mortality was not statistically significant.
SD on day 1 was significantly higher in non survivors as compared to survivors (40.41 vs. 30.34mg/dL, p=0.039).
In the first week of ICU stay the SD, CV and GLI were significantly higher in the non-survivors when compared to survivors (SD: 34 vs. 30; p=0.023; CV: 20.2 vs. 17.6; p=0.021; GLI: 836 vs. 639; p=0.027 in non survivors vs. survivors respectively).
Comparison of glucometrics based on diabetic statusA similar comparison of glucometrics was done in diabetics and nondiabetics (Table 3). On day 1, maximum BG (236 vs. 224.5mg/dL, p=0.025) and CV (21.3 vs. 15%, p=0.003) were significantly higher in diabetics when compared to non-diabetics. However, when glucometrics for the week were compared, maximum BG, SD, CV and GLI were significantly higher in diabetics. Diabetic patients had lower TIR: 50% (44.75–60.50) when compared to non-diabetics: 62% ([50–72]; p=0.003).
Glucometrics of Day 1 and 1st week of ICU stay based on diabetic status.
| Glucometric | Day 1 of ICU stay | p value | 1st week of ICU stay | p value | ||
|---|---|---|---|---|---|---|
| Diabetic (n=42) | Non diabetic (n=58) | Diabetic (n=42) | Non diabetic (n=58) | |||
| Maximum BG (mg/dL) | 236 (217.75–299.75) | 224.50 (209–254.50) | 0.025 | 226 (213.75–261.25) | 215.50 (200.50–228.50) | 0.001 |
| Minimum BG (mg/dL) | 151 (109.50–174.25) | 137 (108.75–165.25) | 0.190 | 119 (108.75–128.50) | 120 (110.75–129) | 0.268 |
| Median BG (mg/dL) | 188 (171.75–248.37) | 186.75 (177.87–196.25) | 0.600 | 174 (162–183) | 168 (160.75–176) | 0.052 |
| SD (mg/dL) | 35.09 (21.13–49.70) | 30.98 (24.60–42.55) | 0.670 | 34.50 (28–43) | 29 (25–35.25) | 0.003 |
| CV | 21.3 (14.7–27.8) | 15.0 (11.7–20.8) | 0.003 | 20.07 (17.46–24.96) | 17.79 (16.44–21.48) | 0.021 |
| Lability indexa/GLI | 351.25 (163.98–762.92) | 359 .50 (165.54–842.13) | 0.804 | 910 (558.75–1297.50) | 653.50 (526.25–1020.25) | 0.044 |
| TIR % | 38 (10.50–51.75) | 40 (25–50) | 0.341 | 50 (44.75–60.50) | 62 (50–72) | 0.003 |
All values are shown in median (interquartile range). BG: blood glucose; SD: Standard deviation; CV: coefficient of variation; GLI: glycemic lability index; TIR: time in range.
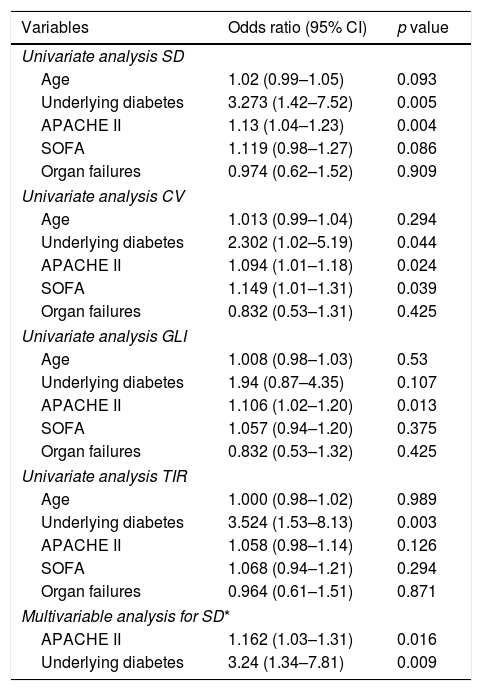
Various admission characteristics including age, presence of diabetes, severity of illness scores (APACHE II and SOFA) and organ failures were assessed to determine the factors associated with worse glucometrics. The results of the univariate and multivariate regression are shown in Table 4.
Univariate and multivariate analysis of factors associated with worse glucometrics.
| Variables | Odds ratio (95% CI) | p value |
|---|---|---|
| Univariate analysis SD | ||
| Age | 1.02 (0.99–1.05) | 0.093 |
| Underlying diabetes | 3.273 (1.42–7.52) | 0.005 |
| APACHE II | 1.13 (1.04–1.23) | 0.004 |
| SOFA | 1.119 (0.98–1.27) | 0.086 |
| Organ failures | 0.974 (0.62–1.52) | 0.909 |
| Univariate analysis CV | ||
| Age | 1.013 (0.99–1.04) | 0.294 |
| Underlying diabetes | 2.302 (1.02–5.19) | 0.044 |
| APACHE II | 1.094 (1.01–1.18) | 0.024 |
| SOFA | 1.149 (1.01–1.31) | 0.039 |
| Organ failures | 0.832 (0.53–1.31) | 0.425 |
| Univariate analysis GLI | ||
| Age | 1.008 (0.98–1.03) | 0.53 |
| Underlying diabetes | 1.94 (0.87–4.35) | 0.107 |
| APACHE II | 1.106 (1.02–1.20) | 0.013 |
| SOFA | 1.057 (0.94–1.20) | 0.375 |
| Organ failures | 0.832 (0.53–1.32) | 0.425 |
| Univariate analysis TIR | ||
| Age | 1.000 (0.98–1.02) | 0.989 |
| Underlying diabetes | 3.524 (1.53–8.13) | 0.003 |
| APACHE II | 1.058 (0.98–1.14) | 0.126 |
| SOFA | 1.068 (0.94–1.21) | 0.294 |
| Organ failures | 0.964 (0.61–1.51) | 0.871 |
| Multivariable analysis for SD* | ||
| APACHE II | 1.162 (1.03–1.31) | 0.016 |
| Underlying diabetes | 3.24 (1.34–7.81) | 0.009 |
SD: standard deviation; CV: coefficient of variation; GLI: glycemic lability index; TIR: time in blood glucose range; APACHE II: acute physiology and chronic health evaluation II; SOFA: sequential organ failure assessment.
On univariate analysis, diabetes and higher APACHE II were associated with higher SD (p<0.05). On multivariate analysis, diabetes (OR: 3.24; 95% CI: 1.34–7.81, p=0.009) and APACHE II score were independently associated with higher SD.
On univariate analysis, diabetes, higher APACHE II and SOFA scores were associated with higher CV (p<0.05); higher APACHE II score was associated with higher GLI (OR 1.16; p=0.016) and diabetes was associated with lower TIR (OR 3.52; p=0.003).
Overall, patients who were diabetic had worse glucometrics. A higher APACHE II was among the other factors associated with worse glucometrics.
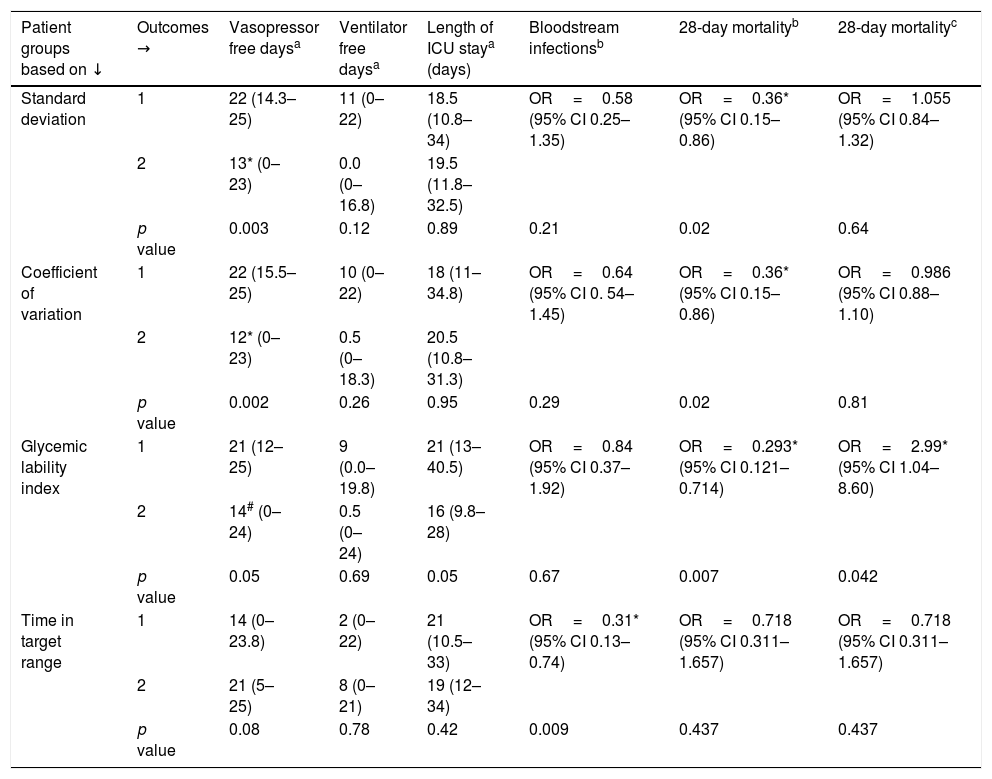
Glucometrics and patient outcomesThe various glucometrics were assessed for association with patient outcomes. These include days free of organ supports, need for renal replacement therapy, length of ICU stay, adverse outcomes such as blood stream infections and 28-day mortality.
Table 5 shows these results. Patients with values of glucometrics below the cohort median are labeled group 1 and those with values above the cohort median as group 2; hence group 1 SD, CV and GLI are patients with better glucometrics while group 1 TIR is with worse glucometrics.
Patient groups based on glucometrics and outcomes i.e., organ support free days, length of ICU stay, blood stream infections and 28-day mortality.
| Patient groups based on ↓ | Outcomes → | Vasopressor free daysa | Ventilator free daysa | Length of ICU staya (days) | Bloodstream infectionsb | 28-day mortalityb | 28-day mortalityc |
|---|---|---|---|---|---|---|---|
| Standard deviation | 1 | 22 (14.3–25) | 11 (0–22) | 18.5 (10.8–34) | OR=0.58 (95% CI 0.25–1.35) | OR=0.36* (95% CI 0.15–0.86) | OR=1.055 (95% CI 0.84–1.32) |
| 2 | 13* (0–23) | 0.0 (0–16.8) | 19.5 (11.8–32.5) | ||||
| p value | 0.003 | 0.12 | 0.89 | 0.21 | 0.02 | 0.64 | |
| Coefficient of variation | 1 | 22 (15.5–25) | 10 (0–22) | 18 (11–34.8) | OR=0.64 (95% CI 0. 54–1.45) | OR=0.36* (95% CI 0.15–0.86) | OR=0.986 (95% CI 0.88–1.10) |
| 2 | 12* (0–23) | 0.5 (0–18.3) | 20.5 (10.8–31.3) | ||||
| p value | 0.002 | 0.26 | 0.95 | 0.29 | 0.02 | 0.81 | |
| Glycemic lability index | 1 | 21 (12–25) | 9 (0.0–19.8) | 21 (13–40.5) | OR=0.84 (95% CI 0.37–1.92) | OR=0.293* (95% CI 0.121–0.714) | OR=2.99* (95% CI 1.04–8.60) |
| 2 | 14# (0–24) | 0.5 (0–24) | 16 (9.8–28) | ||||
| p value | 0.05 | 0.69 | 0.05 | 0.67 | 0.007 | 0.042 | |
| Time in target range | 1 | 14 (0–23.8) | 2 (0–22) | 21 (10.5–33) | OR=0.31* (95% CI 0.13–0.74) | OR=0.718 (95% CI 0.311–1.657) | OR=0.718 (95% CI 0.311–1.657) |
| 2 | 21 (5–25) | 8 (0–21) | 19 (12–34) | ||||
| p value | 0.08 | 0.78 | 0.42 | 0.009 | 0.437 | 0.437 |
1=patients with values <50th centile; 2=patients with values ≥50th centile. RRT=renal replacement therapy; OR=odds ratio; CI=confidence interval.
Patients with lower SD (group 1) had higher number of vasopressor free days (22 vs. 13 days, p=0.003) and lower 28-day mortality (OR 0.36, 95% CI 0.15–0.86; p=0.021).
Patient outcomes based on CVPatients with lower CV (group 1) had higher number of vasopressor free days (22 days vs. 12 days, p=0.002) and lower 28-day mortality (OR 0.36, 95% CI 0.15–0.86; p=0.02).
Patient outcomes based on GLIThose with a lower GLI had a lower 28-day mortality (OR 0.29, 95% CI 0.0.12–0.71; p=0.007).
Patient outcomes based on TIRIn patients with lower TIR (group 1), a greater number of BSI were seen (24 vs. 10, p=0.009).
Patients with higher SD and CV required vasopressors for longer duration. Higher 28-day mortality was seen in patients with higher SD, CV and GLI. Patients with lower TIR had more BSI. The minimum, median and maximum BG were not associated with any adverse patient outcomes.
On multivariate analysis for 28-day mortality, GLI alone was associated with a higher mortality (OR 2.99, 95% CI 1.04 to 8.6, p=0.04).
DiscussionIn this prospective observational study, we have described glucometrics in the first week of ICU stay in critically ill adults, the factors associated with worse glucometrics and their association with adverse clinical outcomes. Diabetics and patients with higher admission APACHE II had worse glucometrics. Higher SD on day 1, and higher SD, CV and GLI in the first week of ICU admission were associated with mortality. This was similar to that seen in recent retrospective studies assessing GV.10,13,25,26
The prevalence of hyperglycemia in our study was 39%, similar to previous studies ranging from 40 to 54%.27 Analysis of repeated measures revealed a higher mean BG and lower TIR on day 1 as compared to each of the other days. This is probably due to the time taken to stabilize the patient's physiological derangements and titrate the insulin infusion.
The median BG in the cohort was 169mg/dL and the mean was 174mg/dL. Several investigators have combined mean BG with SD, GLI and other similar measures of GV to study the association with adverse outcomes. In earlier studies, it was found that patients with higher GLI, but with lower median BG, had higher mortality.28,29 In our study we found no association between mean or median BG and outcome.
Amongst the factors associated with worse glucometrics at admission, the presence of diabetes mellitus was independently associated with worse SD as well as worse TIR, similar to the studies by Chao et al.,13 and Krinsley et al.16 APACHE II at admission was also associated with a worse SD with an odds ratio of 1.16, suggesting that sicker patients have worse glucometrics.16
Glycemic variability (GV) is considered an important target in the glycemic management of ICU patients. Various measures of variability, i.e., mean BG, SD, CV and GLI have been studied. Egi et al. were the first to study the relationship of GV and outcome in critically ill patients.30 Egi et al. and subsequently Krinsley et al.31 found that the SD of glucose values is strongly associated with ICU and hospital mortality respectively. Similarly, Krinsley et al.31 and Lanspa et al.32 found CV as an important measure of glucose variability. A value above 20mg/dL for SD and 20% for CV have been associated with increased mortality.
In our study, we studied SD, CV, GLI and TIR of a cohort of critically ill patients and found each of these glucometrics associated with different adverse outcomes. Higher SD and CV were associated with higher vasopressor requirement. SD, CV and GLI were associated with 28-day mortality; on multivariate analysis, GLI was associated with almost 3 times higher risk of ICU mortality. The other important finding is the higher risk of BSI in patients whose TIR was below the cohort median.
The median GLI in our study was 718.5[mg/dL]2/h/week (540.50–1131.50), comparable to a recent multicentre study done in Australia and Sweden (GLI was 720 (mg/dL)2/h/week).25 Todi et al. showed that deciles of increasing GLI were associated with increasing ICU mortality.28 In our study, GLI above cohort median was associated with higher mortality (23% in higher GLI vs. 10% in lower GLI); this was significant on univariate and multivariate analysis.
The TIR in our study was 57%, which is similar to previous studies done.14,15 There was no significant difference in requirements of organ supports, length of ICU stay and mortality between patients with higher TIR versus with lower TIR. Diabetics spent less time in target range; however recent research suggests that the target BG levels should be different for diabetics as compared to non-diabetics.13,16,17 A TIR >80% is considered a desirable target in critically ill non-diabetics; however, the TIR in critically ill diabetics needs to be further defined.15
Our study suggests a significant association between low TIR and increased risk of bacteremia. To the best of our knowledge, it is the first to explore this association between TIR and bacteremia. This can be explained by the worsening in immunity and oxidative stress generated by glucose level variation, as shown in previous studies.33–35 Van den Berghe et al. found significant reduction in BSI in surgical ICU patients who received intensive insulin therapy; perhaps because these patients achieved a high TIR.1
Several mechanisms have been postulated in which high GV could be harmful in critically ill patients. Analyses of patients with diabetes mellitus requiring insulin have suggested an association between glycemic variability and end-organ damage through endothelial dysfunction.11 It is likely that poorer outcomes among critically ill patients may be due to increased oxidative stress.11 Such oxidative stress has been found to lead to endothelial dysfunction and vascular injury. Apoptosis of cells has also been observed with rapid correction of hyperglycemia to normoglycemia as against sustained hyperglycemia.11,34
SD, CV and GLI were significantly higher in diabetics, while TIR was lower in diabetics when compared to non-diabetics. This finding was similar to the study done by Lanspa et al. and Krinsley et al. where diabetics had more GV when compared to non-diabetics.15−17,32 This increased GV in diabetic patients does not result in higher mortality, whereas the reverse is true for non-diabetics.16,17 Hence, any glucometric analysis in critically ill patients should be matched for diabetic status and it needs to be personalized in the phase of critical illness.16,25,36 In our study, analysis of adverse outcomes in diabetic patients did not yield results different from outcomes in the entire cohort (results not shown), perhaps due to the small numbers.
Strengths of the studyIt is a prospective study and the first to specifically address the dynamicity of glucose measurements in the first week of critical illness.
Limitations of the studyThis was a single center study with a small number of patients. We have not assessed the effect of glucose intake (i.e., nutrition) and/or steroids on BG abnormalities. Almost 2/5th of the patients were diabetic. We did not assess adherence to the insulin infusion protocol.
ConclusionAmong critically ill patients receiving insulin infusion, glycemic lability index was independently associated with 28-day mortality. Diabetics were found to have worse glucometrics. TIR was significantly associated with the risk of BSI.
Authors’ contributionsVS: Designed the study, collected the data, did the preliminary analysis and wrote the first draft of the manuscript.
BP: Conceived and designed the study, finalized the analysis and the manuscript and is the corresponding author.
SS: Helped in finalizing the analysis and the manuscript.
SKJ: Collected the data and helped VS in analysing the results.
AA: Critiqued the manuscript and suggested modifications.
MG: Helped in designing the study and made major changes to the manuscript.
RKS: Critiqued the manuscript and suggested changes.
AKB: Critiqued the study design, manuscript and suggested changes.
All authors have read the manuscript and approved the manuscript for submission.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNo conflict of interest declared by any of the authors.