We aimed to anlayse the relationship between anti-Xa activity below range and thomboembolic events.
DesignSingle center prospective observational longitudinal cohort study (February–November 2021).
SettingPatients admitted to the ICU of a University Hospital.
ParticipantsPatients with severe COVID-19 pneumoniae.
InterventionsEnoxaparin was used for prophylactic and therapeutic anticoagulation. Enoxaparin dosing and dose adjustment were based on anti-Xa activity according to the hospital protocol.
Main variables of interestTarget: thomboembolic events.
Predictors: demographics, pharmacotherapy, anti-Xa measurements, clinical data, and laboratory results.
Logistic regression was used to identify independent risk factors for thomboembolic events.
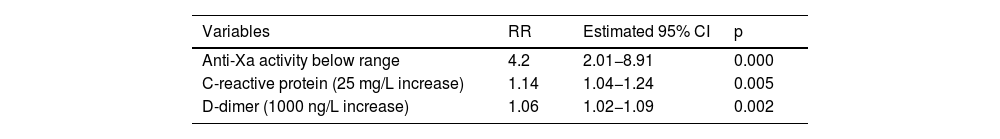
ResultsData were available for 896 serum anti-Xa measurements from 228 subjects. Overall, 71.9% were male, with a median age of 62. Most patients needed invasive mechanical ventilation (87.7%) and mortality was 24.1%. A total of 28.9% new thomboembolic events were diagnosed. There were 27.1% anti-Xa measesurements below range. When multivariable logistic regression analysis was performed anti-Xa activity below range (RR, 4.2; p = 0.000), C-reactive protein (25 mg/L increase) (RR, 1.14; p = 0.005) and D-dimer (1000 ng/L increase) (RR, 1.06; p = 0.002) were the independent factors related to new thomboembolic events in patients with severe COVID-19.
ConclusionsAnti-Xa activity below range, C-reactive protein and D-dimer were the independent factors related to thomboembolic events in patients with severe COVID-19. Purposely designed clinical trials should be carried out to confirm the benefit of an anti-Xa monitoring.
Analizar la relación entre los niveles de anti-Xa por debajo del rango objetivo con los eventos trombóticos.
DiseñoEstudio de cohortes, unicéntrico, prospectivo, longitudinal y observacional (Febrero–Noviembre 2021).
ÁmbitoPacientes ingresados en la UCI de un Hospital Universitario.
ParticipantesPacientes críticos con neumonía COVID-19.
IntervencionesLa enoxaparina se utilizó como anticoagulación profiláctica y terapéutica. Su dosificación y ajuste de dosis se basó en la actividad anti-Xa según el protocolo hospitalario.
Variables de interés principalsObjetivo: eventos trombóticos.
Predictoresdemográficos, farmacoterapia, mediciones de anti-Xa, datos clínicos y resultados de laboratorio.
Se realizó un análisis de regresión logística para identificar los factores de riesgo independientes de trombosis.
ResultadosSe realizaron 896 mediciones séricas de anti-Xa en 228 pacientes. La mayoría fueron varones (71,9%), con una edad mediana de 62 años. La mayoría precisaron de ventilación mecánica invasiva (87,7%) y la mortalidad fue del 24,1%. Se diagnosticó un 28,9% de nuevos eventos trombóticos. El 27,1% de las mediciones se encontraron por debajo del rango objetivo. El análisis de regresión logística demostró que la actividad anti-Xa por debajo del rango objetivo (RR: 4,2; p = 0,000), la PCR (incremento de 25 mg/L) (RR:1,14; p = 0,005) y, el dímero D (incremento de 1000 ng/L) (RR: 1,06; p = 0,002) fueron los factores predictores independientes relacionados con nuevos eventos trombóticos.
ConclusionesLa actividad anti-Xa por debajo del rango objetivo, la PCR y, el dímero D fueron los factores independientes que se relacionaron con eventos trombóticos en pacientes con neumonía grave por COVID-19. Se deberían realizar ensayos clínicos al respecto para confirmar el beneficio de la monitorización de la actividad anti-Xa.
SARS-CoV-2, also known as COVID-19 (COronaVIrus Disease 19), was initially detected in China in December 2019,1 and it was declared a pandemic by the WHO on March 2020.2 The spectrum of COVID-19 presentations ranges from a mild self-limited flulike illness to life-threatening multiorgan failure, reaching up to 49% mortality.3 In severe COVID-19, thromboses are common, due to a prothrombotic state, vascular inflammation and endothelial injury.4–8 Venous thromboembolism is the most frequently reported thrombotic complication, occuring in up to 42.6% of patients,8–11 despite the use of prophylactic heparin treatment since the intensive care unit (ICU) admission. However, limited evidence exists to guide the prophylactic and therapeutic antithrombotic regimens.12 Low-molecular-weigth heparin (LMWH) is the preferred heparin because of its safety profile, efficacy, bioavailability and persistent anticoagulant response.12–15
Throughout the pandemic, guidelines have been constantly updated. Initaliy, prophylactic, intermediate and therapeutic doses, were considered.9,10,12,16 Currently, randomized clinical trials have demonstrated that anticoagulation at therapeutic doses, without evidence of thrombosis, increases bleeding complications and mortality.17 Likewise, the INSPIRATION clinical trial showed that anticoagulation at intermediate doses did not reduce the incidende of thromboembolic events.18 Currently, clinicians are preventing and treating thromboembolic events in patients with severe COVID-19 with prophylactic and therapeutic doses of enoxaparin, as suggested by clinical practice guidelines for venous thromboembolism.13,14,19–21
However, we are concerned about its accuracy. It would likely be more precise to monitor anti-Xa activity with the aim of guiding LMWH prophylaxis and treatment. Critically ill patients may be heparin-resistant22,23 and may need higher doses of LMWH to achieve the desired prophylactic and therapeutic anticoagulation effect. Some studies have shown that the mean level of anti-Xa activity in patients with severe COVID-19 is subtherapeutic.24–27 Until now, because of the safety profile of LMWH, monitoring has only been indicated in special situations, such as those involving obesity, renal injury, pregnancy and elderly individuals.13–15
Due to the high incidence of thromboembolic events and the likely resistance to heparin in these patients, the aim of this study was to analyse the relationship between anti-Xa activity below range and the diagnosis of new thromboembolic events in patients with severe COVID-19 admitted to the ICU.
Patients and methodsStudy designThis was an observational prospective longitudinal cohort study. Patients were recruited from the ICU of the Germans Trias i Pujol University Hospital between 18th February and 4th November, 2021 and were followed until discharge from the ICU. The study was started after the approval of the thromboprophylaxis protocol for severe COVID-19 by the hospital thrombosis committee (Germans Trias i Pujol University Hospital. Document Code: CMT-PM-008, revised February 1, 2021).
The eligibility criteriaThe inclusion criteria were patients eighteen years or older and admitted to the ICU because of severe COVID-19. The exclusion criteria were active bleeding, a glomerular filtration rate (GFR) less than 15 mL/min per 1.73 m2 and a platelet count less than 30 × 10^9/L.
Initial dosing of enoxaparinThe initial dose of enoxaparin was based on weight and renal function (Table S1, supplementary material). Prophylactic enoxaparin was administered at 6 am, and therapeutic enoxaparin was administered at 6 am and 6 pm.
Anti-Xa monitoringThe draw time of the anti-Xa assay is generally 4 h after the dose of enoxaparin so sample extractions were carried out at 10 am. Blood draws were performed on Mondays and Thursdays until patient discharge from the ICU. Anti-Xa monitoring was performed after at least two enoxaparin injections to ensure the achievement of a functional drug steady state.13,14 The levels of procoagulant (platelets, D-dimer, fibrinogen) and inflammatory biomarkers [C-reactive protein, procalcitonin (PCT), monocyte distribution width (MDW), interleukin-6 (IL-6), ferritin, and lactate dehydrogenase (LDH)] and the glomerular filtration rate (GFR) were determined at the same time as the anti-Xa levels. Data on pharmacotherapy (tocilizumab, dexamethasone, and enoxaparin) and support therapies [invasive mechanical ventilation and extracorporeal membrane oxygenation (ECMO)] were collected on the same day that blood samples were collected for anti-Xa monitoring. The anti-Xa levels were available after four hours of sample collection to adjust the dose of enoxaparin dosing on the same day.
Anti-Xa assay characteristics: Blood samples were collected in 3.8% (0.129 M) sodium citrate (anticoagulant) tubes (BD Vacutainer) and plasma was obtained by 10-minute centrifugation at 3000 rpm. The anti-Xa activity of enoxaparin was measured using a chromogenic assay (Liquid AntiXa®, Instrumentation Laboratory, Bedford, MA, USA) on an automated coagulation analyser (ACL TOP 750, Instrumentation Laboratory, Bedford, MA, USA) and was reported in IU/mL.
The expected anti-Xa prophylactic and therapeutic ranges were 0.2 to 0.4 IU/mL and 0.5–1 IU/mL, respectively.13,14 Anti-Xa activity was categorized as subprophylactic, prophylactic or supraprophylactic and subtherapeutic, therapeutic and supratherapeutic (Table S2, supplementary material).
Enoxaparin dose adjustmentEnoxaparin dose adjustment was based on anti-Xa activity according to Manresa et al.13 and the hospital protocol (Table S3, supplementary material).
Screening for new thromboembolic eventsUS screening for DVTUS screening for DVT was always done upon ICU admission; routinely on Tuesday and Thursday (simultaneously with the determination of anti-Xa activity), and on Saturday or Sunday; when there were significant differences between D-dimer results and when thrombosis was suspected on physical examination (pain or swelling).
Intensive care physicians performed the screening. These physicians had a long-standing experience in US (more than ten vascular examinations performed per week and more than one year of experience in performing and interpreting US examinations). Veins were scanned transversally over their entire length, with probe compression applied at 1−2 cm intervals.
If needed, augmentation manoeuvers were performed to find spontaneous or reverse-flow intraluminal color filling. The veins scanned in the lower extremities included the common femoral, femoral, popliteal, tibial, peroneal, gastrocnemius, soleal and saphenous veins. The veins scanned in the upper extremities were the radial, ulnar, axillary, basilic and cephalic veins. Additionally, the subclavian, internal, and external jugular veins were scanned. The arteries were scanned in the same way as the veins but only when ischemic complications occurred.
US studies were performed using a Canon Ultrasound CUS-X100 G (Xario 100 G), with a linear transducer (4.2−14 MHz) and a FUJIFILM SonoSite M-Turbo Ultrasound, with a linear transducer (6−13 MHz).
Transthoracic echocardiography screening for PETransthoracic echocardiography was performed upon ICU admission and when PE was clinically suspected (worsening hypoxemia and/or unexpected shock). Both intensive care physicians trained in Focus Cardiac Ultrasound and cardiologists performed the echocardiograms.
The US studies were performed using a Canon Ultrasound CUS-X100 G (Xario 100 G), with a sector transducer (1.8–5.2 MHz) and a FUJIFILM SonoSite M-Turbo Ultrasound, with a sector transducer (1−5 MHz).
Angio-CT screening for PETo confirm PE, angio-CT was performed when severe hypoxemia and/or shock could not be explained by other causes, if the patient’s condition allowed it. The radiology department was far from the ICU, and the transfer required taking the elevator and placing the patient on a transfer stretcher.
OutcomesThe primary aim was to determine the relative risk (RR) of new thromboembolic events in patients with severe COVID-19 with anti-Xa activity below range compared to anti-Xa activity within range.
The secondary outcome was to indentify the independent factors related to thomboembolic events in patients with severe COVID-19.
Institutional review board statementThe study was carried out in accordance with the ethical principles for medical research involving human subjects (revised Declaration of Helsinki, Brazil, 2013)28 and the Good Clinical Practice Guidelines of the International Conference of Harmonization (2016).29 The data were stored securely, and all procedures related to data management complied with the General Data Protection Regulation.30
The clinical research ethics committee of the Germans Trias i Pujol Hospital approved the development of the study (resolution number: PI-22-056) in February 2021.
Sample sizeConsidering an estimated 42.6% incidence of thromboembolic events in patients with severe COVID-19 patients admitted to the ICU,7 and an estimated 10% reduction in the incidence of thromboembolic events with enoxaparin dose adjustment based on anti-Xa activity, two-tailed statistical significance, 5% alpha risk, 85% power, and assuming a 2% of losses, we estimated a sample size of 228 patients (nsize Stata® v.16).
Data collectionDemographics, prognosis [Acute Physiology, Age and Chronic Health Evaluation II (APACHE II)], body-mass index and comorbidities were collected from the patient’s clinical health records. The following parameters were prospectively collected: antiplatelet therapy, COVID-19 anti-inflammatory treatment (tocilizumab, dexamethasone), enoxaparin dosing, anti-Xa activity, need for invasive mechanical ventilation, shock, GFR, new thromboembolic events (DVT, PE and AT), severe hemorrhage, length of ICU stay and mortality.
Statistical analysisThe statistical analysis of the data was performed in Stata® v.16. The means and medians with their respective 95% confidence intervals (CIs) were calculated for quantitative variables and percentages with their 95% CIs were calculated for qualitative variables. The Kolmogorov-Smirnov test was applied to confirm the normal distribution of the data.
Simple logistic regression was used to determine the bivariate analysis of possible factors associated with the probability of developing new thromboembolic events and to identify potential confounders.
Multivariable logistic regression analysis was used to determine the significant factors associated with new thromboembolic events and to construct a predictive model, illustrated with a receiver operating characteristic (ROC) curve. To perform the multivariable logistic regression analysis, we considered those variables that were significantly associated with new thromboembolic events in the simple logistic regression. Those variables with a large percentage of missing values (>60%) were discarded (Table S4, supplementary material).
The statistical method used to choose the best model was the all-possible-subset method (based on the cut-off point of 0.1) for logistic regression, and the final model chosen was the most parsimonious model with the best Hosmer–Lemeshow score and best area under the curve. A ROC curve was generated.
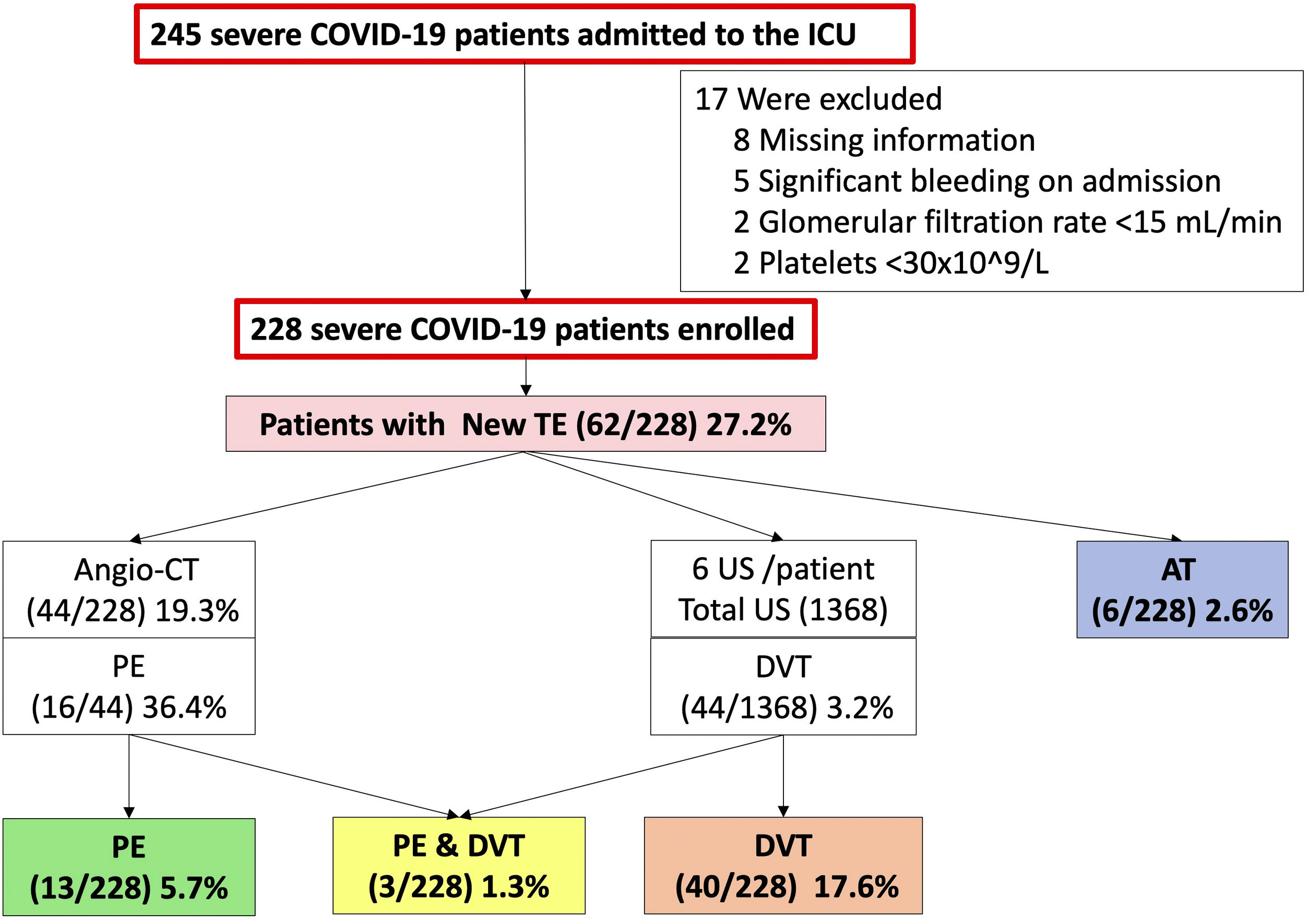
ResultsStudy population and thromboembolic eventsA total of 245 patients were screened between 18th February and 4th November, 2021 and a total of 228 patients were enrolled.
At the end of the follow-up, 85 (37.2%; 95% CI: 31–44) thromboembolic events were diagnosed in 75 patients, so 32.9% (95% CI: 26.8–39.4) of these patients had a thrombosis. Nineteen thromboembolic events (8.3%) were diagnosed pre-ICU in 15 patients [6 (2.6%) with PE, 5 (2.2%) with DVT and 4 (1.8%) with PE plus DVT], so 6.6% patients were enrolled with a thromboembolic event already diagnosed.
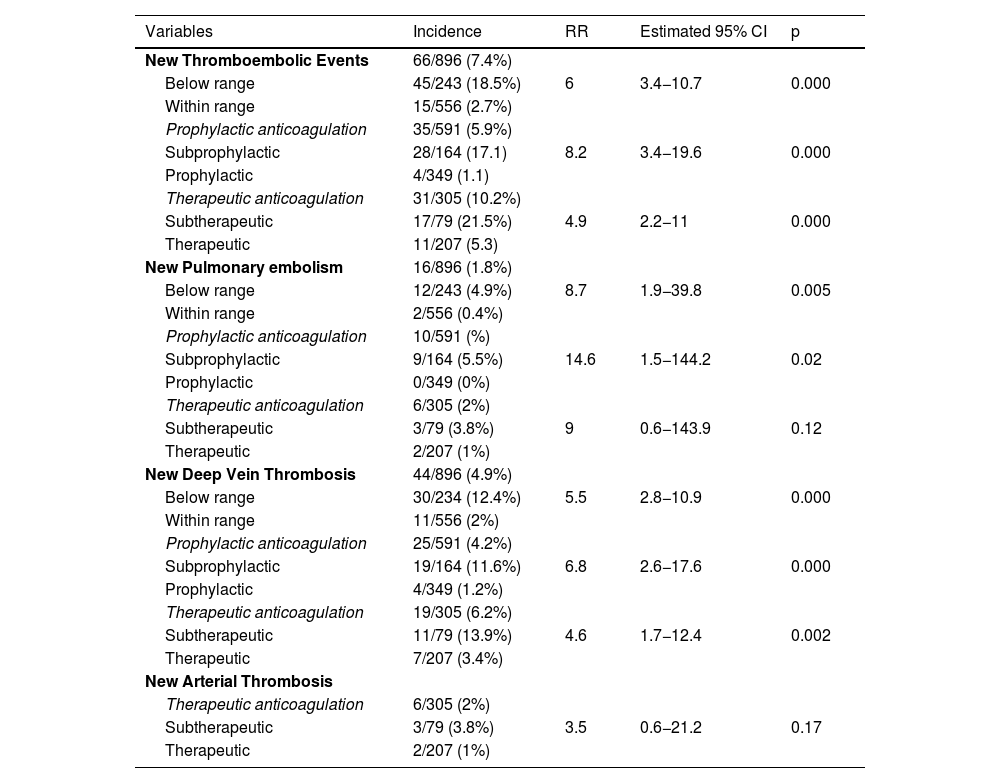
Sixty-six (28.9%; 95% CI: 23.2–35.3) new thromboembolic events were diagnosed after enrolment in the ICU in 62 patients, so 27.2% (95% CI: 21.5–33.5) of patients had a new thromboembolic event [13 (5.7%) PE, 40 (17.6%) DVT, 3 (1.3%) PE plus DVT and 6 (2.6%) AT] (Fig. 1).
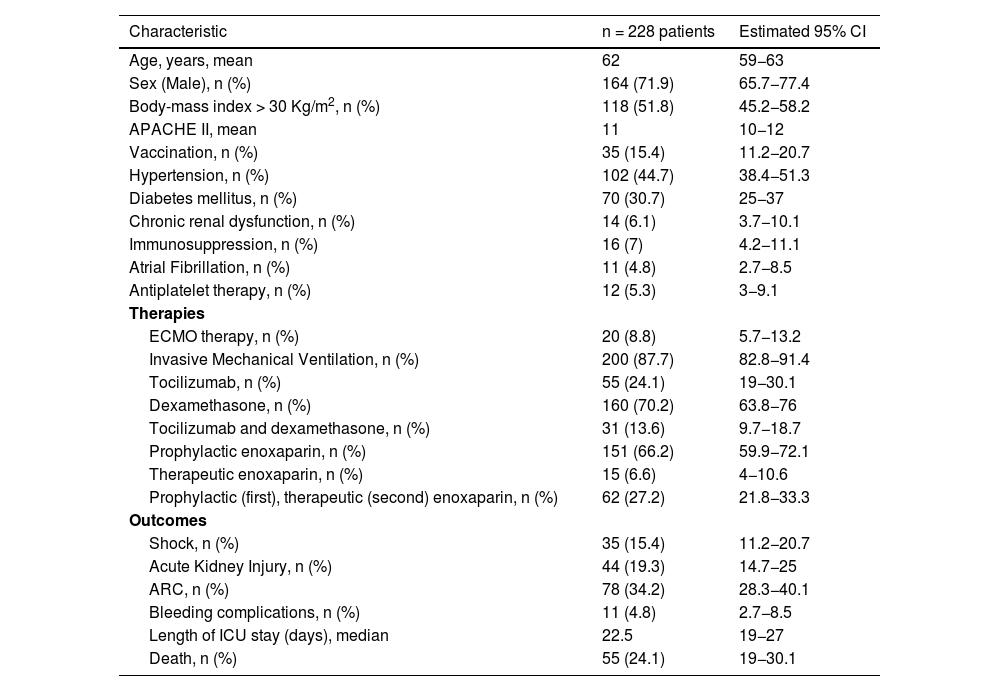
Patient baseline characteristicsOverall, 71.2% (95% CI, 65.7−77.4) were male and the median age was 62 years old (95% CI, 59−63). A body-mass index >30 kg/m2 (obesity) was the most common coexisting condition (51.8%; 95% CI, 45.2−58.2) followed by hypertension (44.7%; 38.4−51.3) (Table 1).
Patient baseline characteristics at ICU enrolment.
| Characteristic | n = 228 patients | Estimated 95% CI |
|---|---|---|
| Age, years, mean | 62 | 59−63 |
| Sex (Male), n (%) | 164 (71.9) | 65.7−77.4 |
| Body-mass index > 30 Kg/m2, n (%) | 118 (51.8) | 45.2−58.2 |
| APACHE II, mean | 11 | 10−12 |
| Vaccination, n (%) | 35 (15.4) | 11.2−20.7 |
| Hypertension, n (%) | 102 (44.7) | 38.4−51.3 |
| Diabetes mellitus, n (%) | 70 (30.7) | 25−37 |
| Chronic renal dysfunction, n (%) | 14 (6.1) | 3.7−10.1 |
| Immunosuppression, n (%) | 16 (7) | 4.2−11.1 |
| Atrial Fibrillation, n (%) | 11 (4.8) | 2.7−8.5 |
| Antiplatelet therapy, n (%) | 12 (5.3) | 3−9.1 |
| Therapies | ||
| ECMO therapy, n (%) | 20 (8.8) | 5.7−13.2 |
| Invasive Mechanical Ventilation, n (%) | 200 (87.7) | 82.8−91.4 |
| Tocilizumab, n (%) | 55 (24.1) | 19−30.1 |
| Dexamethasone, n (%) | 160 (70.2) | 63.8−76 |
| Tocilizumab and dexamethasone, n (%) | 31 (13.6) | 9.7−18.7 |
| Prophylactic enoxaparin, n (%) | 151 (66.2) | 59.9−72.1 |
| Therapeutic enoxaparin, n (%) | 15 (6.6) | 4−10.6 |
| Prophylactic (first), therapeutic (second) enoxaparin, n (%) | 62 (27.2) | 21.8−33.3 |
| Outcomes | ||
| Shock, n (%) | 35 (15.4) | 11.2−20.7 |
| Acute Kidney Injury, n (%) | 44 (19.3) | 14.7−25 |
| ARC, n (%) | 78 (34.2) | 28.3−40.1 |
| Bleeding complications, n (%) | 11 (4.8) | 2.7−8.5 |
| Length of ICU stay (days), median | 22.5 | 19−27 |
| Death, n (%) | 55 (24.1) | 19−30.1 |
APACHE II: Acute physiology and chronic health evaluation II. ECMO: Extracorporeal membrane oxygenation. ARC: Augmented renal clearance (GFR above 130 ml/min/1.73 m2).
Most patients needed invasive mechanical ventilation (87.7%; 95% CI, 82.8−91.4) and 8.8% (95% IC, 5.7−13.2) needed ECMO support. Many patients (70.2%; 95% CI, 63.8−76) were receiving dexamethasone treatment and 24.1% (95% CI, 19−30.1) received tocilizumab. All patients were under enoxaparin treatment: 6.6% (95% CI, 4−10.6) at therapeutic doses, 66.2% (95% CI, 59.9−72.1) at prophylactic doses because no new thromboembolic event was diagnosed, and 27.2% (21.8−33.3) at prophylactic doses first and at therapeutic doses when a new thromboembolic event was diagnosed.
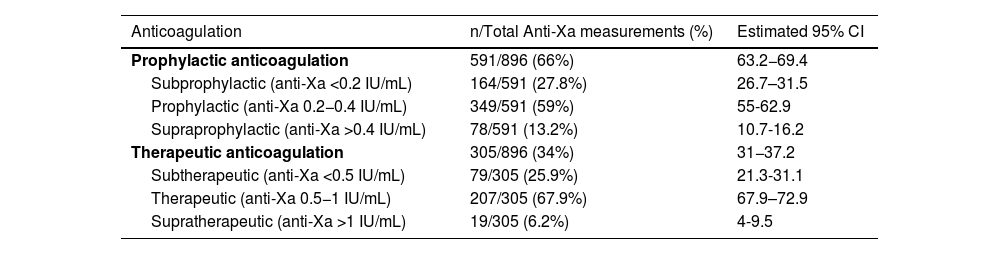
Anti-Xa measurementsA total of 896 anti-Xa measurements were performed, and 27.1% (95% CI, 24.3–30.1) of them were below range, 62.1% (95% CI, 58.8–65.2) were within range and 10.8% (8.9–13) were above range.
When prophylactic enoxaparin was used, 27.8% (95% CI, 26.7–31.5) were subprophylactic and when therapeutic enoxaparin was used, 25.9% (95% CI, 21.3–31.1) were subtherapeutic (Table 2).
Descriptive ranges of anti-Xa levels.
| Anticoagulation | n/Total Anti-Xa measurements (%) | Estimated 95% CI |
|---|---|---|
| Prophylactic anticoagulation | 591/896 (66%) | 63.2−69.4 |
| Subprophylactic (anti-Xa <0.2 IU/mL) | 164/591 (27.8%) | 26.7–31.5 |
| Prophylactic (anti-Xa 0.2−0.4 IU/mL) | 349/591 (59%) | 55-62.9 |
| Supraprophylactic (anti-Xa >0.4 IU/mL) | 78/591 (13.2%) | 10.7-16.2 |
| Therapeutic anticoagulation | 305/896 (34%) | 31−37.2 |
| Subtherapeutic (anti-Xa <0.5 IU/mL) | 79/305 (25.9%) | 21.3-31.1 |
| Therapeutic (anti-Xa 0.5−1 IU/mL) | 207/305 (67.9%) | 67.9–72.9 |
| Supratherapeutic (anti-Xa >1 IU/mL) | 19/305 (6.2%) | 4-9.5 |
The median number of measurements was 3 per patient. Only four patients had one measurement, and two patients had fifteen measurements.
Many new thromboembolic events were diagnosed at the first (39.4%; 95% CI, 28.5–51.5), second (34.8%; 95% CI, 24.5–46.9) and third (16.7%; 9.6–27.4) anti-Xa measurements (Table S5, supplementary material). Most anti-Xa level were within range after the third measurement.
Bivariate analysisCrude relative risk of new thromboembolic eventsThe incidence of new PE and new DVT when anti-Xa activity was below range was 4.9% (crude RR 8.7; p = 0.005) and 12.4% (crude RR 5.5; p = 0.000), respectively (Table 3).
Incidence and RR of new thromboembolic events when anti-Xa is below range.
| Variables | Incidence | RR | Estimated 95% CI | p |
|---|---|---|---|---|
| New Thromboembolic Events | 66/896 (7.4%) | |||
| Below range | 45/243 (18.5%) | 6 | 3.4−10.7 | 0.000 |
| Within range | 15/556 (2.7%) | |||
| Prophylactic anticoagulation | 35/591 (5.9%) | |||
| Subprophylactic | 28/164 (17.1) | 8.2 | 3.4−19.6 | 0.000 |
| Prophylactic | 4/349 (1.1) | |||
| Therapeutic anticoagulation | 31/305 (10.2%) | |||
| Subtherapeutic | 17/79 (21.5%) | 4.9 | 2.2−11 | 0.000 |
| Therapeutic | 11/207 (5.3) | |||
| New Pulmonary embolism | 16/896 (1.8%) | |||
| Below range | 12/243 (4.9%) | 8.7 | 1.9−39.8 | 0.005 |
| Within range | 2/556 (0.4%) | |||
| Prophylactic anticoagulation | 10/591 (%) | |||
| Subprophylactic | 9/164 (5.5%) | 14.6 | 1.5−144.2 | 0.02 |
| Prophylactic | 0/349 (0%) | |||
| Therapeutic anticoagulation | 6/305 (2%) | |||
| Subtherapeutic | 3/79 (3.8%) | 9 | 0.6−143.9 | 0.12 |
| Therapeutic | 2/207 (1%) | |||
| New Deep Vein Thrombosis | 44/896 (4.9%) | |||
| Below range | 30/234 (12.4%) | 5.5 | 2.8−10.9 | 0.000 |
| Within range | 11/556 (2%) | |||
| Prophylactic anticoagulation | 25/591 (4.2%) | |||
| Subprophylactic | 19/164 (11.6%) | 6.8 | 2.6−17.6 | 0.000 |
| Prophylactic | 4/349 (1.2%) | |||
| Therapeutic anticoagulation | 19/305 (6.2%) | |||
| Subtherapeutic | 11/79 (13.9%) | 4.6 | 1.7−12.4 | 0.002 |
| Therapeutic | 7/207 (3.4%) | |||
| New Arterial Thrombosis | ||||
| Therapeutic anticoagulation | 6/305 (2%) | |||
| Subtherapeutic | 3/79 (3.8%) | 3.5 | 0.6−21.2 | 0.17 |
| Therapeutic | 2/207 (1%) |
The incidence of new AT when therapeutic enoxaparin was used was 3.8% (crude RR 3.5; 95% CI 0.6–21.5; p = 0.17) (Table 3).
Crude relative risk of severe hemorrhageOnly 1 patient suffered a severe hemorrhage when anti-Xa was above range and the patient was under therapeutic anticoagulation.
Bivariate analysis for risk of new thromboembolic eventsNew thromboembolic events were related to illness severity (APACHE II; RR, 1.1; 95% CI, 1.01–1.12; p = 0.03), ECMO support (RR, 3.06; 95% CI, 1.2–7.77; p = 0.02), the ARC (augmented renal clearance) (glomerular filtration rate above 130 ml/minute per 1.73 m2) (RR, 3; 95% CI,1.42–6.29; p = 0.004), increased D-dimer (RR, 1.0001, 95% CI, 1.00003–1.0001; p = 0.000), serum inflammation-related parameters such as C-reactive protein (RR, 1.007, 95% CI, 1.004–1.009; p = 0.000) and anti-Xa activity below range (RR, 6, 95% CI, 3.4−10.7; p = 0.000) (Table S6).
Clinical situations that were found to protect against new thromboembolic events included a GFR between 90 and 130 ml/minute/ 1.73 m2 (RR, 0.49; 95% CI, 0.26−0.94; p = 0.03) compared with a GFR under 90 ml/minute per 1.73 m2 and tocilizumab treatment (RR 0.51; 95% CI, 0.25−0.99; p = 0.04) (Table S6, supplementary material).
Multivariable analysisThe variables included to perform the best model for multivariable analysis were APACHE II score, ECMO, ARC, tocilizumab treatment, D-dimer, C-reactive protein, IL-6, LDH and anti-Xa levels below range. After applying the all-possible-subset method for logistic regression, the model included ARC, D-dimer, C-reactive protein and anti-Xa levels below range. Multicollinearity was checked for all those variables and because the variance inflation factors (VIFs) between the ARC and anti-Xa level and between the ARC and C-reactive protein were high (6.2 and 5.4, respectively), the ARC was discarded from the final model. Therefore, the best final model included anti-Xa below range, D-dimer and C-reactive protein levels.
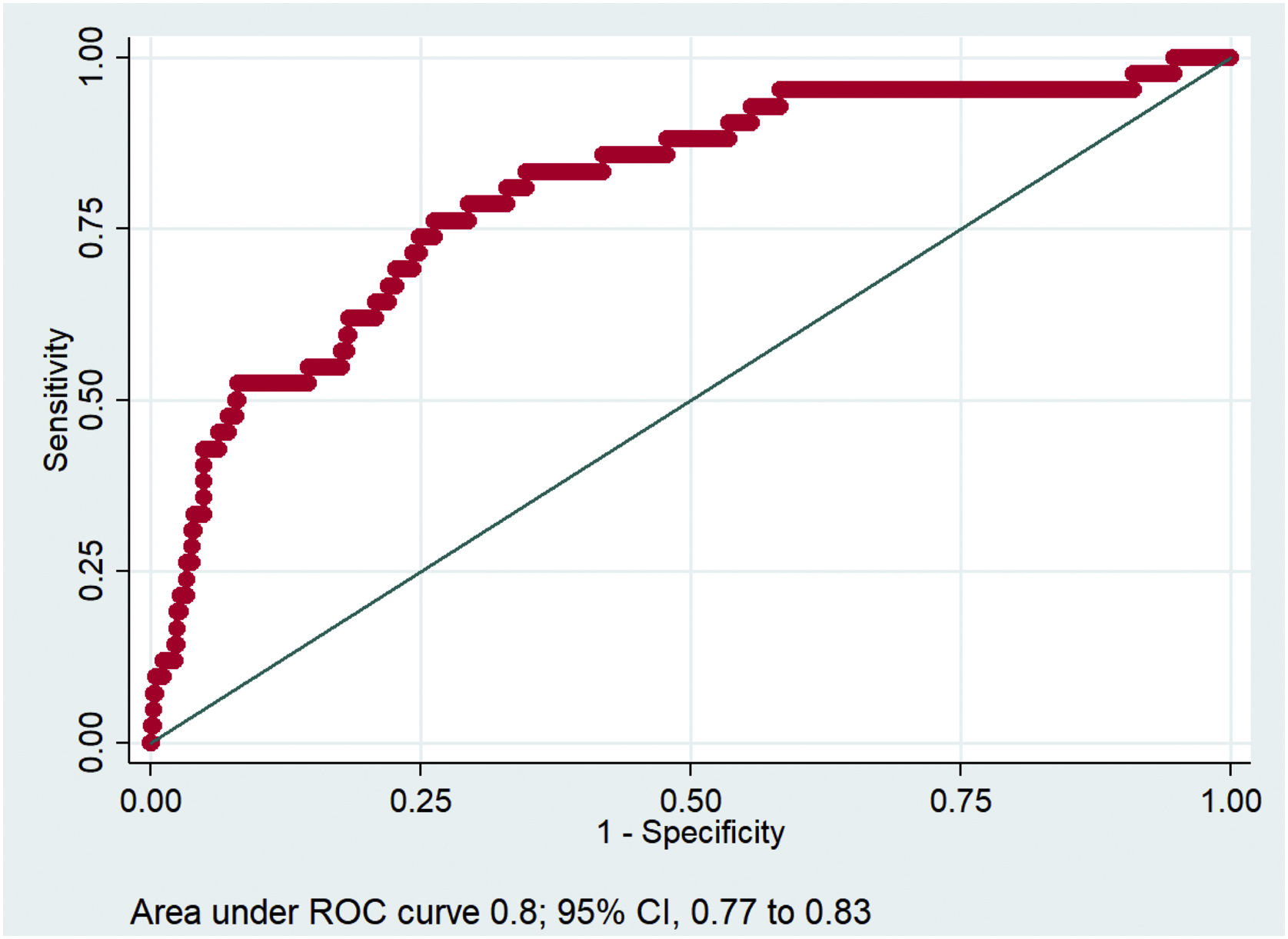
Multivariable logistic regression analysis revealed that anti-Xa activity below range (RR, 4.2; 95% CI, 2.01−8.91; p = 0.000), C-reactive protein (25 mg/L increase] (RR 1.14; 95% CI, 1.04−1.24; p = 0.005) and D-dimer (1000 ng/L increase) (RR, 1.06; 95% CI, 1.02.1.09; p = 0.002) were the independent factors related to new thromboembolic events in patients with severe COVID-19 (Table 4).
The resulting predictive model forecasted the probability of developing new thromboembolic events with a good ROC curve (Fig. 2).
DiscussionOur findings suggested that patients admitted to the ICU because of severe COVID-19 were at high risk of developing thromboembolic events, partly due to inadequate subprophylactic or subtherapeutic anticoagulation treatment. Because the real incidence of thromboembolic events was lower (27.2%) than the expected (42.6%), the power of the study was better (close to 99%), so these findings should be considered. This is the largest study relating COVID-19 thrombosis to anti-Xa activity below range.
Taccone et al.31 suggested that high-dose thromboprophylaxis may decrease the occurrence of pulmonary embolism in ventilated COVID-19 patients, and observed that patients treated with high-dose thromboprophylaxis had significantly greater anti-Xa activity than those treated with standard thromboprophylaxis, but the INSPIRATION trial18 failed to demonstrate a reduction in thromboembolic events with intermediate-dose prophylactic anticoagulation compared to standard prophylactic anticoagulation, without taking into account anti-Xa measurements. Probably, the main outcome of these studies should not have been standard, intermediate or high-dose thromboprophylaxis, but rather targeted anti-Xa activity.
The message from our study is that we should not only anticoagulate patients with severe COVID-19 according to weight and renal function, but also individualize anticoagulation (prophylactic and therapeutic) according to proper monitoring, as we do in routine clinical practice with other drugs such as antibiotics.32
Our study revealed that many new thromboembolic events were diagnosed during the first three anti-Xa adjustments. If we did not monitor the anti-Xa level, we would not have changed the enoxaparin dose, and more thromboses would likely have been diagnosed.
Most anti-Xa levels were within range after the third measurement, so it may not have been necessary to monitor anti-Xa levels throughout ICU stay, if the patient remained stable.
Because anti-Xa monitoring may be important for reducing the incidence of thromboembolic events in severe COVID-19 patients, a second analysis from this study will be performed to determine which factors were related to anti-Xa activity below range.
In this study, the cumulative incidence of thromboembolic events was high (37.3%; 95% CI, 31.3–43.7), as were the incidence of new thromboembolic events diagnosed (28.9%; 95%, CI 23.4–35.1). These results agree with other reports (30%,8 42.6%10 and 40% in a cohort of 100 COVID-19 autopsies11). In our cohort, arterial thrombosis (2.6%) was only related to the catheter line, differing from other studies that reported up to 3% myocardial or cerebral thromboses.9
As other authors reported,10 the crude relative risk of thrombosis in COVID-19 patients receiving ECMO therapy was high (RR, 3.06; 95% CI, 1.2–7.77) because of their inflammatory state and the absence of filter clotting during therapy.
New thromboembolic events were also related to increased fibrinogen degradation products (D-Dimer), reflecting the hypercoagulation state of patients with severe COVID-19,5,6,8,10 and to classical inflammatory biomarkers, such as C-reactive protein, leading to heparin-resistance because of inflammation and impaired enoxaparin absorption due to vasoconstriction induced by shock and vasopressors.22,23,27,33
Our study had several limitations. The results should be viewed with caution, as an observational study is unable to control potentially confounding variables. Furthermore, the multivariable analysis could have been influenced by other unknown variables related to severe COVID-19. In addition, there was only one treatment group because we did not consider it ethical to conduct the study with a control group without anti-Xa monitoring, because it was a usual clinical practice, and we did not consider it appropriate to conduct the study with a retrospective control group because data collection in previous COVID-19 waves was not strictly reported. Moreover, mortality was not considered a competing endpoint and PE may be underdiagnosed.
Purposely designed clinical trials should be carried out to confirm the benefit of anti-Xa monitoring.
ConclusionsIn conclusion, anti-Xa activity below range, increased C-reactive protein and D-dimer levels were the independent factors related to new thromboembolic events in patients with severe COVID-19.
Author contributionsStudy design: PMN, CMI, MFC, TTI, LBL.
Data acquisition: PMN, CMI, VRA.
Analysis and interpretation of data: PMN, CMI, MAFC, LBL.
Quality assessment: CMI, MFC, TTI, LBL.
Drafting the manuscript: PMN, CMI, MFC, TTI, VRA.
Revision for important intellectual content: PMN, CMI, MFC, LBL, TTI.
FundingThe current work was not funded.
Competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributionsStudy design: Pilar Marcos-Neira, Cristian Morales-Indiano, Mariana Fernández-Caballero, Teresa Tomasa-Irriguible, Luisa Bordejé-Laguna.
Data acquisition: Pilar Marcos-Neira, Cristian Morales-Indiano, Víctor Ruíz-Artola.
Analysis and interpretation of data: Pilar Marcos-Neira, Cristian Morales-Indiano, Mariana Fernández-Caballero, Luisa Bordejé-Laguna.
Quality assessment: Cristian Morales-Indiano, Mariana Fernández-Caballero, Teresa Tomasa-Irriguible, Luisa Bordejé-Laguna.
Drafting the manuscript: Pilar Marcos-Neira, Cristian Morales-Indiano, Mariana Fernández-Caballero, Teresa Tomasa-Irriguible, Víctor Ruíz-Artola.
Revision for important intellectual content: Pilar Marcos-Neira, Cristian Morales-Indiano, Mariana Fernández-Caballero, Luisa Bordejé-Laguna, Teresa Tomasa-Irriguible.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors gratefully thank the collaboration of the ICU nurses, the staff of the Department of Clinical Analysis and Biochemistry and the intensive care doctors for collecting many blood samples, determining anti-Xa activity and performing ultrasound during the large third wave of the pandemic in Spain.