Antiplatelet therapy (AT) is increasingly used for treating or preventing vascular diseases, especially as a consequence of population aging. However, the risks may sometimes outweigh the benefits, mostly in relation to intracranial hemorrhage (ICH). Our aim was to determine whether AT is associated with hematoma enlargement and increased mortality in ICH.
DesignA prospective, observational cohort study.
SettingThe Intensive Care Unit (ICU) of Arrixaca University Hospital (Murcia, Spain).
PatientsWe studied 156 patients admitted with non-traumatic ICH between January 2006 and August 2008.
InterventionsNone.
Main variablesDemographic data, medical history and clinical and laboratory parameters were recorded, along with hematoma volume upon admission and after 24h, and mortality.
ResultsA total of 37 patients (24%) received AT. These subjects were older (69±11 vs. 60±15 years, p=0.001) and more frequently diabetic (38% vs. 15%, p=0.003) than those without AT. We detected no difference in hematoma volume upon admission between the two groups, though the volume was significantly greater after 24h in the AT group (66.7 [IQR 42–110] vs. 27 [4.4–64.6]cm3, p=0.03), irrespective of surgical intervention. Moreover, hematoma volume increased by more than a third in AT-users (69% vs. 33%, p=0.002), and AT was the only significant predictor of hematoma enlargement. Patients on AT also had higher mortality during their ICU stay (78% vs. 45%, p<0.001). In addition, of the patients with hematoma enlargement, over one-third had higher overall mortality (62.5 vs. 28.8%, p=0.001). Independent risk factors for death were the Glasgow Coma Scale score, blood glucose upon admission, and AT.
ConclusionsOur results show an association between AT and subsequent hematoma enlargement, as well as increased mortality in patients presenting with ICH who were receiving AT.
Con el envejecimiento progresivo de la población cada es más frecuente la toma de fármacos antiagregantes para el tratamiento o la prevención de las enfermedades vasculares. El beneficio, en ocasiones, está contrarrestado por el riesgo de hemorragias, especialmente la hemorragia intracraneal (HIC). Nuestro objetivo fue determinar si el tratamiento antiagregante (TAG) provoca un aumento del tamaño del hematoma y de mortalidad en la HIC.
DiseñoEstudio de cohortes prospectivo y observacional.
ÁmbitoUnidad de cuidados intensivos (UCI) del Hospital Universitario Virgen de la Arrixaca (Murcia).
PacientesEstudiamos a 156 pacientes que ingresaron por HIC no traumática entre Enero de 2006 y Agosto de 2008.
IntervencionesNinguna.
Principales variablesSe recogieron datos demográficos, antecedentes personales, parámetros clínicos y analíticos, así como, el volumen del hematoma al ingreso y a las 24 horas, además de la mortalidad.
ResultadosEntre los pacientes estudiados, 37 (24%) tomaban TAG. Los antiagregados eran de mayor edad (69±11 vs 60±15 años, p=0,001) y con mayor frecuencia diabéticos (38 vs 15%, p=0,003). No hubo diferencias en el volumen del hematoma al ingreso entre los dos grupos pero este fue significativamente mayor a las 24 horas en los antiagregados (66.7 [IQR 42-110] vs 27 [4.4-64.6] cm3, p=0.03), independientemente de si fueron intervenidos o no. Además, el volumen del hematoma creció en más de un 33% en los antiagregados (69 vs 33%, p=0,002) y el TAG fue el único predictor significativo del crecimiento del hematoma. Los pacientes antiagregados también presentaron una mayor mortalidad durante su estancia en la UCI (78 vs 45%, p<0,001). Además, los pacientes con crecimiento del hematoma mayor de un 33% tuvieron una mayor mortalidad global (62,5 vs 28,8%, p=0,001). Los factores independientemente asociados con la mortalidad fueron la puntuación en la escala de Glasgow, la glucemia al ingreso y el TAG.
ConclusionesNuestros resultados muestran tanto la asociación entre el TAG y el crecimiento del hematoma como con el incremento de la mortalidad en los pacientes que sufren una HIC y están tomando antiagregantes.
Non-traumatic intracranial hemorrhage (ICH) is caused by acute blood extravasation in cerebral parenchyma due to a spontaneous vascular rupture that usually originates from arterioles or small arteries. Its position is variable and it can be limited to cerebral parenchyma or it may reach both the ventricular system or subarachnoid space. Despite hemorrhagic stroke being less frequent (15–20%) than ischemic stroke (atherothrombotic and embolic), its mortality (35–52% at 30 days) and morbidity are higher and only 10% of patients are living independently after one month and 20% after six months.1–6
Among the overall risk factors for ICH, the most important is hypertension7,8 irrespective of age, gender and ethnicity. Others include alcohol, smoking, previous anticoagulant and antiplatelet therapy, cerebral amyloid angiopathy and genetic factors (mutations of the gene codifying coagulation factor XIII alpha subunit).3,8,9 With an increasingly aging population, there are more patients with spontaneous ICH who are taking either anticoagulant or antiplatelet therapy (AT) for chronic diseases.10,11
Despite AT being the main pharmacological option for vascular disease prevention,12–14 its benefits may be undermined by the risk of bleeding.15–17,11 Previous studies have shown that patients taking anticoagulant therapy at ICH onset have an increased risk of hematoma enlargement and have a worse prognosis compared with those not on anticoagulant treatment.11,18,19 Additionally, it has been reported that hematoma enlargement is an independent predictor of mortality and poor functional outcome.20–22 In a recent meta-analysis of 218 patients with spontaneous ICH, patients had a head computerized tomographic (CT) scan within 3h of onset and another after 24h and hematoma enlargement was found to be associated with a higher mortality and poorer functional outcome.22 Notwithstanding the above, the possible influence of AT on ICH outcome remains controversial.15,23 The purpose of our study, therefore, was to establish if previous AT is associated with a higher mortality and hematoma enlargement in patients with ICH.
Material and methodsWe prospectively and consecutively studied all patients with ICH admitted to the Intensive Care Unit (ICU) of the Arrixaca University Hospital Murcia, Spain between January 2006 and August 2008. This is the only regional neurosurgical referral centre in Murcia, serving eight secondary hospitals with a total population of 1.4 million.
All patients with a spontaneous ICH were included in the study (ICH located in lobes, hemispheric deep structures, brainstem and cerebellum). Patients with traumatic and subarachnoid hemorrhage, hemorrhagic transformation following an ischemic stroke, cerebral tumors and cerebral venous thromboses, as well as central nervous system vasculitis were excluded.
Neurological deficit on admission was assessed using the National institute of Health Stroke Scale (NIHSS) and consciousness level was graded according to the Glasgow Coma Scale (GCS)24 with coma defined as GCS≤8. A CT scan was performed on admission and again at 24–48h.
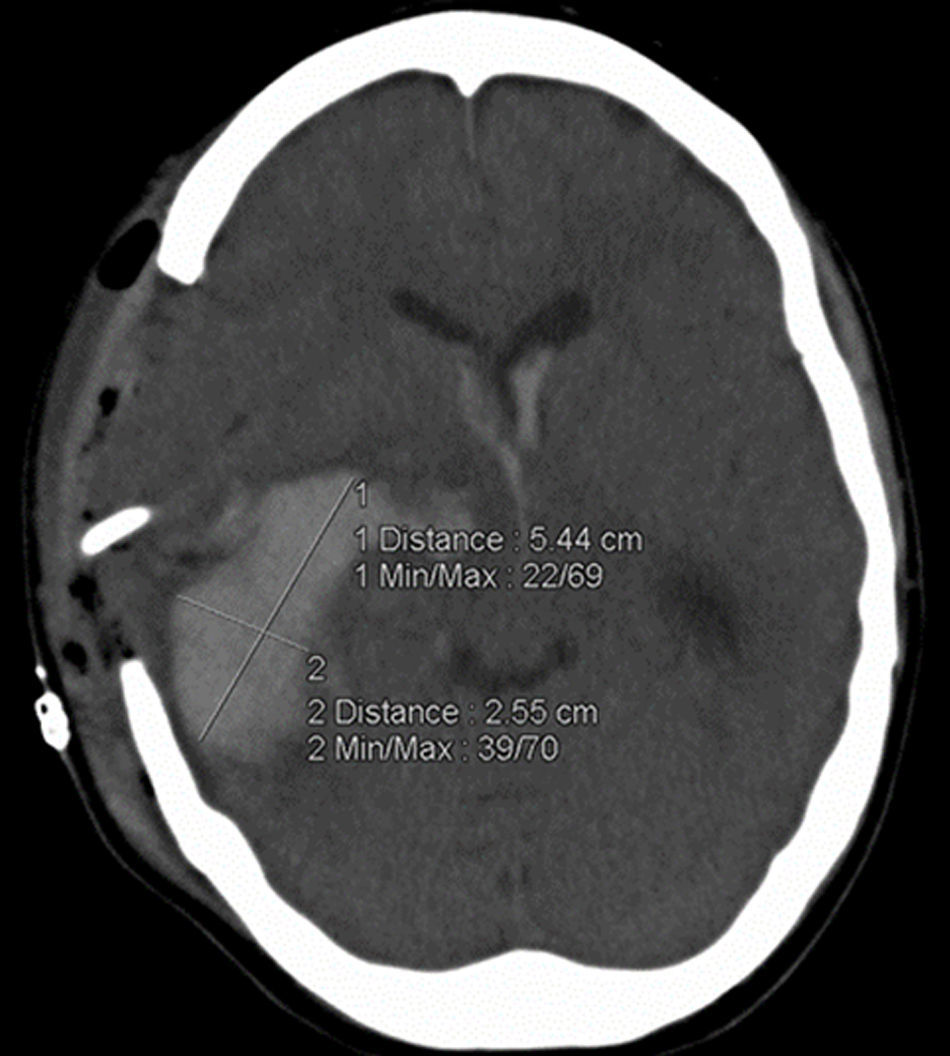
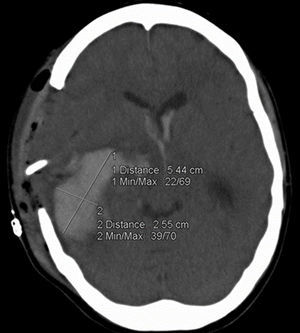
Demographic data, including age, gender, functional status at hospital discharge (measured by the modified Rankin Scale, mRS), history of hypertension, diabetes mellitus (DM), smoking, alcohol and non-prescription drug history, and current or previous anticoagulant or antiplatelet treatment were recorded on admission. Measurements of systolic and diastolic blood pressure, GCS score, NIHSS score, pupillary reflexes and morphology and acute physiology and chronic health evaluation (APACHE) II score were also recorded. Laboratory analysis included random blood glucose, platelet count, prothrombin activity, activated partial thromboplastin time and International Normalized Ratio (INR). Hematoma volume was measured on the admission CT scan and on that performed approximately 24h later by experienced ICU clinicians using the ABC/2 method (fig. 1).25 Timing between ICH onset and CT scan was measured in minutes and that between admission CT and follow-up CT in hours. If the patient was initially admitted to another hospital and later transferred, we considered the first CT scan in the admitting hospital as the admission scan. Hematoma enlargement was defined as the difference in hematoma volume between the admission and follow-up CT and a 33% increase in volume between both scans was regarded as clinically significant.17,20,22,26 Mortality during ICU admission was recorded as was surgical intervention.
Patients were grouped according to whether or not they were taking AT at ICH onset. Those taking aspirin and/or clopidogrel up to seven days before the hemorrhagic event were included in the AT group. Patients were subsequently analyzed and compared according to whether or not they had a significant hematoma enlargement and mortality. No patient received a platelet transfusion during hospitalization.
End-points were defined as significant hematoma enlargement within and between AT and non-AT patients and mortality during their ICU stay.
Statistical analysisFor statistical analysis, we used the statistical program SPSS, version 15.0 and we considered p≤0.05 as significant. Normally distributed continuous quantitative variables are reported as means±standard deviation (SD) and non-normally distributed data as median and interquartile range [IQR]. Qualitative variables are presented as numbers and percentages (%). Quantitative variables were compared between the groups using Student t test when normally distributed and non-parametric Mann–Whitney U-test in cases of deviation from normal distribution. Qualitative variables were compared by χ2 test. The influence of AT or evacuation surgery on changes in hematoma volume was analyzed by a multivariate general linear model using Wilks’ lambda statistic test. Similarly, data analysis was performed using analysis of covariance (ANCOVA), with AT, volume on admission and evacuation surgery as covariates, to evaluate differences in follow-up hematoma volume. In addition, in order to control for possible confounding factors and to identify those associated independently with significant hematoma enlargement and mortality, we performed a logistic regression analysis, including variables reaching p<0.05 in univariate analysis as a candidate selection screening criterion.
ResultsDuring the study period 1426 patients were admitted to our ICU of which 471 had a neurological condition and spontaneous non-traumatic hemorrhagic stroke was diagnosed in 221. Of these, 55 cases of subarachnoid hemorrhage, five of hemorrhage following an ischemic stroke and three from a tumor as well as two venous sinus thromboses were excluded thus leaving 156 patients with a confirmed diagnosis of spontaneous ICH as the study population.
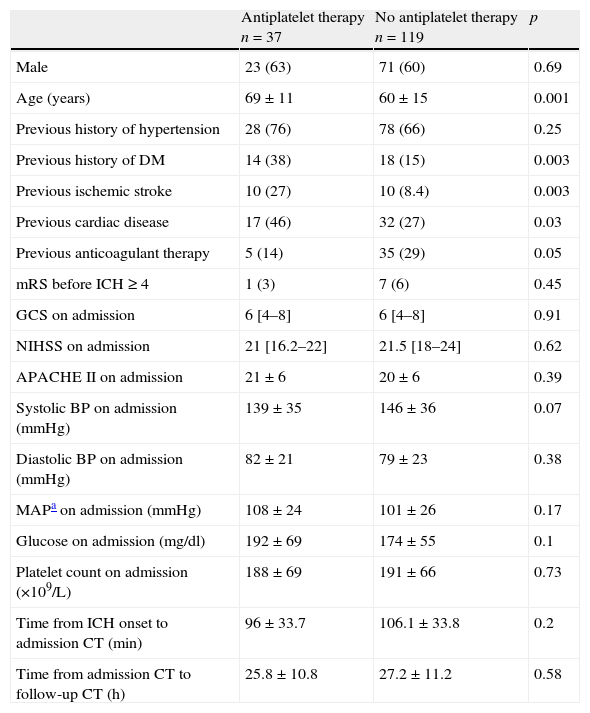
Ninety-four (60.3%) were men and 62 (39.7%) were women and, at the time of ICH onset, 37 patients (23%) were on AT taking either aspirin (67.7%), clopidogrel (13.5%) or both (18.9%). Patients on AT were older and more frequently diabetic, whereas non-AT patients were more frequently on anticoagulant therapy than patients on AT (Table 1). There was no significant difference in the intervals between ICH onset and the admission CT in both groups nor between their admission and follow-up CT. Emergency evacuation surgery was performed on 64 patients, 12 in the AT group (32.4%) and 52 in the non-AT group (43.7%) (p=0.22).
Baseline patient characteristics.
| Antiplatelet therapy n=37 | No antiplatelet therapy n=119 | p | |
| Male | 23 (63) | 71 (60) | 0.69 |
| Age (years) | 69±11 | 60±15 | 0.001 |
| Previous history of hypertension | 28 (76) | 78 (66) | 0.25 |
| Previous history of DM | 14 (38) | 18 (15) | 0.003 |
| Previous ischemic stroke | 10 (27) | 10 (8.4) | 0.003 |
| Previous cardiac disease | 17 (46) | 32 (27) | 0.03 |
| Previous anticoagulant therapy | 5 (14) | 35 (29) | 0.05 |
| mRS before ICH≥4 | 1 (3) | 7 (6) | 0.45 |
| GCS on admission | 6 [4–8] | 6 [4–8] | 0.91 |
| NIHSS on admission | 21 [16.2–22] | 21.5 [18–24] | 0.62 |
| APACHE II on admission | 21±6 | 20±6 | 0.39 |
| Systolic BP on admission (mmHg) | 139±35 | 146±36 | 0.07 |
| Diastolic BP on admission (mmHg) | 82±21 | 79±23 | 0.38 |
| MAPa on admission (mmHg) | 108±24 | 101±26 | 0.17 |
| Glucose on admission (mg/dl) | 192±69 | 174±55 | 0.1 |
| Platelet count on admission (×109/L) | 188±69 | 191±66 | 0.73 |
| Time from ICH onset to admission CT (min) | 96±33.7 | 106.1±33.8 | 0.2 |
| Time from admission CT to follow-up CT (h) | 25.8±10.8 | 27.2±11.2 | 0.58 |
Variables are presented as number (%), mean±SD or median [IQR].
Mean arterial pressure.
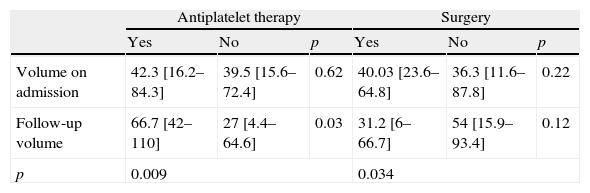
As regards the admission CT, there was no significant difference in the hematoma volume between those patients taking AT and those who were neither (42.3 [IQR 16.2–84.3] vs. 39.5 [IQR 15.6–72.4]cm3, p=0.62), nor was between patients who had evacuation surgery and those who did not (40.03 [IQR 23.6–64.8] vs. 36.3 [IQR 11.6–87.8]cm3, p=0.22) (Table 2). However, the follow-up scan showed a significant difference in volume between patients on AT and not on AT (66.7 [IQR 42–110] vs. 27 [IQR 4.4–64.6]cm3, p=0.03). In addition, patients taking AT had an increase of hematoma volume in the follow-up CT (42.3 [IQR 16.2–84.3] vs. 66.7 [IQR 42–110]cm3, p=0.009), whereas it was smaller in patients who had evacuation surgery (40.03 [IQR 23.6–64.8] vs. 31.2 [IQR 6–66.7]cm3, p=0.034) (Table 2). The interaction between AT and surgery on hematoma enlargement was not significant (Wilks’ Lambda=0.99, p=0.91). Similarly, the ANCOVA modeling performed to test the effect of AT in hematoma volume in follow-up scans shows that AT is related to a volume increase (B=29.54, 95% CI: 10.5–48.5, p=0.003), independently of volume on admission (B: 0.52 95% IC 0.3–0.74, p<0.001) and evacuation surgery (B: −24.2, 95% CI: −41.8 to −6.5, p=0.008).
Effect of AT and evacuation surgery on hematoma volume (cm3) on admission and follow-up CT.
| Antiplatelet therapy | Surgery | |||||
| Yes | No | p | Yes | No | p | |
| Volume on admission | 42.3 [16.2–84.3] | 39.5 [15.6–72.4] | 0.62 | 40.03 [23.6–64.8] | 36.3 [11.6–87.8] | 0.22 |
| Follow-up volume | 66.7 [42–110] | 27 [4.4–64.6] | 0.03 | 31.2 [6–66.7] | 54 [15.9–93.4] | 0.12 |
| p | 0.009 | 0.034 | ||||
Values shown as median [IQR].
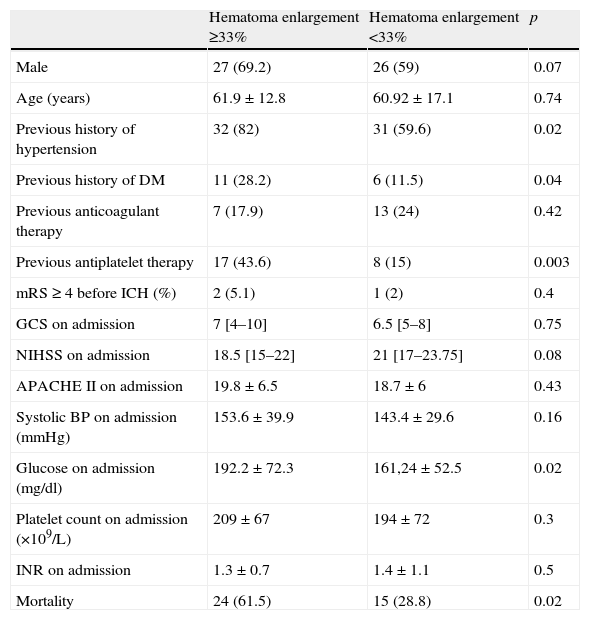
As regards hematoma enlargement, 26% of our study patients reached the criteria of an increase of over a third in hematoma volume between the admission and follow-up scans and almost 45% of the AT group reached this criteria compared with only 18% of those not on AT (p=0.003). Patients with significant hematoma enlargement had more often a history of hypertension (82% vs. 59.6%, p=0.02) and diabetes (28.2% vs. 11.5%, p=0.04), had a higher blood glucose level on admission (192.2±72.3 vs. 161.24±52.5mg/dl, p=0.02), were more often on AT before ICH onset (43.6% vs. 15%, p=0.003) and had a higher mortality (61.5 vs. 28.8%, p=0.02) (Table 3). In the logistic regression analysis of hematoma enlargement of greater than a third, only previous AT was identified as being independently associated (OR: 3.49, CI 95%: 1.19–10.20, p=0.023).
Hematoma volume enlargement of more than a third in ICH patients using univariate analysis.
| Hematoma enlargement ≥33% | Hematoma enlargement <33% | p | |
| Male | 27 (69.2) | 26 (59) | 0.07 |
| Age (years) | 61.9±12.8 | 60.92±17.1 | 0.74 |
| Previous history of hypertension | 32 (82) | 31 (59.6) | 0.02 |
| Previous history of DM | 11 (28.2) | 6 (11.5) | 0.04 |
| Previous anticoagulant therapy | 7 (17.9) | 13 (24) | 0.42 |
| Previous antiplatelet therapy | 17 (43.6) | 8 (15) | 0.003 |
| mRS ≥ 4 before ICH (%) | 2 (5.1) | 1 (2) | 0.4 |
| GCS on admission | 7 [4–10] | 6.5 [5–8] | 0.75 |
| NIHSS on admission | 18.5 [15–22] | 21 [17–23.75] | 0.08 |
| APACHE II on admission | 19.8±6.5 | 18.7±6 | 0.43 |
| Systolic BP on admission (mmHg) | 153.6±39.9 | 143.4±29.6 | 0.16 |
| Glucose on admission (mg/dl) | 192.2±72.3 | 161,24±52.5 | 0.02 |
| Platelet count on admission (×109/L) | 209±67 | 194±72 | 0.3 |
| INR on admission | 1.3±0.7 | 1.4±1.1 | 0.5 |
| Mortality | 24 (61.5) | 15 (28.8) | 0.02 |
Variables are presented as number (%), mean±SD or median [IQR].
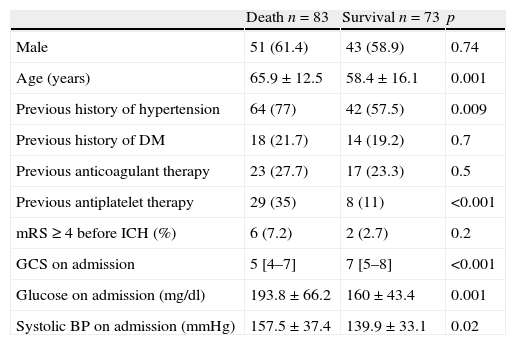
Overall mortality was 53.2%. It was higher in older patients, those with a history of hypertension, those on AT at onset (35% vs. 11%, p<0.001) and those with a higher systolic BP, blood glucose level and lower GCS score on admission (Table 4).
ICU mortality in patients with ICH using univariate analysis.
| Death n=83 | Survival n=73 | p | |
| Male | 51 (61.4) | 43 (58.9) | 0.74 |
| Age (years) | 65.9±12.5 | 58.4±16.1 | 0.001 |
| Previous history of hypertension | 64 (77) | 42 (57.5) | 0.009 |
| Previous history of DM | 18 (21.7) | 14 (19.2) | 0.7 |
| Previous anticoagulant therapy | 23 (27.7) | 17 (23.3) | 0.5 |
| Previous antiplatelet therapy | 29 (35) | 8 (11) | <0.001 |
| mRS≥4 before ICH (%) | 6 (7.2) | 2 (2.7) | 0.2 |
| GCS on admission | 5 [4–7] | 7 [5–8] | <0.001 |
| Glucose on admission (mg/dl) | 193.8±66.2 | 160±43.4 | 0.001 |
| Systolic BP on admission (mmHg) | 157.5±37.4 | 139.9±33.1 | 0.02 |
Variables are presented as number (%), mean±SD or median [IQR].
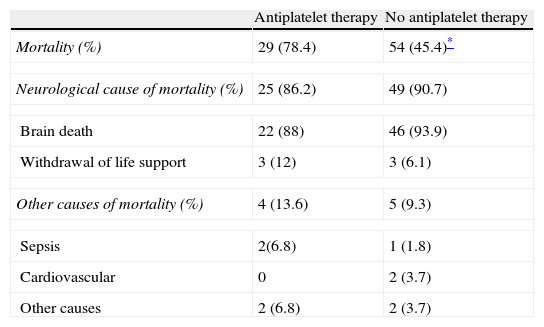
Mortality in AT patients was higher than in those not on AT (78.4% vs. 45.4%, p<0.001). In both groups, the most frequent cause of death was related to the underlying neurological lesion and there was no difference between both groups (86% vs. 91%, p=0.7) (Table 5). There was also a higher mortality in those patients with a significant change in hematoma volume (61.5% vs. 28.8%, p=0.001).
Mortality in AT and non-AT patients.
| Antiplatelet therapy | No antiplatelet therapy | |
| Mortality (%) | 29 (78.4) | 54 (45.4)* |
| Neurological cause of mortality (%) | 25 (86.2) | 49 (90.7) |
| Brain death | 22 (88) | 46 (93.9) |
| Withdrawal of life support | 3 (12) | 3 (6.1) |
| Other causes of mortality (%) | 4 (13.6) | 5 (9.3) |
| Sepsis | 2(6.8) | 1 (1.8) |
| Cardiovascular | 0 | 2 (3.7) |
| Other causes | 2 (6.8) | 2 (3.7) |
Variables are presented as number (%).
p<0.001
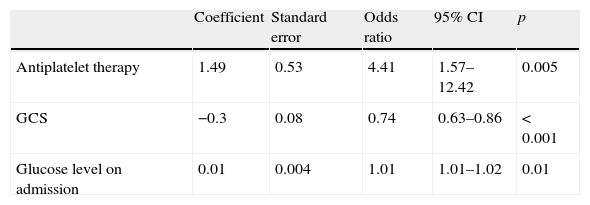
After multivariate adjustment, lower GCS score and blood glucose level on admission were significant independent predictors of mortality, as was AT (Table 6).
DiscussionAtherothrombotic disease, which includes coronary heart, cerebrovascular and peripheral arterial disease, is the leading cause of death in most developed countries.12 Evidence from epidemiological studies and randomized clinical trials has provided strong support for the role of AT in reducing the risk of morbidity and mortality in diverse patient groups.12,14 Nowadays, given the growing prevalence of atherothrombotic disease, AT is being used with increasing frequency, despite the risk of bleeding.12–14
The percentage of patients in our study admitted with ICH and taking AT was 23%, similar to that of other studies (24.8%),26 (24.2%)15 and (23%).16 We found no significant gender difference between those taking AT or not although other authors have reported a higher usage of AT in males,16,26,27 probably due to their higher prevalence of coronary heart disease.28 Our patients on AT were also older, consistent with previous studies16,17,27,29 and were more often diabetic16,29 compared with those not on AT, which could be explained by population ageing. However, there was no difference in the clinical status on admission (GCS, NIHSS, APACHE II) between the groups, as also reported in other studies.17,26,29 Neither were there any differences in the time interval between symptom onset and admission CT scan between the AT and non-AT groups, consistent with the studies of Sansing et al. (111.5 vs. 120.5min, p=0.35)26 and Moussouttas et al. (146±102 vs. 151±86min, p=0.72).30 Similarly, as with other studies,30 there was no difference in the time interval between the admission and follow-up CT scan in our study.
Hematoma volume is the most important determinant of outcome after ICH.3,22 Our study showed that patients presenting with ICH taking AT had a similar hematoma volume on admission compared with those not on AT. However, the follow-up CT scan in those taking AT not only showed a significant increase in hematoma volume compared with their initial scan but this measurement was also significantly greater than both the initial and follow-up volume of those not on AT. Similarly, AT patients had a significantly higher mortality compared with those not on AT. These results contrast with those of other studies in a number of ways. Moussouttas et al. did not find any difference in the admission or follow-up CT hematoma volume between patients on AT and not on AT.30 Similarly, Sansing et al., in a study of 282 patients with ICH, found that hematoma volume on admission and after 48h was similar in patients taking AT and in controls.26 However, this study, based on the cerebral hemorrhage and NXY-059 treatment (CHANT) trial population,26 only included mild and moderate ICH since an exclusion criterion was unconsciousness on admission and this may explain the lack of difference in hematoma volume between the two groups. In contrast, Toyoda et al. found a larger mean hematoma volume in the AT group both on admission and on follow-up.16,29 Consistent with our results, Saloheimo et al. found that patients on AT did not have a larger admission CT hematoma volume than those not on AT (22% vs. 16.1%, p=0.006) and that an antecedent history of regular aspirin use was significantly associated with hematoma growth.17
Unlike previous reports, our study addressed the important issue of changes in hematoma volume after ICH in relation to evacuation surgery and we found that patients on AT had a greater hematoma enlargement irrespective of evacuation surgery, thus again highlighting the importance of AT in hematoma enlargement.
In addition, recent studies17,20,22,26 have defined a hematoma enlargement of more than a third between admission and follow-up scans as clinically significant. Using this criterion, the AT group in our study had a significantly higher percentage of patients with hematoma enlargement, irrespective of surgical intervention. Naidech et al. reported that reduced platelet activity on admission (usually, but not always, related to aspirin use) is associated with ICH volume enlargement and poor outcomes.31 In contrast, Moussouttas et al. reported that patients on AT experienced a similar absolute and relative increase in hematoma volume compared with patients not on AT.30 However, our results are similar to those of Sorimachi et al., where substantial hematoma enlargement (>20%) was more frequent in patients on AT than in those not on AT (63% vs. 8%, p=0.0003).32 Moreover, multivariate regression analysis showed that, in our study, previous AT was the only factor associated with this clinically significant enlargement.
Significant hematoma expansion in ICH usually occurs during the first 3h and previous studies have reported that between 14% and 38% of patients show hematoma enlargement within the first 48h.33–35 Brott et al., using sequential CT imaging, observed that ICH volume expanded by 26% in the first hour and 38% at 3h20 and Davis et al. reported similar results.22 Toyoda et al., likewise, found that early CT scanning is an independent predictor of hematoma enlargement in the subsequent CT, describing a greater than 40% increase between both scans.16 Therefore, very early CT scanning may sometimes not show the full extent of hematoma expansion because of continuing bleeding in that period following the admission CT scan. This may explain our findings of similar hematoma volume on admission in both AT and non-AT groups because of our hospital's strategic geographical location which facilitates rapid access to emergency neurological/neurosurgical diagnostic services allowing early CT scanning, sometimes within 2h from onset of ICH.
As regards mortality, Saloheimo et al. reported a two and a half times increased relative risk of early mortality for patients using regular aspirin prior to ICH and they attributed this increase to hematoma enlargement.17 Our overall rate of 53% was at the upper limit compared with that reported elsewhere in patients with ICH (33–52% in the first month3). This figure may be explained by the fact that our patients’ clinical status was more severe than that of patients from other studies (the majority had GCS≤8). Unlike those studies,26,27 our patients on AT had a significantly higher mortality compared to those not on AT (78% vs. 45%, p<0.001). Caso et al.27 concluded that AT was not associated with a higher mortality (23.4% vs. 23.2%) and both Moussouttas et al.30 and Creutzfeld et al.36 reported similar conclusions (18% vs. 17% and 33% vs. 35%, respectively). Foerch et al. initially described a higher in-hospital mortality in patients on AT (43% vs. 22%, p=0.005) but, after adjustment for age and preadmission mRS, it was found that previous AT was not in fact an independent predictor of in-hospital death (OR: 1.12; p=0.5).23 In contrast, the study by Toyoda et al. supports our findings of a significantly higher mortality in patients on AT (22% vs. 16%, p<0.001).29 In relation to mortality and hematoma volume enlargement, we found that, using the aforementioned criteria of greater than a third, enlargement was associated with significantly increased mortality (62.5% vs. 28.8%, p=0.001).
A logistic regression analysis of other variables found that, in addition to AT, GCS score and blood glucose level on admission were also associated with increased mortality, similar to the findings of Roquer et al.15 Interestingly, using this analysis, AT was indeed found to be an independent factor for mortality in other studies including that of Saloheimo et al. (OR: 2.5, 95% CI 1.3–4.6, p<0.01),17 Creutzfeld et al. (OR: 2.4, 95% CI 1.1–5.6)36 and Toyoda et al. (OR: 2.04, 95% CI 1.32–3.14, p=0.001).29
Of particular interest is the finding that, despite the preponderance of anticoagulant users in the non-AT group, this group had a lower rate of hematoma enlargement and mortality. Although perhaps surprising, this may be explained by the routine administration on admission of fresh frozen plasma and vitamin K to all ICH patients taking anticoagulants. No patient received any treatment that could influence platelet aggregation.
Our study has some limitations including the fact that it was not multicenter and it only included critically ill ICH patients. Although our results cannot be generalized to every patient with hemorraghic stroke, they add relevant information to other studies published so far on the role of AT in this critically ill subgroup of patients. Another limitation could be that our analysis did not take into account the type of AT or indeed whether patients were taking antiplatelet mono- or polytherapy. Nevertheless, it does show a significant association between AT and subsequent hematoma enlargement in ICH as well as an increased mortality in AT users and a higher mortality in relation to significant hematoma volume enlargement after ICH. If corroborated, our results suggest the need to investigate potential future therapies aimed at reducing hematoma volume enlargement and, therefore, mortality in these patients. Some areas of research are already focusing on antiplatelet reversion in ICH including the use of Desmopresin,37 recombinant activated factor VII38,39 and administration of platelets.30,40,41 If proved successful, such therapies could have a major impact on prognosis in patients on AT presenting with ICH.
ConclusionsOur results show a strong association between AT and subsequent hematoma volume enlargement as well as increased mortality in patients taking AT presenting with ICH. Further studies are necessary with a view to a search for potential antiplatelet reversion therapies.
Conflict of interestThere are no conflict, interest or any type of funding.
We are grateful to Dr. M V Tobin for checking the manuscript.