To analyze the evolution of patients subjected to renal replacement therapy (RRT), and to determine risk factors associated with mortality and the recovery of renal function.
DesignA prospective, observational study of critically-ill patients.
SettingClinical–surgical Intensive Care Unit (ICU) of Sabadell Hospital (Spain).
PatientsInclusion of all patients treated in our unit due to acute renal failure (ARF) requiring RRT.
Primary variables of interestWe recorded epidemiological data, severity using the APACHE II score, days of the technique, ICU mortality, and renal function recovery. The study period was divided into 2 parts: part 1 (2000–2004) and part 2 (2005–2009). The 2 periods were compared using the Student's t-test for continuous variables and the chi-squared test for categorical variables. Multiple regression analysis was performed to determine the risk factors for mortality and recovery of renal function.
ResultsA total of 304 patients were treated. Sepsis was the main etiology of ARF (61%), involving principally respiratory and abdominal foci. In the second period the convective technique and community-acquired ARF were far more prevalent than in the first period. There were fewer days of therapy in the second period (19.7 versus 12.3 days; p=.015). Total ICU mortality was 52.3%, with a decrease in the last period (61.9–45.5%: p=.003). The risk factors associated with mortality were creatinine upon admission (odds ratio [OR] 0.77; 95% confidence interval [95%CI] 0.61–0.97) and treatment with IHD alone (OR 0.37, 95%CI 0.16–0.87). Survivors had normal renal function at ICU discharge in 56.7% of the cases in the second period, vs in 72.9% in the first period, with more patients subjected to IHD in the second period (10.4% versus 26.8%). The factors related to the recovery of renal function were creatinine upon admission (OR 1.98, 95%CI 1.12–3.48), acute renal failure (OR 0.11, 95%CI 0.04–0.34) and treatment with continuous techniques (OR 0.18, 95%CI 0.03–0.85).
ConclusionsMortality among critically-ill patients subjected to RRT has improved in recent years.
Analizar la evolución de los pacientes con insuficiencia renal aguda tratados con terapia de reemplazo renal (TRR) y determinar los factores de riesgo asociados a mortalidad y recuperación de la función renal.
DiseñoEstudio prospectivo y observacional en pacientes críticos.
ÁmbitoUnidad de Cuidados Intensivos (UCI) polivalente del Hospital de Sabadell.
PacientesInclusión de los pacientes con insuficiencia renal que precisaron TRR en nuestra unidad.
Principales variables de interésRegistro de variables epidemiológicas, de gravedad (APACHE II) así como el tipo y duración de la TRR, mortalidad y recuperación de la función renal al alta de UCI. El periodo de estudio comprende 10 años, repartiendo la muestra en 2 periodos: inicial (2000-2004) y reciente (2005-2009). Análisis estadístico comparativo de ambos periodos y análisis de regresión logística múltiple para determinar factores de riesgo de mortalidad y de recuperación de función renal.
ResultadosAnálisis de 304 pacientes. Principal causa de ingreso la sepsis (61%), siendo el foco respiratorio y el abdominal los más frecuentes. El origen comunitario de la insuficiencia renal y la técnica convectiva se incrementaron en el periodo reciente. Destaca un descenso de días de terapia (19,7 a 12,3; p=0,015). La mortalidad global en UCI fue de 52,3%, siendo la principal causa el fallo multiorgánico, objetivando un descenso entre ambos periodos (61,9 a 45,5%; p=0,003). Los factores relacionados con la mortalidad fueron la creatinina al ingreso (odds ratio [OR] 0,77; intervalo de confianza del 95% [IC95%] 0,61-0,97) y el tratamiento solo con HDI (OR 0,37; IC95% 0,16-0,87). De los supervivientes, al alta de UCI, en el periodo reciente destaca un aumento de los pacientes que quedan con dependencia de HD (10,4 versus 26,8%). Los factores relacionados con la recuperación de la función renal fueron la creatinina al ingreso (OR 1,98; IC95% 1,12-3,48), la insuficiencia renal aguda versus la crónica agudizada (OR 0,11; IC95% 0,04-0,34) y el tratamiento con técnicas continuas (OR 0,18; IC95% 0,03-0,85).
ConclusionesLa mortalidad de los pacientes críticos tratados con TRR ha mejorado en los últimos años.
The different studies conducted to date reveal high incidences of acute renal failure (ARF) in hospitalized patients, and particularly among critically-ill patients.1 Moreover, since ARF in the critical patient is associated with multiorgan dysfunction syndrome (MODS), the mortality rate among such individuals is much higher (35–53% depending on the source) than in patients without ARF admitted to the Intensive Care Unit (ICU).2,3 Even the need for renal replacement therapy (RRT) in the critical patient has been shown to be an independent predictor of mortality.4,5
In recent years there have been many changes in RRT which in turn have led to important improvements. Since the publication in the year 2000 of the article by Ronco et al.,6 in which increased dialysis doses were correlated to improved survival, continuous RRT mainly has been designed to apply high convective dialysis doses to the patient, and this subsequently has led to the introduction of new catheters and machines allowing such high flows.
Despite the knowledge obtained, the improvements in the management of these patients, and the years of experience gained in the use of renal replacement techniques, the mortality rate remains high in these patients. This could be explained by the fact that the patients treated today are older, with increased comorbidities and in more serious condition than in the past.5 Indeed, given the current characteristics of the patients, the needs for intermittent hemodialysis (IHD) at discharge have increased.7–9
Among those patients who survive, most will recover from failure with good quality of life at discharge, while 5–20% will require IHD after leaving hospital.4
The primary objective of this study was to describe the characteristics of the patients admitted to the ICU with ARF and who required RRT, and to analyze the evolutive changes of the patients and of the treatment received over the years. The secondary objective was to identify the risk factors associated to mortality and the recovery of renal function in the study cohort.
Patients and methodsStudy population and periodWe prospectively included all the patients admitted to our Unit with ARF or exacerbated chronic renal failure (previous creatinine>1.8mg/dl) requiring RRT (both intermittent and continuous) during their stay in Intensive Care.
We only excluded those patients with chronic renal failure who were already enrolled in a previous IHD program. Ours is a polyvalent Unit with 26 beds (16 in the ICU plus 10 in Semicritical Care) that receives clinical, postsurgical and trauma cases. Given the logistics of our Unit, and depending on the nursing activity burden, we can perform IHD and continuous renal replacement techniques (CRRTs) in all 16 boxes of the ICU. In the Semicritical Care area we can only perform IHD (supervised by Nephrology nursing personnel) in one of the boxes; alternatively, the patients are moved to the acute patients area of Nephrology for IHD. Patients on IHD while in the Semicritical Care area and who suffer clinical worsening with the need to switch to CRRT are moved to the ICU.
Acute renal failure was defined as a creatinine increase to >2mg/dl (if previously normal), with urea 150–200mg/dl and preserved diuresis, oliguria or anuria (at the time of data collection referred to these patients, the RIFLE criteria had not yet been defined).
Exacerbated chronic renal failure in turn was diagnosed in those patients with worsening of basal creatinine at the time of admission, with a concentration of >1.8mg/dl. This parameter was checked from previous admissions of the patients or on the basis of their antecedents. Creatinine clearance of the patients was not registered.
In a very low percentage of patients, and due to the absence of prior data or reports, the basal creatinine was not known.
Critical patients of septic origin were treated according to the guidelines of the Surviving Sepsis Campaign10 from the time when they were published in 2004.
The inclusion period of the study extended from January 2000 to December 2009.
Renal replacement therapyThe indications of RRT were hypervolemia with respiratory involvement refractory to diuretic treatment, uremia 150–200mg/dl with clinical involvement, hyperpotassemia, pericarditis and/or uremic encephalopathy and severe metabolic acidosis (pH<7).
In recent years, and on the basis of the published literature, RRT (particularly in continuous mode) was indicated both based on the previously defined classical criteria and in the context of ARF with multiorgan failure secondary to septic shock. No septic shock patients without ARF were treated.
The type of RRT (IHD, continuous venous–venous hemofiltration (CVVHF), continuous venous–venous hemodiafiltration (CVVHDF), high-volume continuous venous–venous hemofiltration) was decided according to medical criterion, following a homogeneous protocol used in the unit.
According to the mentioned protocol, continuous therapy was provided in all patients with hemodynamic instability (requiring vasoactive drugs) and in those subjects presenting intolerance (hypotension with systolic blood pressure (SBP)<90mmHg) to the intermittent technique. As per protocol, the continuous technique was always started in septic patients in the form of CVVHF, and starting in 2006 with the switch to PrismaFlex®, it was started in the form of high-volume CVVHF (35ml/kg/h). In patients with obesity, severe catabolism, or hyperpotassemia with clinical involvement, CVVHDF was started.
The administration and supervision of continuous therapy were carried out by the nurses and physicians of the ICU.
In those patients exhibiting a good course following vasoactive drug withdrawal and with persistent needs for RRT, we switched from continuous to intermittent techniques.
IHD in our center is carried out by the nurses and physicians of the Department of Nephrology, with daily discussion of the case by both medical teams.
Double-lumen 11.5 RF catheters were inserted in the stable patients programmed for IHD, while double-lumen 13 RF catheters were used in the patients subjected to continuous techniques. These latter catheters were introduced in our Unit in the year 2006. The insertion site was usually the internal jugular vein and femoral vena.
During the study period we initially used the BSM monitor, followed in the period 2003–2004 by the Prisma® monitor, and since 2005 we have only used the PrismaFlex® system for continuous therapy (all from Gambro-Hospal). The filter used from the time of introduction of the PrismaFlex® system has been the M100 filter (AN69) with a biocompatible polyacrylonitrile membrane (0.9m2).
During the years of treatment with the Prisma® monitor, 20–25ml/kg/h ultrafiltration was performed (the daily dialysis doses are not registered) with arterial pump settings of 150–180ml/min. After introduction of the PrismaFlex® device, ultrafiltration was increased to 35ml/kg/h, with arterial pump settings of 280–330ml/min.
In the absence of contraindications, the anticoagulation used during therapy consisted of heparin sodium at a dose of 300–500IU/h, according to the activated partial thromboplastin time (aPTT) controls.
Data collectionFrom the time of patient admission, and after confirming compliance with the inclusion criteria, we recorded the following variables on a daily basis: epidemiological parameters (gender, age), risk factors for renal failure (hypertension, diabetes mellitus, dyslipidemia, postoperative period, associated neoplasm), APACHE II score, origin of ARF (nosocomial or community acquired), etiology of ARF (prerenal, renal or obstructive) and urine output (anuria<100ml/24h, oliguria<400ml/24h, and preserved diuresis). Likewise, we documented the reason for admission to the ICU, the therapy received (intermittent, continuous or both) and the duration of RRT in days, mortality (in the ICU), and recovery of renal function prior to discharge from the ICU. The complications of RRT were not recorded in the effects of the study.
The recovery of renal function was defined in the descriptive data and in the comparative analysis as full recovery of renal function (normal creatinine concentration at discharge), or partial recovery of renal function but with no need for IHD (creatinine concentration at discharge>1.5mg/dl) or with the need for IHD at discharge from the ICU.
In order to establish the predictors of the recovery of renal function, and in relation to the previously published literature, we divided the patients into only two groups: IHD dependency or non-dependency at discharge from the ICU.
Because of the complexity caused by the variability of the onset of ARF, we were unable to precisely document the start of RRT. Furthermore, the unit protocol does not precisely define the time for introducing such therapy.
Since the publication (in 2004) of the RIFLE score,11 we started to record the latter along with the rest of the data, on a prospective basis. A review was moreover made of the previously entered case histories, conducting a retrospective analysis of the RIFLE score of these patients.
The study interval covers 10 years, divided into two periods: initial (2000–2004) and recent (2005–2009). This division was made with the purpose of comparing the two periods, since it was in the recent period when therapy with high-volume CVVHF was started in our unit.
Statistical analysisA descriptive statistical study was made of the study population data, reporting the quantitative variables as the mean and standard deviation, and the categorical variables as percentages.
After dividing the sample into the two above mentioned periods, a comparative study was made of both periods (initial versus recent), using the chi-squared test for the qualitative variables, and the Student's t-test for the quantitative variables.
The results are shown comparing the initial period versus the recent period.
For the variable therapy provided, we divided the sample into three subgroups: patients receiving only IHD; patients receiving only the continuous modality; and patients receiving both techniques.
The predictors of mortality and of recovery of renal function were established using the Student's t-test, chi-squared test and Fisher exact test. The survivors were compared versus the patients who died, and on the other hand, comparisons were also made between those patients who upon discharge from the ICU remained dependent on IHD versus those who were not dependent upon IHD.
Multiple logistic regression analysis was made of the variables found to be significant in the univariate analysis (p<0.05), as well as of those believed to be significant on the basis of the previously described literature–with a view to determining possible predictors of the dependent variable under study. The results are reported as the odds ratio (OR) and corresponding 95% confidence interval (95%CI).
ResultsCharacteristics of the patients/evolutive analysisBaseline population characteristicsDuring the study period, 304 patients with ARF or exacerbated chronic renal failure required RRT.
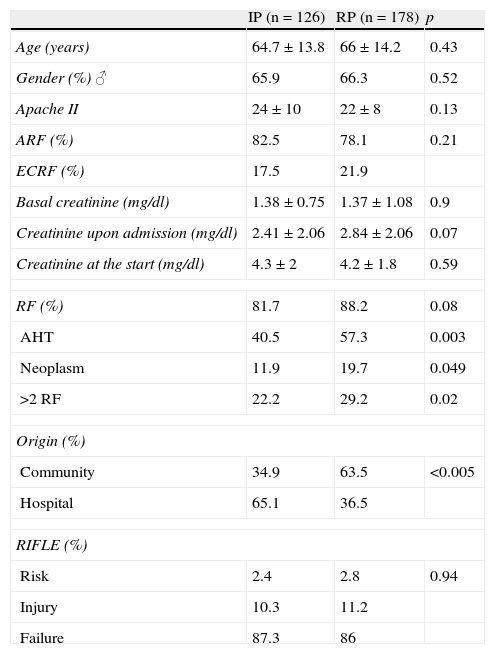
The demographic data and clinical characteristics of the patients, comparing both periods, are shown in Table 1.
Basal characteristics of the patients.
| IP (n=126) | RP (n=178) | p | |
| Age (years) | 64.7±13.8 | 66±14.2 | 0.43 |
| Gender (%) ♂ | 65.9 | 66.3 | 0.52 |
| Apache II | 24±10 | 22±8 | 0.13 |
| ARF (%) | 82.5 | 78.1 | 0.21 |
| ECRF (%) | 17.5 | 21.9 | |
| Basal creatinine (mg/dl) | 1.38±0.75 | 1.37±1.08 | 0.9 |
| Creatinine upon admission (mg/dl) | 2.41±2.06 | 2.84±2.06 | 0.07 |
| Creatinine at the start (mg/dl) | 4.3±2 | 4.2±1.8 | 0.59 |
| RF (%) | 81.7 | 88.2 | 0.08 |
| AHT | 40.5 | 57.3 | 0.003 |
| Neoplasm | 11.9 | 19.7 | 0.049 |
| >2 RF | 22.2 | 29.2 | 0.02 |
| Origin (%) | |||
| Community | 34.9 | 63.5 | <0.005 |
| Hospital | 65.1 | 36.5 | |
| RIFLE (%) | |||
| Risk | 2.4 | 2.8 | 0.94 |
| Injury | 10.3 | 11.2 | |
| Failure | 87.3 | 86 | |
RF: risk factors for renal failure; AHT: arterial hypertension; ARF: acute renal failure; ECRF: exacerbated chronic renal failure; IP: initial period; RP: period recent.
The main cause of admission was sepsis, with the respiratory system and abdominal region as the most frequent foci. Risk factors (RFs) for renal failure upon admission were recorded in 85.5% of the patients. Of note in this sense was an increase in arterial hypertension in the recent period, the presence of neoplasms, and an increase in the number of patients with two or more RFs. A community origin of ARF was seen to increase in the recent period. Regarding the RIFLE criteria, at the start of RRT, the most predominant was “failure”. ARF was prerenal in 94% of the cases–the main underlying causes being septic and cardiogenic shock.
Types and duration of renal replacement therapyOne-half of the patients in the study underwent IHD, mainly because the latter is the method used in the unit for weaning from the technique.
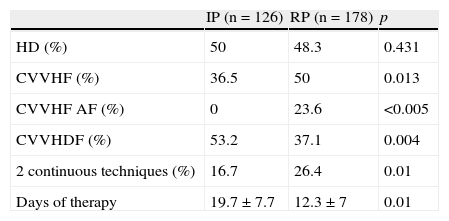
Regarding the continuous techniques, CVVHF and high-volume CVVHF were seen to increase significantly on comparing both periods, with a decrease in CVVHDF. Up to 75% of the patients used continuous techniques (alone or combined with IHD), and an increase was recorded in the number of patients combining more than one continuous technique (16.7% versus 26.4%; p=0.01).
Regarding the days of treatment, a significant decrease was observed in the recent period on summing all the techniques received by the patients (Table 2).
Renal replacement therapy (RRT) techniques and days of therapy.
| IP (n=126) | RP (n=178) | p | |
| HD (%) | 50 | 48.3 | 0.431 |
| CVVHF (%) | 36.5 | 50 | 0.013 |
| CVVHF AF (%) | 0 | 23.6 | <0.005 |
| CVVHDF (%) | 53.2 | 37.1 | 0.004 |
| 2 continuous techniques (%) | 16.7 | 26.4 | 0.01 |
| Days of therapy | 19.7±7.7 | 12.3±7 | 0.01 |
HD: hemodialysis; CVVHDF: continuous venous–venous hemodiafiltration; CVVHF: continuous venous–venous hemofiltration; IP: period initial; RP: recent period.
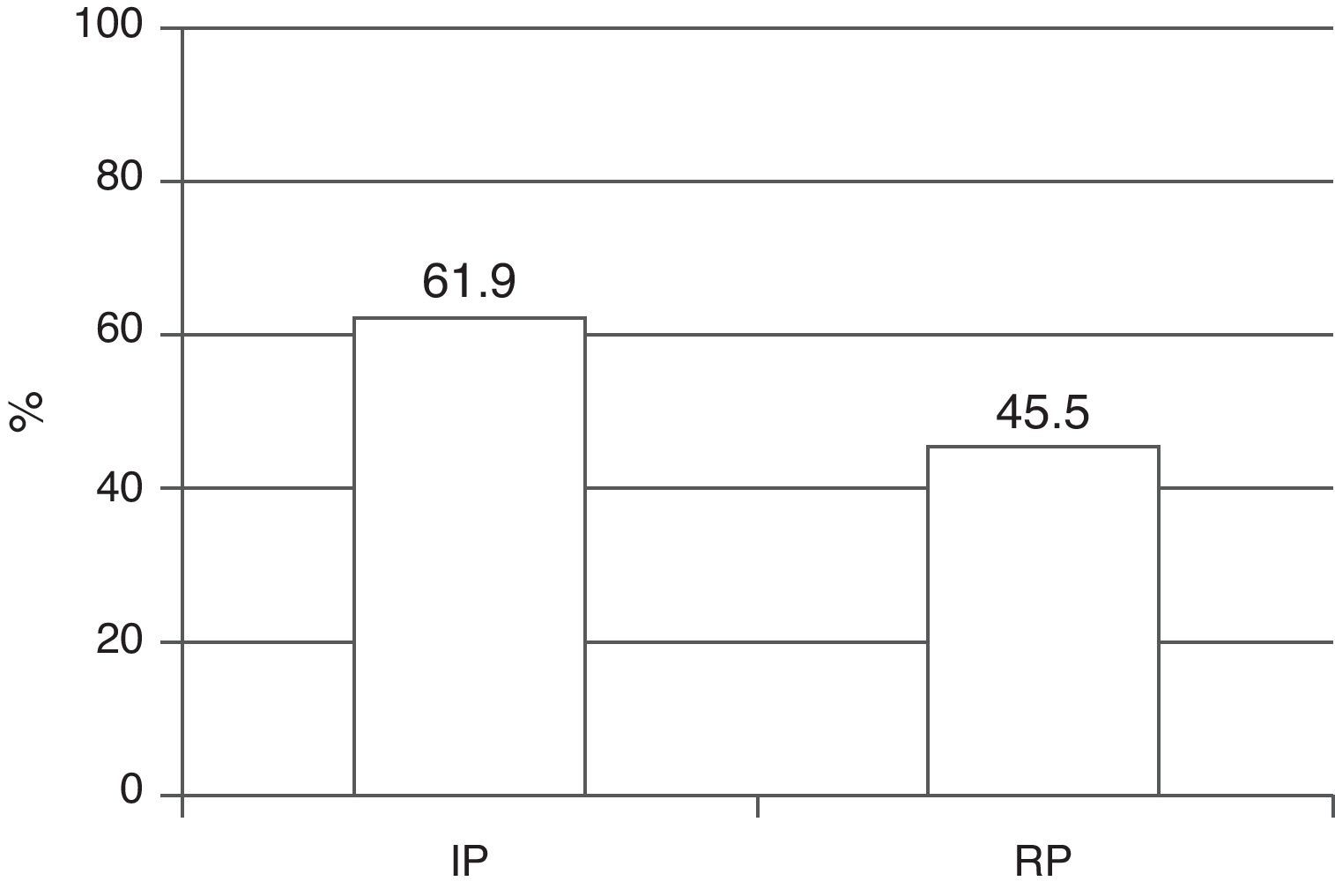
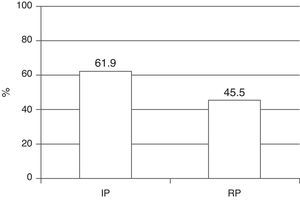
The global mortality rate in the study cohort was 52.3%–the main cause of death being MODS, with the observation of a significant decrease between the two periods (61.9% versus 45.5%; p=0.003) (Fig. 1).
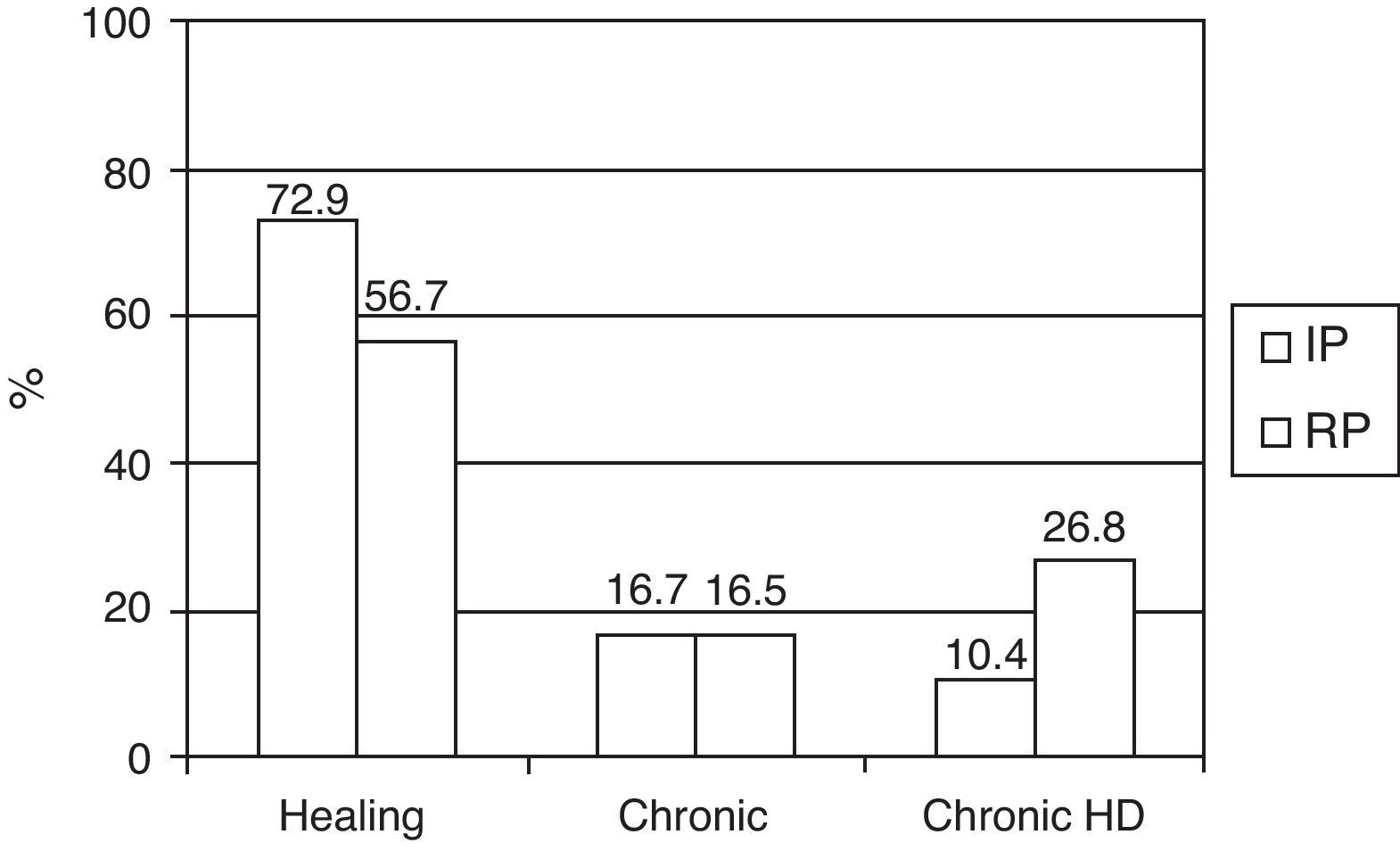
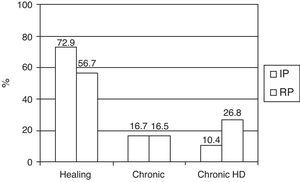
Referred to the survivors (145 patients) at discharge from the ICU, we recorded a decrease over time in the resolution of renal failure, an increase in the number of patients dependent upon IHD, and a stable number of chronic cases with no need for IHD (Fig. 2).
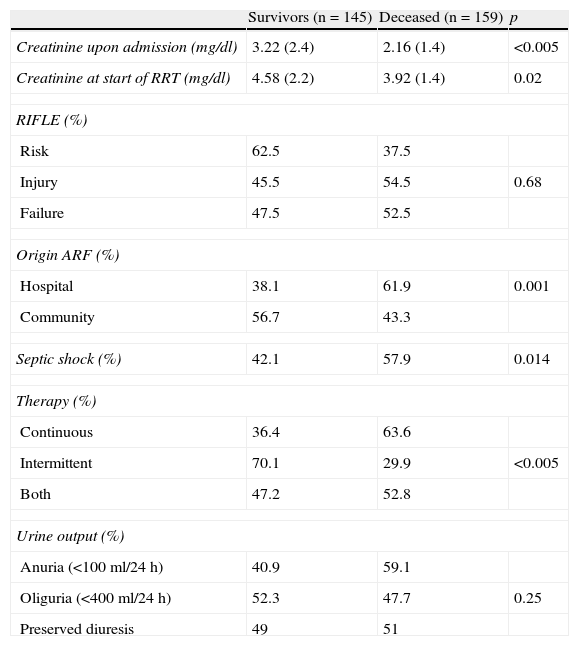
Predictors of mortality and renal recoveryIn the 10 years of the study, a total of 159 of the 304 patients died (52.3%). The variables found to be significant predictors of mortality in the univariate analysis were creatinine upon admission and creatinine at the start of the technique–both being higher among the survivors. Likewise, the origin of renal failure was identified as a significant variable; specifically, patients with ARF originating in hospital suffered greater mortality than those with community-acquired ARF (Table 3).
Variables related to mortality.
| Survivors (n=145) | Deceased (n=159) | p | |
| Creatinine upon admission (mg/dl) | 3.22 (2.4) | 2.16 (1.4) | <0.005 |
| Creatinine at start of RRT (mg/dl) | 4.58 (2.2) | 3.92 (1.4) | 0.02 |
| RIFLE (%) | |||
| Risk | 62.5 | 37.5 | |
| Injury | 45.5 | 54.5 | 0.68 |
| Failure | 47.5 | 52.5 | |
| Origin ARF (%) | |||
| Hospital | 38.1 | 61.9 | 0.001 |
| Community | 56.7 | 43.3 | |
| Septic shock (%) | 42.1 | 57.9 | 0.014 |
| Therapy (%) | |||
| Continuous | 36.4 | 63.6 | |
| Intermittent | 70.1 | 29.9 | <0.005 |
| Both | 47.2 | 52.8 | |
| Urine output (%) | |||
| Anuria (<100ml/24h) | 40.9 | 59.1 | |
| Oliguria (<400ml/24h) | 52.3 | 47.7 | 0.25 |
| Preserved diuresis | 49 | 51 | |
The creatinine values are reported as the mean and standard deviation.
Septic shock as a cause of ARF also proved significant in the univariate analysis (57.9% versus 44.4%; p=0.014).
Another factor adding to mortality was the renal replacement technique used. In effect, the mortality rate was higher among the patients subjected to continuous techniques versus only the intermittent mode or those patients subjected to both treatment modes.
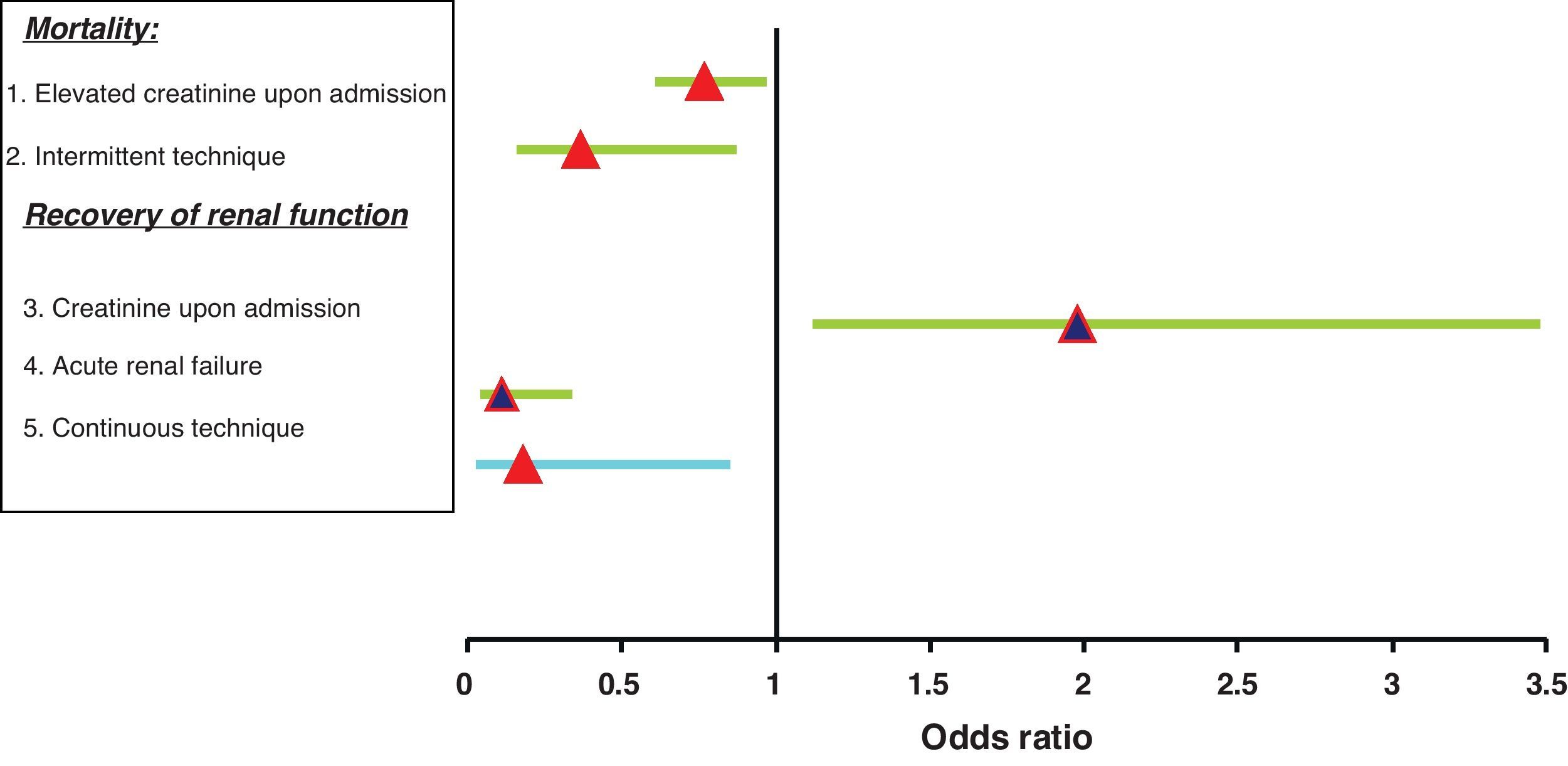
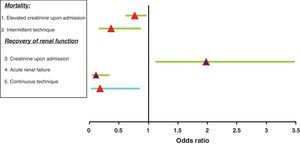
In the multivariate analysis (Fig. 3), and after adjusting for age and the APACHE II score upon admission, the variables independently related to mortality were the following:
- -
Creatinine upon admission (p=0.02; OR 0.77; 95%CI 0.61–0.97). The survivors showed greater creatinine upon admission.
- -
The replacement therapy received. Specifically, intermittent treatment was a predictor of mortality versus those subjected to continuous therapy or both techniques (p=0.015; OR 0.37; 95%CI 0.16–0.87).
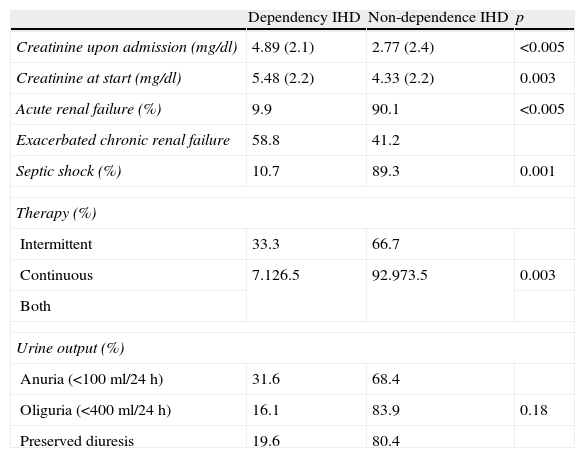
Regarding the recovery of renal function among the survivors (145 patients), only 21.4% of the total patients (31 subjects) required IHD at discharge. After excluding the patients (34 subjects) who already presented previous known renal failure (creatinine>1.8mg/dl) from the group of survivors, the percentage of patients requiring IHD at discharge decreased to 11%.
The variables identified by the univariate analysis as being significantly associated to the need for IHD at discharge were creatinine upon admission, creatinine at the start of the technique, and patients with previous chronic renal failure.
The variables significantly associated to the recovery of renal function were septic shock as the origin of ARF and the replacement therapy received. Specifically, the subjects who received continuous treatment required IHD at discharge less often than those who received both techniques (7.1% versus 26.5%; p=0.003). There were no significant differences between the continuous and intermittent techniques (Table 4).
Variables related to the recovery of renal function.
| Dependency IHD | Non-dependence IHD | p | |
| Creatinine upon admission (mg/dl) | 4.89 (2.1) | 2.77 (2.4) | <0.005 |
| Creatinine at start (mg/dl) | 5.48 (2.2) | 4.33 (2.2) | 0.003 |
| Acute renal failure (%) | 9.9 | 90.1 | <0.005 |
| Exacerbated chronic renal failure | 58.8 | 41.2 | |
| Septic shock (%) | 10.7 | 89.3 | 0.001 |
| Therapy (%) | |||
| Intermittent | 33.3 | 66.7 | |
| Continuous | 7.126.5 | 92.973.5 | 0.003 |
| Both | |||
| Urine output (%) | |||
| Anuria (<100ml/24h) | 31.6 | 68.4 | |
| Oliguria (<400ml/24h) | 16.1 | 83.9 | 0.18 |
| Preserved diuresis | 19.6 | 80.4 | |
The creatinine values are reported as the mean and standard deviation.
In the multivariate logistic regression analysis (Fig. 3), the variables shown to be independently related to the need for IHD at discharge from the ICU were the following:
- -
Creatinine upon admission (p=0.01; OR 1.98; 95%CI 1.12–3.48).
- -
The type of renal failure: acute versus exacerbated chronic failure (p<0.005; OR 0.11; 95%CI 0.04–0.34).
- -
The continuous technique as treatment received versus the group subjected to both techniques (p=0.03; OR 0.18; 95%CI 0.03–0.85).
The present study shows that the survival of critical patients requiring RRT due to renal failure has improved over time. All the patients were treated according to the homogeneous protocol used in our Unit, with variability being limited to changes in the therapy provided in accordance with the literature published during these years and the improvements in the global treatments provided in our Unit.
Although the global mortality of our patients has been similar to that described in the literature, the main finding in our study was the decrease in mortality observed despite the fact that these are older patents, with increased comorbidity and in very serious condition (APACHE II>20). These findings are in contrast to the published data affirming that mortality in patients with ARF remains high despite the medical advances, because of the greater age of the patients, greater comorbidity, and a more serious patient condition.5
While old, several publications offer results similar to our own.12,13 Turney et al.14 compared patients with ARF (admitted or not to the ICU) treated in two different time periods, and reported a decrease in mortality rate from 51% to 42%, despite an increase in age and in the seriousness of the patient condition. Bisenbach et al.15 in turn compared three consecutive time periods and likewise found a progressive drop in mortality rate from 69% to 54% and 48%, despite an increase in patient age.
In addition to the decrease in mortality, we recorded a significant reduction in the days of therapy between the two time periods. In our case, considering similar characteristics in both groups and knowing that most patients presented ARF secondary to septic shock, we attributed the decrease in mortality and in days of therapy to implementation of the treatment recommendations established from publication of the sepsis management guides.10
This is justified by the greater number of cases of ARF originating in the community during the second time period, which would correspond to the septic patients admitted during that period.
Of note is the observation that despite the decrease in days of therapy and in mortality, the number of patients dependent upon IHD at discharge was higher in the recent period. This is probably related to the larger number of patients with exacerbated chronic renal failure, older age and a greater number of RFs for the development of renal failure.
Although this may be incongruent, fewer days of therapy but more patients requiring IHD at discharge could be explained by the small number of patients needing RRT at discharge from the ICU, together with the fact that many of these patients will not require IHD prior to hospital discharge.
These conclusions are complex and may be due to the difficult and scant definition of the concepts of ARF and exacerbated chronic renal failure.
Regarding the applied technique, it is well known that the article published by Ronco et al. in the year 20006 led to important changes in the management of our patients, with the incorporation of an increased use of convection, and a decrease in diffusion. Furthermore, the Acute Dialysis Quality Initiative, on occasion of its third consensus conference, recommended a dose of 35ml/kg/h in septic patients (evidence level II and degree of recommendation C).16
This caused many Units to replace their RRT machines with systems characterized by higher ultrafiltration flows, and consequently involving higher pressures, and to the great increase in the utilization of convective therapy. A decade later, in 2008 and 2009,17,18 two studies have been published where despite the limitations involved, the efficacy and safety of the treatment applied in recent years has been questioned, and even new concepts have emerged such as “dialytrauma”–causing us to reflect upon and analyze how the high dialysis doses affect our patients and the rest of their treatment (antibiotics, nutrition, etc.).19 At present, this has led us to assess the dialysis dose requirements of our patients on a daily basis, introducing changes according to their evolution over time.
On analyzing the mortality predictors in our study population, one of the variables correlated to increased mortality was creatinine upon admission–with higher values among the survivors. Since most of the patients were septic cases, we probably could deduce that since these subjects had higher creatinine levels, they were placed on dialysis earlier (though in our work, and as a limiting element of the study, the RRT starting time was not documented).
Recently, however, Chou et al.20 have published a propensity score analysis of the relationship between the RIFLE criteria11 and the early or late start of replacement therapy. The authors conclude that the mentioned classification is a poor predictor of the benefits of early or late initiation of RRT in the septic patient.
The other important finding in our study was that the therapy provided is independently associated with increased mortality–the provision of intermittent therapy only being a protective factor against mortality compared with continuous treatment or a combination of both techniques (OR 0.77). Ours is an observational study; this result therefore cannot be inferred from the logistic regression analysis. Despite adjustment for the APACHE II score and age, there are very important limitations; given the protocol used in our Unit, it was obvious that those patients who were only subjected to intermittent treatment, as less seriously ill individuals, also suffered lesser mortality.
Regarding the predictors of the recovery of renal function at discharge from the ICU, our findings are not very different from those published to date.4,5,7 At discharge from the ICU, only 21.4% of the patients required IHD, and if from these we exclude the chronic cases (basal creatinine>1.8mg/dl), then the percentage drops to 11%.
In our case, elevated creatinine values upon admission represented a risk factor for dependency upon IHD at discharge (OR 1.98). On the other hand, ARF versus exacerbated chronic renal failure was identified as a protective factor, in the same way as continuous techniques as RRT versus the group of patients receiving both treatment modes.
Another important limitation appears here, since the group of survivors did not include the patients who died, and the great majority of those who died did so while receiving treatment with continuous techniques. The patients only subjected to continuous treatment and which improved were therefore more likely to recover better renal function than the patients who were previously on IHD. Here again, however, we cannot infer that the continuous techniques are related to improved recovery of renal function.
Our study has a number of important limitations. A first limitation is the complexity of the variables and of the definitions involved–a situation still in wait of improvement after all these years of research in the field of renal failure. On the other hand, the time of the start of RRT has not been registered, and no analysis has been made of the evolution of the SOFA score of the patient in the ICU, or of other severity scores at the time of initiation of RRT. As a result, no extrapolation can be made to the APACHE II score of the same patient 24h after admission to the ICU.
Despite the results of the regression analysis, we cannot independently relate the different techniques to patient mortality and/or the recovery of renal function, since this is an observational study, and the protocol used in our Unit precludes such inference.
Lastly, another important limitation is the fact that no registry has been made of the complications of RRT, for although such complications are well defined and are few, they could also have been analyzed according to the technique used.
In conclusion, critical patients requiring RRT have shown lower mortality rates in recent years, and require fewer days of therapy. This situation is probably attributable to improvements in the global management of these patients, since many other factors in addition to RRT influence patient outcome.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Navas A, et al. Terapia de reemplazo renal en paciente crítico: cambios evolutivos del tratamiento en los últimos años. Med Intensiva. 2012;36:540–7.