To assess the level of implementation of medication safety practices in Intensive Care Units (ICUs) and to identify opportunities for improvement.
DesignA descriptive multicenter study was carried out.
SettingIntensive Care Units.
Participants/ProcedureA total of 40 ICUs voluntarily completed the “Medication use-system safety self-assessment for Intensive Care Units” between March and September 2020. The survey comprised 147 items for evaluation grouped into 10 key elements.
Main variablesCalculation was made of the mean scores and mean percentages based on the maximum possible values for the overall survey, referred to the key elements and to each individual item for evaluation.
ResultsThe mean score of the overall questionnaire among the participating ICUs was 436.8 (49.2% of the maximum possible score). No differences were found according to functional dependence, size of the hospital or type of ICU. The key elements referred to the incorporation of clinical pharmacists in these Units, as well as the competence and training of the professionals in safety practices yielded the lowest values (31.2% and 33.2%, respectively). Three other key elements related to accessibility to information about patients and medicines; to the standardization, storage and distribution of medicines; and to the quality and risk management programs, yielded percentages <50%.
ConclusionsNumerous effective safety medication practices have been identified with a low level of implementation in ICUs. This situation must be addressed in order to reduce medication errors in critically ill patients.
Conocer el grado de implantación de las prácticas seguras con los medicamentos en los Servicios de Medicina Intensiva e identificar oportunidades de mejora.
DiseñoEstudio descriptivo multicéntrico.
ÁmbitoServicios de Medicina Intensiva.
Participantes/Procedimiento40 Servicios de Medicina Intensiva que voluntariamente cumplimentaron el “Cuestionario de Autoevaluación de la Seguridad del Uso de los Medicamentos en los Servicios de Medicina Intensiva” entre marzo y septiembre de 2020. El cuestionario contiene 147 ítems de evaluación agrupados en 10 elementos clave.
Variables principales de interésPuntuación media y porcentaje medio sobre el valor máximo posible en el cuestionario completo, en los elementos clave y en los ítems de evaluación.
ResultadosLa puntuación media del cuestionario completo en los Servicios de Medicina Intensiva fue de 436,8 (49,2% del valor máximo posible). No se encontraron diferencias según dependencia funcional, tamaño del hospital y tipo de servicio. Los elementos clave referentes a la incorporación de farmacéuticos en estos servicios, así como a la competencia y formación de los profesionales en prácticas de seguridad mostraron los valores más bajos (31,2% y 33,2%, respectivamente). Otros tres elementos clave relativos a la accesibilidad a información sobre los pacientes y los medicamentos; a la estandarización, el almacenamiento y la distribución de los medicamentos; y a programas de calidad y gestión de riesgos mostraron porcentajes inferiores al 50%.
ConclusionesSe han identificado numerosas prácticas seguras efectivas cuyo grado de implantación en los Servicios de Medicina Intensiva es bajo y que es preciso abordar para reducir los errores de medicación en el paciente crítico.
Medication mistakes are one of the leading causes of morbidity and mortality in critically ill patients. According to the SYREC trial—conducted in 79 Intensive Care Units (ICU)—in Spain medication mistakes were the most common harmless incidents (31.2%), and cause for 11.6% of adverse events recorded.1 In studies conducted in the United States it has been reported that medication mistakes in these patients are more common compared to other hospitalized patients with a 2–3 times higher risk of adverse events and a 2.5 higher mortality rate.2,3 Several factors contribute to the higher risk of adverse events preventable with medication at the ICU setting, among them: the greater severity of critically ill paients and the complexity of treatments they need: the use of numerous drugs—many high-risk drugs—and the IV administration of a large number of drugs that require dose estimation, and are often administered via continuous IV perfusion.4,5
To improve the safety of drugs at the ICU setting a multimodal and multidisciplinary approach4,5 is needed to facilitate the implementation of specific mistake prevention practices in all the processes that determine the safety of such practices. Proactive assessment tools have proven very effective to help health centes analyze their processes, identify areas of risk, and prioritize practices for improvement that should be implemented. In the United States, the Institute for Safe Medication Practices (ISMP) developed the ISMP Medication Safety Self Assessment for Hospitals,6 a self-evaluation survery to conduct a comprehensive and extensive analysis of the safety of drugs administered at the hospital setting that has been used in different countries now.7–11 With support and funding from the Spanish Ministry of Health and the technical collaboration of an expert working group from different hospitals, the ISMP-España adapted this survey to the Spanish healthcare practice. Therefore, in 2007 the «ISMP Medication Safety Self Assessment for Hospitals»12 was published. It was updated a years later.13 From this latest iteration of this document, ISMP-España, the Intensive Medicine and Critical Care Pharmacist (FarMIC) working group of the Spanish Society of Hospital Pharmacists (SEFH) and the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) drafted the «ISMP Medication Safety Self Assessment for Intensive Care Units»14 to have a specific tool that ICUs could use to make detailed assessments of drug safety in this specific setting, identify the critical areas of risk, and with this information, plan safe practices that should be implemented at local level in each center to minimize the risk of making mistakes.
Once the survey for ICU was published, SEMICYUC, SEFH, and ISMP-España decided to conduct a nationwide study to know the degree of implementation of safe drug practices at the ICU setting and identify any opportunities for improvement in which collaborative effort is required to improve the safety of critically ill patients.
MethodsDescriptive multicenter study of the degree of involvement of the safety practices included in the «ISMP Medication Safety Self Assessment for Intensive Care Units».14 It was based on the self-assesment conducted by ICUs and Hospital Pharmacy Services that volunteered to participate in the study and completed the questionnaire from March to September 2020.
The study was published on SEMICYUC and SEFH website and e-mail lists. To register responses to the survey a computer application installed in the ISMP-España website was used to guarantee the confidentialiy of the information provided.
Survey and assessmentThe «ISMP Medication Safety Self Assessment for Intensive Care Units»14 was developed from the latest version of the «ISMP Medication Safety Self Assessment for Hospitals»12 by a group of experts from SEFH and SEMICYUC and was coordinated by ISMP-España. Using the Delphi technique with 2 evaluation rounds, the evaluation items of this survey applicable to the ICU setting were selected. Also, new items from other tools from the ISMP were studied.15,16 The specific survey for ICUs includes 147 evaluation items with practical or concrete measures destined to prevent medication mistakes. A total of 103 out these 147 are items from the general survey of hospitals, 31 are items adapted to the ICU setting, and 13 are new items specific to ICUs. Items are grouped in 10 sections corresponding to the the 10 key elements that are more closely associated with the safety of drugs at the ICU setting, according to the conceptual model of the ISMP.
Survey should be completed throughout different sessions by a multidisciplinary team that should assess the degree of implementation of every evaluation item at the ICU setting by using a scale that includes 5 different possibilities. The possible answers are:
- A
No initiative was performed to implement this item.
- B
This item has been discussed for its possible implementation, but it has not been implemented yet.
- C
This item has been implemented partially in some or in all areas, patients, drugs or healthcare workers.
- D
This item has been implemented in some areas, patients, drugs or healthcare workers.
- E
This item has been implemented in its entirety in all areas, patients, drugs or healthcare workers.
Items from the survey are associated with different scores depending on their efficacy preventing medication mistakes and they impact they have on the safety of the entire system. Therefore, option A always scores 0 while the possible scores for options B, C, D or E from the score increase gradually, and include different values that can go from 0 (lowest) for option B to 16 (highest) for option E. Also, there is a total of 5 evaluation items in the survey whose responses have the option of being «non-applicable» for those situations where a given ICU does not perform the activity referred to in such item (eg, in the absence of automated distribution systems). These items are subtracted from the overall count if they were responded as «non-applicable».
Multidisciplinary teams from the participant ICUs did not know the score of the items during the self-evaluation phase. Once completed, the representatives from each center registered the responses in the computer application developed to evaluate and register the responses given to the survey obtaining an automated individual analysis of their data. This application allows all users to access their own data and the information added to the remaining ICUs for result comparison purposes.
The aggregate analysis of thev results from ICU participants in the study included a determination of mean scores in absolute values obtained for the entire survey, for every key element, and for the 147 items under study. Also, the rates on the maximum possible values of the entire questionnaire on every key element and every item under study were estimated since these rates allow us to make comparisons among these key elements or items under study.
Statistical analysisA descriptive analysis of the characteristics of each participant center was performed in the study while the scores and rates associated with the maximum values reached were compared in the entire survey among the sample ICUs that were stratified based on their characteristics. The variables uner consideration were: 1) functional dependency with the public and private hospital categories; 2) number of hospital beds with the following categories: ≥500 beds, from 200 to 499 beds, and <200 beds; 3) type of ICU: polyvalent, and other, and 4) postgraduate education teaching activity or not.
The mean rates of dichotomic variables were compared using the Student t test. For the variable “number of beds”, the ANOVA test was used. P values <.05. were considered statistically significant.
ResultsA total of 39 ICUs from 12 different Spanish autonomous communities participated in the study plus ICUs from Andorra. The characteristics of these ICUs are shown on Table 1. A total of 20% of these ICUs belonged to hospitals with 99–199 beds, 37.5% to hospitals with 200–499 beds, and 42.5% to large hospitals with ≥500 beds. Regarding the type of ICU included in the study, most were polyvalent units and only 3 other type of units (2 doctors and 1 coronary artery disease specialist).
Characteristics of the Intensive Care Units that participated in the study (N = 40).
| Characteristics | Participants | |
|---|---|---|
| N | % | |
| Functional dependency | ||
| National Healthcare System and other public services | 34 | 85.0 |
| Private (charity and non-charity) | 6 | 15.0 |
| No. of beds | ||
| 99−199 beds | 8 | 20.0 |
| 200−499 beds | 15 | 37.5 |
| ≥500 beds | 17 | 42.5 |
| Type of Intensive Care Unit | ||
| Polyvalent | 37 | 92.5 |
| Other | 3 | 7.5 |
| Education | ||
| Postgraduate education | 31 | 77.5 |
| No education | 9 | 22.5 |
| Location of Intensive Care Unit | ||
| Andalusia | 3 | 7.5 |
| Aragon | 1 | 2.5 |
| Balearic Islands | 3 | 7.5 |
| Castile and León | 3 | 7.5 |
| Castile La Mancha | 4 | 10.0 |
| Catalonia | 9 | 22.5 |
| Galicia | 2 | 5.0 |
| Madrid | 5 | 12.5 |
| Murcia | 1 | 2.5 |
| Navarre | 1 | 2.5 |
| Basque Country | 5 | 12.5 |
| Valencia | 2 | 5.0 |
| Andorra | 1 | 2.5 |
Table 2 shows the overall results obtained for the survey in the overall 40 ICUs and in the different groups of ICUs established based on characteristics of functional dependency, size of the hospital, type of ICU, and teaching activity. The mean score to the overall survey in all ICUs was 436.8 points, which is equivalent to 49.2% of the theoretical maximum value observing a wide range of values (20.9%–82.3%). When rates on the maximum possible value observed were compared in the different groups of ICU, no statistically significant differences were found among the established groups (P > .05).
Overall results obtained from the survey conducted in all Intensive Care Units (N = 40), and groups under consideration.
| Characteristics | Score | Rates on the maximum value (%) | |||
|---|---|---|---|---|---|
| Mean | σ | Mean | σ | Range | |
| Functional dependency | |||||
| National Healthcare System and other public services (N = 34) | 438.2 | 103.1 | 49.3 | 11.6 | 20.9−82.3 |
| Private (charity and non-charity) (N = 6) | 429.3 | 89.2 | 48.3 | 10.0 | 34.5−63.2 |
| No. of beds | |||||
| 99−199 beds (N = 8) | 409.7 | 84.9 | 46.1 | 9.5 | 34.5−59.4 |
| 200−499 beds (N = 15) | 457.6 | 89.0 | 51.5 | 10.0 | 20.9−63.7 |
| ≥500 beds (N = 17) | 431.3 | 116.4 | 48.6 | 13.1 | 32.9−82.3 |
| Type of Intensive Care Unit | |||||
| Polyvalent (N = 37) | 444.0 | 100.6 | 50.0 | 11.3 | 20.9−82.3 |
| Other (N = 3) | 349.5 | 31.6 | 39.4 | 3.56 | 36.1−43.2 |
| Education | |||||
| Postgraduate education (N = 31) | 440.1 | 95.0 | 49.6 | 10.7 | 32.9−82.3 |
| No education (N = 9) | 425.9 | 121.9 | 48.0 | 13.7 | 20.9−63.7 |
| Overall (N = 40) | 436.8 | 100.1 | 49.2 | 11.2 | 20.9−82.3 |
σ, standard deviation.
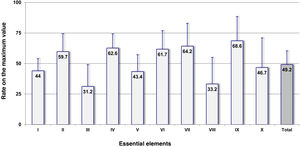
Fig. 1 shows the mean rates on the maximum possible value obtained for the 10 key elements in the overall number of ICUs, which graphically shows areas of a higher risk. Elements iii and viii on the addition of pharmacy specialists to ICUs and on the competence and training of healthcare workers in drugs and safety practices showed the lowest rates of all (31.2% and 33.2%, respectively). The key elements on the availability and accessibility to information on patients and drugs (element i), standardization, storage, and distribution of drugs (element v), and quality and risk management programs (element x) also showed values <50% (44.0%; 43.4%, and 46.7%, respectively). The remaining key elements showed rates above 50%.
Results obtained in the overall amount of Intensive Care Units (ICU) (N = 40) for the 10 key elements and for the complete survey expressed as rates on the maximum possible value.
Abridged description of key elements: I. Availability and accessibility to information on patients and drugs. II. Communication of prescriptions and other type of information on medication. III. Adition of pharmacy specialists to ICUs. IV. Labeling, packaging, and name of drugs. V. Standardization, storage, and distribution of drugs. VI. Acquisition, use, and follow-up of the different devices for the administration of drugs. VII. Environmental factors and human resources. VIII. Competence and training of healthcare workers in drugs and safety practices. IX. Education to patients and families. X. Quality and risk management programs.
The analysis of the results obtained for each evaluation item provides information at a more detailed level that can be useful to know the degree of implementation of specific practices and be able to identify those that should be approached prioritarily to improve safety. A thorough analysis of the 147 evaluation items discussed would exceed the length of the article. Tables 3 to 5 show the values of several items on the addition of new technologies to healthcare continuity and use of high-risk drugs. The overall results can bee read in a report published by SEFH, SEMICYUC, and ISMP-España.17
Overall results obtained from the survey conducted in all Intensive Care Units (N = 40) regarding several evaluation items associated with the implementation of new technologies.
| Evaluation items | Score | Maximum possible value | Rate on the maximum value | ||
|---|---|---|---|---|---|
| Mean | σ | ||||
| 6 | Electronic prescription systems for hospitalized and outpatient patients are interconnected and intergrated into the patients’ electronic health records. | 4.50 | 5.21 | 12 | 37.5 |
| 30a | Electronic prescription systems have support systems for the clinical decision-making process. | 6.93 | 10.51 | 16 | 43.3 |
| 15 | Code reader is used to check drugs prior to their administration. | 0.55 | 1.43 | 16 | 3.4 |
| 108 | Smart infusion pumps are used with all the safety functionalities activated for the administration of, at least, high-risk drugs. | 8.40 | 6.64 | 16 | 52.5 |
σ, standard deviation.
Overall results obtained from the survey conducted in all Intensive Care Units (N = 40) regarding several evaluation items associated with continuing healthcare.
| Evaluation item | Score | Maximum possible value | Rate on the maximum value | ||
|---|---|---|---|---|---|
| Mean | σ | ||||
| 17 | The complete pharmacotherapeutic history of all the patients admitted to the ICU is obtained | 2.30 | 1.22 | 4 | 57.5 |
| 18 | A standard procedure is used to match the patient’s drugs upon his ICU admission. | 8.20 | 6.07 | 16 | 51.3 |
| 19 | A standard procedure is used to match drugs when a patient is transferred from a different healthcare unit at the ICU setting. | 4.65 | 3.01 | 8 | 58.1 |
| 20 | After ICU discharge, a standard procedure is used to inform the next healthcare workers who will be assisting the patient on the drugs administered, possible adverse events, and proposed medication. | 11.40 | 5.32 | 16 | 71.3 |
| 96 | At the beginning of a new shift or at any time during the transfer of patients between hospitals a standard procedure is performed to match different lines and eventually check all connections. | 3.05 | 2.17 | 6 | 50.8 |
ICU, Intensive Care Unit; σ, standard deviation.
Overall results obtained from the survey conducted in all Intensive Care Units (N = 40) regarding several evaluation items associated with the use of high-risk drugs.
| Evaluation item | Score | Maximum possible value | Rate on the maximum value | ||
|---|---|---|---|---|---|
| Mean | σ | ||||
| 24 | High-risk drugs are perfectly defined, and mistake prevention practices have been established. | 2.02 | 1.54 | 4 | 50.5 |
| 25 | Protocols, guidelines, dosing sclaes or checklists are available and used to prescribe, dispense, and administer high-risk drugs | 5.20 | 2.21 | 8 | 65.0 |
| 26 | Maximum doses for high-risk drugs have been established and added to the software of the technology used (electronic prescription, infusion pumps, etc.). | 4.93 | 3.86 | 10 | 49.3 |
| 68 | The concentrations of different solutions regarding the infusion of high-risk drugs have been standardized. | 3.47 | 0.90 | 8 | 43.4 |
| 69 | The Hospital Pharmacy Service prepares standard IV solutions of high-risk drugs that are not available at the market | 2.53 | 3.08 | 10 | 25.3 |
| 82 | Vials or ampoules with concentrated electrolytes are not available or are kept separately with other additional safety measures. | 1.40 | 2.48 | 12 | 11.7 |
| 85 | Neuromuscular blocking drugs are stored and labelled in separate drawers. | 2.35 | 3.17 | 8 | 29.4 |
| 108 | Smart infusion pumps are used with all safety functionalities on for the administration of, at least, high-risk drugs. | 8.40 | 6.64 | 16 | 52.5 |
σ, standard deviation.
On the implementation of technologies that impact the safety of drugs with the corresponding elevated maximum values in the survey (Table 3), low rates of 37.5% were seen for item #6 on electronic prescriptions integrated into the hospital information systems while 43.3% rates were reported for item #30a on the availability of systems to support the clinical decision-making process. The use of a code reader in the administration showed rates of 3.4% only while the use of smart infusion pumps to administer high-risk drugs had a higher value of 52.5%.
Regarding the implementation of standard procedures to match patients’ medication and ICU admission when transferred from a different ICU or discharged from the ICU (items #18, #19, and #20), the mean rates on the maximum possible value obtained were 51.3%, 58.1%, and 71.3%, respectively (Table 4).
Several items associated with the safe use of high-risk drugs had low implementation rates (Table 5). This is the case of item #82 (11.7%) on the elimination of vials or ampoules of electrolyte concentrations from the ICU setting or else be stored separately from other drugs with additional safety measures. Also, item #85 on the storage of neuromuscular blocking drugs (29.4%), and item #69 on the preparation by the Hospital Pharmacy Service of standard IV solutions of high-risk drugs that are not available in the market (25.3%).
Finally, we should mention that item #44 on the availability of a pharmacist assigned to the ICU as part of the healthcare team long enough to perform the clinical activities that the ICU requires, had a mean score of 5.80 ± 5.27 for a maximum value of 16 (36.3%).
DiscussionBack in 2017, SEMICYUC and SEFH signed a collaboration agreement to lead and support common initiatives in the healthcare, teaching, and research settings.18 Both societies worked on a multidisciplinary basis to establish the safe use of drugs in critically ill patients, recommendations, and clinical practice guidelines. Also, to delve into the epidemiology of mistakes and drug-related adverse events. Drafting the «ISMP Medication Safety Self Assessment for Intensive Care Units», and conducting this study are the early results of this initiative.
This study results provide an overall perspective on the safety of the process of using drugs at the ICU setting and reveal that there is significant room for improvement. Although a wide variety of scores to the overall survey were obtained in the different ICUs of the sample—indicative of the exisence of differences in the degree of implementation of safe practices—the analysis of data revelaed that there are safe and effective practices whose rate of implementation is very low or practically zero in many different ICUs. In our opinion, the information collected is very uself for SEMICYUC and SEFH to prioritize areas where their efforts should be oriented to improve the safety of critically ill patients.
Intensive care unit pharmacists play an important role in the safety profile of the drugs administered at the ICU setting. Evidence supports including them as part of the ICU multidisciplinary team to minimize mistakes, adverse events, and mortality.19,20 Some of the functions associated with intensive care unit pharmacists are helping in the clinical decision-making process, being involved in quality programs to improve drug management, participating in the development, implementation, and follow-up of the ICU pharmacotherapeutic protocol, and implementing new technologies.21 However, in Spain, the involvement of intensive care unit pharmacists in multidisciplinary teams is fairly limited.18,22 The results obtained for key element iii of the survey were the lowest of them all (31.2%), indicative of the importance of the role of intensive care unit pharmacists for SEFH and SEMICYUC.
Element viii on competence and training of healthcare workers regarding drugs and safety practices also had a low degree of implementation despite being one of the fundamental pillars to minimize the risks associated with healthcare.23,24 The use of educational strategies based on clinical simulation and a multidisciplinary approach to reduce medication mistakes at the ICU setting is advised25,26 since teamwork and training have both proven capable of minimizing errors and reducing the mortality rate.25,27
This study demonstrates the need for encouraging the implementation of different technologies to minimize prescription and administration mistakes that are the most common errors at the ICU setting.2–4 Only half of the ICUs have infusion pumps with smat technology available to minimize dose administration mistakes or incorrect velocities. Only 37.5% and 43.3% of ICUs have integrated electronic prescriptions and clinical decision-making support systems, respectively. We should mention that the use of a bar codes in the administration of drugs—considered the mot effective barrier to prevent errors and guarantee traceability—is minimum. In Spain, the development of this technology has always been hindered by the lack of bar code unique identifiers in drugs.
Other safe practices recommended by the World Health Organization in its third global patient safety challenge—“Medication Without Harm”—and by other organizations are aimed at minimizing drug mistakes in healthcare transfares and high-risk drugs,26,28,29 common mistakes at the ICU setting associated with an elevated risk of damaging the patients.30,31 Study data indicates that, little by little, ICUs are establishing practices to guarantee the correct continuity of medication. However, its implementation still needs to be improved. On the safety practices associated with the management of high-risk drugs, it is surprising to see the low rate of implementation of practices considered a priority or emblematic regarding patient safety like the elimination of vials and ampoules of potassium chloride, which can be attributed to the lack of potassium concentration solutions in bags in Spain.
As far as we know, no self-evaluation surveys have ever been conducted specific to the ICU setting or with the same tools, which is why, although desirable, we cannot compare the results of this study to the results of other studies of similar characteristics. However, we should mention that, in the latest study on the implementation of safe practices conducted in 2011 among 165 Spanish hospitals, with the early version of the hospital general survey,32 the lowest values of all were also obtained for the element associated with competence and training of the healthcare personnel in both drugs and safe practices (29.8%). Although the items of both surverys are not the same, there is no doubt both deal with measures considered essential to transform healthcare systems and that these measures have not been implemented in the Spanish healthcare practice like we said before. Other key elements (i, ii, iv, vii, and ix) showed slightly higher rates in this study at the ICU setting probably because, over the years, several safe measures have been implemented in our country. In this sense, we should mention that element vi on drug administration devices showed rates of 61.7%, compared to 46.7% reported in the hospital setting, indicative that significant improvements have been made in this area. Therefore, it has been confirmed that more smart infusion pumps, pumps with free-flow protection and specific systems for the administration of oral solutions and enteral nutrition have been used.
The study has several limitations associated with the methodology used. In the first place, the sample could be non-representative of the activity developed at the ICU setting because it was not randomized. Also, the number of ICUs involved in the study was smaller compared to the number of ICUs initially anticipated since the study was disclosed shortly before the COVID-19 pandemic started, which has impacted ICUs so negatively. That is why different units that wished to participate in the survey could not eventually do so due to the high and continuous care load sustained by the healthcare system. However, the study was closed because its primary endpoint was to identify those areas with highest risk for the ICUs. Also, because the results proved the existence of common problems in most centers, which is why we think that the information obtained from areas with most room for improvement can be generalized.
Other limitations we should mention here are those associated with this type of self-evaluation tools. Therefore, the instructions to conduct the self-evaluation survey indicate that it should be conducted by a multidisciplinary team aware of the reality at the ICU setting. However, noone checked any of this. Also, we should mention that there could have been variability regarding the interpretation of the different items of the survey by the teams of each hospital, which can eventually affect the results. Although the hospitals included in the working group were instructed to confirm that everybody had understood the evaluation items proposed, the reproducibility of the survey was never validated.
In conclusion, this study allowed us to identify numerous safe practices whose degree of implementation at the ICU setting is low but should be dealt with to reduce medication mistakes in critically ill patients. Similarly, we believe that this study promoted the use of self-evaluation surveys at the ICU settings, which contributed to making all healthcare workers familiar with safe practices regarding the use of drugs and then be able to launch actions at local level.
AuthorsAll the undersigned authors contributed to the design and methodology of the study. Also, they directed the survey and contributed to the publication of the study. Dr. Otero and Dr. Martín Muñoz performed the preliminary analysis of data that were reviewed by all the authors. Dr. Otero and Dr. Merino de Cos were involved in the manuscript first draft. All the authors reviewed the results and contributed to the manuscript final version that was latter approved by all the authors.
FundingThis study has been funded by SEFH and SEMICYUC.
Conflicts of interestNone whatsoever.
Here is the list of all the centers that participated in the study and the coordinators from each hospital:
Andalusia: Manuel Colmenero Ruiz, Rocío Morón Romero (Hospital Universitario San Cecilio, Granada); Isabel Caba Porras, Trinidad Vilches Medina (Hospital Universitario de Jaén), and Noelia Muñoz Guillén, Asunción Vázquez Castellano (Hospital Cruz Roja de Córdoba).
Aragon: Elena Rebollar Torres, José Ignacio Corchero Martín (Hospital Nuestra Señora de Gracia, Zaragoza).
Balearic Islads: María Asunción Colomar Ferra (Hospital Universitario Son Espases, Palma de Mallorca); Paz Merino de Cos, Fernando Becerril (Hospital Can Misses, Ibiza), and Adriana Martín (Clínica Nuestra Señora del Rosario, Ibiza).
Castile and Leon: Ana María López González, Victor Sagredo Meneses (Hospital Universitario de Salamanca); Paola González Pérez (Complejo Asistencial de Ávila), and Caridad Martín López (Complejo Asistencial de Segovia).
Castile-La Mancha: María Luisa Gómez Grande, Raúl Pérez Serrano (Hospital General Universitario de Ciudad Real); Esther Domingo Chiva, José Luís Cortés Monedero (Complejo Hospitalario Universitario de Albacete); Marta Fernández Arévalo, María José Sánchez Carretero (Hospital Virgen de la Salud de Toledo), and Aida Rueda Naharro, Estela Gomez Lorenzo (Hospital Nacional de Parapléjicos de Toledo).
Catalonia: Laura Doménech Moral, Ricard Ferrer Roca (Hospital Universitario Vall d´Hebrón de Barcelona); Laura María Martínez López, Josep Trenado Álvarez (Hospital Universitario Mutua de Terrassa); Ana Ayestarán Altuna, Josana Fierro Banzo (SCIAS Hospital de Barcelona); Mónica Sanmartín Suñer, María Dolores Bosque Cebolla (Hospital General de Cataluña); Francisco Fernández Dorado, Montserrat Medina Blanco (Clínica Diagonal de Barcelona); Silvia Serdá Sánchez, María Planella Cutrina (Hospital Sant Camil, Barcelona); María Bodí Saera, Laura Canadell Vilarrasa (Hospital Universitario Joan XXIII de Tarragona); Pilar Salvador Collado, Immaculada Vallverdu Perapoch (Hospital Sant Joan de Reus), and Silvia Cano Hernández, María Antonia Balet Duat (Fundación Althaia, Manresa).
Galicia: María Sandra Albiñana Pérez, Enrique Alemparte Pardavila (Complejo Hospitalario Universitario A Coruña), and María Sandra Albiñana Pérez, Natalia Mejuto Montero (Complejo Hospitalario Universitario A Coruña, Coronarias).
Madrid: Silvia Manrique Rodríguez, María Luisa Martín Barbero (Hospital Universitario Gregorio Marañón, Madrid); María Luisa Testillano Tarrero, María José Asensio Martín (Complejo Universitario La Paz, Madrid); María Cruz Martín Delgado, Marta Blasco Guerrero (Hospital Universitario de Torrejón, Madrid); Ana María de Pablo Hermida (Hospital Universitario del Sureste, Arganda del Rey, Madrid), and Gema Baldominos Utrilla, Emilio Nevado Losada (Hospital Universitario Príncipe de Asturias, Alcalá de Henares, Madrid).
Murcia: Consuelo García Motos, Maravillas Alcázar Espín (Hospital Universitario Morales Meseguer, Murcia).
Navarre: Amaia Egües Lugea, Joaquín Lobo Palanco (Complejo Hospitalario de Navarra).
Basque Country: Ainhoa Quintana Basterra, Sebastián Iribarren Diarasarri (Hospital Universitario de Álava-Txagorritxu); Fernando Fonseca San Miguel, Sergio Castaño Ávila (Hospital Universitario de Álava-Santiago); Elena Zavala Aizpurua (Hospital Universitario de Donostia); María Milagros Álvarez Lanvín (Hospital Universitario de Basurto, Vizcaya), and Olatz Ibarra Barrueta, Emilia Cámara Quintana (Hospital de Urduliz, Vizcaya).
Valencian Community: Ángel Sánchez Miralles, María Dolores Martínez Juan (Hospital Universitario Sant Joan de Alicante), and Isabel Font Noguera, María Martín Cerezuela (Hospital Universitario y Politécnico La Fe, Valencia).
Andorra: Antonio Margarit Ribas (Hospital Nostra Senyora de Meritxell, Andorra).