A meta-analysis was performed to assesses the effect of storage age of transfused red blood cells (RBCs) upon clinical outcomes in critically ill adults.
MethodsA comprehensive search was conducted in the PubMed, OVID, Web of Science and Cochrane databases for randomized controlled trials (RCTs) comparing the transfusion of fresher versus older RBCs in critically ill adults from database inception to December 2017. The primary endpoint was short-term mortality, and the secondary endpoints were the duration of intensive care unit (ICU) and hospital stay. The pooled odds ratios (OR) and mean differences (MD) were calculated using Stata/SE 11.0.
ResultsA total of six RCTs were identified, of which four were multicenter studies, while two were single-center trials. The pooled results indicated that the transfusion of fresher RBCs was not associated to a decrease in short-term mortality compared with the transfusion of older RBCs (random-effects OR=1.04, 95% confidence interval (CI): 0.96–1.13, P=0.312; I2=0.0%; six trials; 18240 patients), regardless of whether the studies were of a multi-center (random-effects OR=1.04, 95% CI: 0.96–1.13, P=0.292; I2=0.0%) or single-center nature (random-effects OR=1.16, 95% CI: 0.28–4.71, P=0.839; I2=56.7%), or with low risk of bias (random-effects OR=1.04, 95% CI: 0.94–1.16, P=0.445; I2=0.0%). In addition, the transfusion of fresher RBCs did not reduce the geometric mean duration of ICU stay (1.0% increase in geometric mean, 95% CI: −3.0 to 5.1%, P=0.638; I2=81.5%; four trials; 7550 patients) or the geometric mean duration of hospital stay (0.0% increase in geometric mean, 95% CI: −3.9 to 4.1%, P=0.957; I2=7.4%; four trials; 7550 patients) compared with the transfusion of older RBCs.
ConclusionsThe transfusion of fresher RBCs compared with older RBCs was not associated to better clinical outcomes in critically ill adults.
Se llevó a cabo un metaanálisis para evaluar el efecto de la edad de almacenamiento de las transfusiones de glóbulos rojos (GR) sobre los desenlaces clínicos en adultos críticamente enfermos.
MétodosSe realizó una búsqueda exhaustiva en las bases de datos PubMed, OVID, Web of Science y Cochrane, para localizar ensayos controlados y aleatorizados (ECA) donde se comparase la transfusión de GR más recientes frente a otros más antiguos en adultos críticamente enfermos, desde el inicio de la base de datos hasta diciembre de 2017. El criterio de valoración principal fue la mortalidad a corto plazo, mientras que los criterios de valoración secundarios fueron la duración de la estancia en la unidad de cuidados intensivos (UCI) y en el hospital. La oportunidad relativa (OR) agrupada y las diferencias medias (DM) se calcularon mediante Stata/SE 11.0.
ResultadosSe identificó un total de 6 ECA, 4 de los cuales eran estudios multicéntricos, mientras que los 2 restantes se habían llevado a cabo en un único centro. Los resultados agrupados indicaron que la transfusión de GR más recientes no se asociaba con una disminución de la mortalidad a corto plazo en comparación con la transfusión de GR más antiguos (OR de efectos aleatorios=1,04; intervalo de confianza [IC] del 95%: 0,96-1,13, p=0,312; I2=0,0%; 6 ensayos; 18.240 pacientes), con independencia de si los estudios eran multicéntricos (OR de efectos aleatorios=1,04, IC del 95%: 0,96-1,13, p=0,292; I2=0,0%) o si se habían realizado en un único centro (OR de efectos aleatorios=1,16, IC del 95%: 0,28-4,71, p=0,839; I2=56,7%), o con un bajo riesgo de sesgo (OR de efectos aleatorios=1,04, IC del 95%: 0,94-1,16, p=0,445; I2=0,0%). Además, la transfusión de GR más recientes no redujo la media geométrica de la duración de la estancia en la UCI (aumento del 1,0% en la media geométrica, IC del 95%:−3,0 al 5,1%, p=0,638; I2=81,5%; 4 ensayos; 7.550 pacientes) o la media geométrica de la duración de la estancia hospitalaria (aumento del 0,0% en la media geométrica; IC del 95%:−3,9 al 4,1%, p=0,957; I2=7,4%; 4 ensayos; 7.550 pacientes) en comparación con la transfusión de GR más antiguos.
ConclusionesLa transfusión de GR más recientes en comparación con GR más antiguos no se asoció con unos mejores desenlaces clínicos en adultos críticamente enfermos.
Anemia is a very common complication of critical illness, and previous studies reported1,2 that approximately 40% of critically ill patients receive one or more units of red blood cells (RBCs) during their admission to the intensive care unit (ICU). Anemia always leads to a decrease in oxygen delivery and may cause an imbalance between oxygen delivery and consumption; thus, it results in worse clinical outcomes.2 Therefore, critically ill patients with anemia must be transfused with RBCs to maintain adequate oxygen delivery and improve tissue oxygenation, considering their intolerance to tissue hypoxia.
After being collected from donors, RBCs will be stored in the blood bank for up to 42 days for potential recipients. Unfortunately, RBCs will undergo a series of potentially harmful changes to their structure and function over time during storage. These changes include a reduction in 2,3-diphosphoglycerate and adenosine triphosphate and impaired deformability, which can compromise the oxygen transport function of RBCs.3–5 This so-called “storage lesion” is the potential mechanism with respect to the possible adverse effects of older RBCs on prognosis in transfused patients, especially in critically ill patients. Accordingly, a previous meta-analysis involving 409966 hospitalized patients concluded that the use of older stored blood was associated with a significantly increased risk of death.6 However, the results from recent meta-analyses7–9 show a neutral effect of storage age of RBCs on mortality in hospitalized patients.
Although there is sufficient evidence concerning the relationship between the storage age of RBCs and prognosis in general hospitalized patients, it is still uncertain whether the storage age of transfused RBCs affects clinical outcomes in the special population of critical illness to date. Given that critically ill patients are more susceptible to tissue hypoxia than general patients, transfusion of fresher RBCs may, theoretically, bring benefits to prognosis in criticallly ill patients. A multi-center observational study revealed a lower mortality with transfusion of fresher RBCs in critically ill patients.10 However, several recently published randomized controlled trials (RCTs)11,12 failed to identify any improvement in survival rate with the use of fresher RBCs in transfused critically ill patients. The discrepancy in the results from the observational study10 and these RCTs11,12 should be accounted for to inform clinical decision making, and sufficient evidence is still lacking to support or reject transfusion of fresher RBCs in critically ill patients. Hence, we conducted this meta-analysis to investigate the real effect of the storage age of RBCs on clinical outcomes in critically ill patients.
Materials and methodsSearch strategyThis meta-analysis was conducted in accordance with the PRISMA guidelines. Two authors (Zhou X and Xu Z) independently searched the PubMed, OVID, Web of Science and Cochrane databases of clinical trials for RCTs comparing transfusion of fresher versus older RBCs in critically ill adult patients admitted to the ICU from database inception to December 2017. A comprehensive search was conducted to avoid missing relevant studies, and references from other publications were reviewed. The medical subject headings (MeSH) term included: “erythrocyte transfusion”, “erythrocytes”, “blood component transfusion”, “blood transfusion”, “intensive care units”, “intensive care”, “critical illness”, “critical care”, “time factors”. Other non-MeSH terms included: “red cell”, “red blood cell”, “RBC”, “blood”, “retransfus*”, “transfuse*”, “infuse”, “blood preservation”, “age*”, “aging”, “fresh*”, “old*”, “new*”, “young*”, “store*”, “storage”, “storing”, “preserv*”, “critical care”, “critically ill”, “critical*”, “intensive*”. The detailed search strategy is shown in appendix 1. There was no language restriction in this meta-analysis, and all articles in other languages that provided English abstracts were also considered.
Study selectionAll records searched from the above-mentioned four databases were initially filtered through an automated function to exclude duplicates. Three authors (Wang Y, Sun L and Zhou W) independently screened the titles and abstracts of the remaining records by using a questionnaire, and any disagreements were resolved by consensus. Then, eligible articles were included in a full-text review, and we contacted the authors by email for the full text when the full text was unavailable online. The inclusion criteria included the following: prospective RCTs comparing transfusion of fresher RBCs versus older RBCs and critically ill adult patients (aged≥18 years) admitted to the ICU. The primary endpoint was short-term mortality, which included 28-day mortality, ICU mortality and in-hospital mortality. The secondary endpoints were the duration of ICU stay and hospital stay. Articles assessing at least one of the above-mentioned indicators were included in this study. Pediatric studies, animal studies and retrospective, observational, non-randomized or cohort studies were excluded. Studies lacking data on the endpoints and duplicate published studies were also excluded, and the most recent updated data were included in the final analysis.
Data extraction and quality assessmentTwo authors (Zhou X and Liu X) independently extracted data from the included studies using a custom-made form. Data regarding the name of the first author, publication year, study population, study design, number of subjects, number of RBC units transfused per patient, acute physiology and chronic health evaluation (APACHE) II or III score, hemoglobin level before transfusion, duration of RBC storage, short-term mortality and duration of ICU stay and hospital stay were recorded. In studies in which various short-term mortality were reported, the longer follow-up short-term mortality was included for analysis.
The two above-mentioned independent authors (Zhou X and Liu X) evaluated the internal validity and risk of bias of the included studies for the primary outcome (short-term mortality) according to the Cochrane Collaboration methods13 using standardized criteria, which assigned a value of high, unclear, or low to the following six domains: adequate sequence generation, allocation concealment, blinding, incomplete outcome data, free of selective reporting and free of other bias. We defined in advance that studies with a high or unclear risk of bias in less than two domains would be considered to be high quality.14
Statistical analysisThis meta-analysis was performed using Stata/SE 11.0 (StataCorp, College Station, TX, USA). A pooled odds ratio (OR) with corresponding 95% confidence interval (CI) was calculated to assess the effect of storage age of transfused RBCs on mortality using the Mantel–Haenszel method. Data on duration of ICU stay and hospital stay were pooled by using the mean difference (MD) with the inverse variance method. Heterogeneity among included trials was evaluated by visual inspection of forest plots and quantified with inconsistency factor (I2) statistics using chi-squared test. Both fixed-effects and random-effects meta-analyses were conducted for all endpoints (supplementary Table 1). Given the potential clinical and statistical heterogeneity between the trials (in sample size, primary diagnosis and definition of fresher RBCs), we reported the random-effects pooled result as the main result. However, if one or two trials accounts for approximately 80% or more of the total weight in a fixed-effect meta-analysis, we reported the fixed-effects result as the main result.15 For continuous data reported as the median with the interquartile range (IQR), the mean was estimated using the method of Hozo et al.,16 and the standard deviation (SD) was calculated using the formula proposed by the Cochrane handbook as: SD=IQR/1.35.13 The duration of ICU stay and hospital stay were log transformed before being included in meta-analysis due to the skewed distribution. We used approximations to calculate the mean and standard deviation based on logarithmic transformation using method 1 in Higgins et al.,17 and then the difference in geometric means between the fresher RBCs and older RBCs group was exponentiated to obtain the ratio of geometric means on the un-logged scale to offer interpretable results.18 Subgroup analysis was also conducted for the primary endpoint based on the study design (multi-center or single-center) and the risk of bias of the studies included.
We performed a trial sequential analysis (TSA) to assess the risk of type I error and prevent the increased risk of random error from repeated significance testing.19 TSA was conducted using both fixed-effects and random-effects with a 5% risk of type I error and 80% power for mortality in all studies and high-quality studies with low risk of bias, and we reported the random-effects result as the main result. The required information size was also calculated based on a relative risk reduction of 15% in mortality.
Sensitivity analyses were performed by sequentially excluding one trial at a time to assess the robustness of pooled results for the primary endpoint. A two-tailed P-value <0.05 was considered statistically significant.
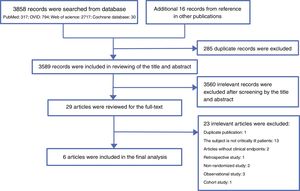
ResultsLiterature searchA total of 3858 records were searched from the above-mentioned four databases through a combination of a MeSH terms search strategy and a liberal terms search strategy; an additional 16 records were also searched from references from the other publications. Among the 3874 total records, 285 duplicate records were filtered; then, 3589 records were included for review of the title and abstract, and 3560 records were ineligible according to our inclusion criteria and were excluded from our study. Subsequently, the remaining 29 records were included for screening the full text. After careful review of the full text, a total of six articles met the eligibility criteria and were included in the final analysis.11,12,20–23 The reasons for excluding the 23 ineligible studies are listed in appendix 2. The PRISMA flow chart of this study is shown in Fig. 1.
Study characteristicsAmong these six studies,11,12,20–23 four studies11,12,20,21 were multi-center RCTs and the remaining two studies22,23 were single-center RCTs; they were all published after 2012, and the number of subjects ranged from 51 to 10578. All six studies reported data on the primary endpoint; however, only four studies reported data on the duration of ICU stay and hospital stay.11,12,21,23 Three studies clearly reported that the subjects in their study were critically ill patients admitted to the ICU.11,12,23 Although the subjects in the other two studies were trauma patients21 and mechanically ventilated patients,22 respectively, all patients in both studies were admitted to the ICU, and therefore, these patients were firmly considered critically ill patients and included in our analysis. However, the subjects in the study by Heddle et al.20 were general hospitalized patients; fortunately, the authors conducted a subgroup analysis for patients in the ICU, and we enrolled these patients in our final analysis. The average number of units of RBCs transfused per patient was 2–4 units and was similar in both groups of patients who received fresher RBCs or older RBCs. The mean or median storage age of transfused RBCs ranged from 4 to 13 days in patients who were allocated to receive fresher RBCs and 22–36 days in patients allocated to receive older RBCs. The detailed study characteristics for the individual trials are summarized in Table 1.
Characteristics of RCTs included in this meta-analysis [mean±SD or median (IQR)].
| First author/publication year | Study population | Study design | Number of subjects | Number of RBC units transfused in per patient | APACHE II/III score | Hemoglobin level before transfusion (g/L or g/dl) | |||
|---|---|---|---|---|---|---|---|---|---|
| Fresher | Older | Fresher | Older | Fresher | Older | ||||
| Cooper/201711 | ICU patients | Multi-center | 4919 | 4.1±6.0 | 4.0±6.2 | 72.6±29.2 | 73.2±29.6 | 74.4±9.8 | 74.3±10.2 |
| Heddle/201620 | ICU patient–subgroup of general hospitalized patients | Multi-center | 10578 | 3 (2–5) | 3 (2–5) | — | — | — | — |
| Lacroix/201512 | ICU patients | Multi-center | 2430 | 4.3±5.2 | 4.3±5.5 | 21.9±7.7 | 21.6±7.6 | 7.69±1.28 | 7.64±1.09 |
| Schreiber/201521 | Trauma patients admitted to ICU | Multi-center | 170 | 2 (1–3) | 2 (1–3) | — | — | — | — |
| Kor/201222 | ICU patients | Single-center | 99 | — | — | 82 (64–108) | 82 (71–112) | 7.8 (7.1–8.8) | 7.9 (7.5–8.4) |
| Aubron/201223 | ICU patients | Single-center | 51 | 3.2±2.6 | 3.8±3.6 | 67.1±30.1 | 55.3±18.3 | — | — |
| First author/publication year | Duration of RBC storage (days) | Mortality | Number of death/total | Length of ICU stay (days) | Length of hospital stay (days) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fresher | Older | Fresher | Older | Fresher | Older | Fresher | Older | ||
| Cooper/201711 | 11.8±5.3 | 22.4±7.5 | 28-day | 476/2457 | 463/2462 | 4.2 (2.0–9.3) | 4.2 (1.9–9.4) | 14.5 (7.4–27.5) | 14.7 (7.4–28.3) |
| Heddle/201620 | 13.4±7.8 | 23.6±9.1 | In-hospital | 472/3553 | 901/7025 | — | — | — | — |
| Lacroix/201512 | 6.1±4.9 | 22.0±8.4 | In-hospital | 403/1212 | 386/1211 | 15.3±15.4 | 15.3±14.8 | 34.4±39.5 | 33.9±38.8 |
| Schreiber/201521 | 7.5 (5–11) | 32 (23–36) | Mortality | 2/86 | 3/84 | 6 (2–11) | 5 (2–11) | 13 (8–20.5) | 13.5 (9–22) |
| Kor/201222 | 4.0 (3.0–5.0) | 26.5 (21.0–36.0) | Mortality | 17/49 | 22/50 | — | — | — | — |
| Aubron/201223 | 12.1±3.8 | 23±8.4 | In-hospital | 5/25 | 2/26 | 11 (5–15) | 7 (3–17) | 21 (12–38) | 17 (8–27) |
ICU, intensive care unit; RBC, red blood cell; APACHE, acute physiology and chronic health evaluation; SD, standard deviation; IQR, interquartile rang.
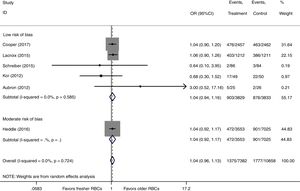
The six domains for assessing the quality and risk of bias for the primary outcome for individual studies are described in detail in Table 2. Regarding random sequence generation, all six trials (100%) had a low risk of bias. In addition, both allocation concealment and selective outcome reporting were assessed as low risk of bias in all trials (100%). Regarding blinding method, five trials11,12,21–23 had a low risk of bias, and one trial by Heddle et al.20 had a high risk of bias. Incomplete outcome data were assessed as unclear risk of bias in all trials (100%). Overall, five trials were judged as having a low risk of bias and were considered to be of high quality.11,12,21–23
Risk of bias assessment for short-term mortality in RCTs included in this meta-analysis.
| First author/publication year | Adequate sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Free of selective reporting | Free of other bias | Overall risk of bias |
|---|---|---|---|---|---|---|---|
| Cooper/201711 | Low | Low | Low | Unclear | Low | Low | Low |
| Heddle/201620 | Low | Low | High | Unclear | Low | Low | Moderate |
| Lacroix/201512 | Low | Low | Low | Unclear | Low | Low | Low |
| Schreiber/201521 | Low | Low | Low | Unclear | Low | Low | Low |
| Kor/201222 | Low | Low | Low | Unclear | Low | Low | Low |
| Aubron/201223 | Low | Low | Low | Unclear | Low | Low | Low |
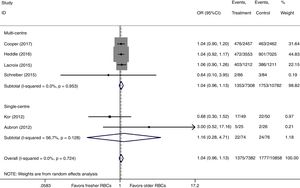
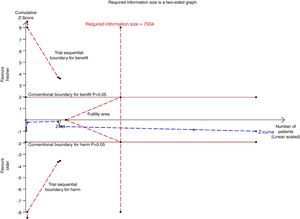
There were six studies, containing 18240 patients in total, that included data on short-term mortality; the pooled results indicated that the transfusion of fresher RBCs was not associated with a reduction in short-term mortality compared with transfusion of older RBCs [random-effects OR=1.04, 95% CI: 0.96–1.13, P=0.312; I2=0.0%] (Fig. 2). The random-effects TSA-adjusted 95% CI was 0.90–1.21, the required information size was 7534 randomized patients and the boundaries for futility were crossed, which indicated that future trials are unlikely to show a 15% relative risk reduction in short-term mortality (Fig. 3).
Trial sequential analysis using random-effects for short-term mortality in all studies included. Short-term mortality of 16.4% in the control arm, diversity of 0.0%, a relative risk reduction of 15%, a risk of type I error of 5% and power of 80%; the required information size of 7534 patients is reached and the boundaries for futility are crossed. The trial sequential analysis adjusted 95% confidence interval for a relative risk of 1.04 is 0.90–1.21.
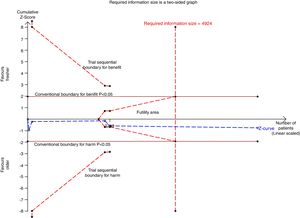
We conducted a stratified analysis based on the study design, and the results revealed a neutral benefit of transfusion of fresher RBCs compared with that of older RBCs on short-term mortality, regardless of whether the studies were multi-center (random-effects OR=1.04, 95% CI: 0.96–1.13, P=0.292; I2=0.0%; four trials; 18090 patients) or single-center (random-effects OR=1.16, 95% CI: 0.28–4.71, P=0.839; I2=56.7%; two trials; 150 patients) (Fig. 2). Furthermore, a similar result was found in the subgroup analysis for high-quality studies with a low risk of bias (random-effects OR=1.04, 95% CI: 0.94–1.16, P=0.445; I2=0.0%; five trials; 7622 patients) (Fig. 4). The TSA was also conducted in the subgroup analysis for high-quality studies with low risk of bias and the random-effects TSA-adjusted 95% CI was 0.89–1.22. Similarly, future trials are also unlikely to detect a 15% relative risk reduction in short-term mortality because the required information size of 4924 patients was reached and the boundaries for futility were crossed (Fig. 5).
Trial sequential analysis using random-effects for short-term mortality in five studies with low risk of bias. Short-term mortality of 22.9% in the control arm, diversity of 0.0%, a relative risk reduction of 15%, a risk of type I error of 5% and power of 80%. The required information size of 4924 patients is reached and the boundaries for futility are crossed. The trial sequential analysis adjusted 95% confidence interval for a relative risk of 1.04 is 0.89–1.22.
There were four studies (7550 patients) that reported data on the duration of ICU stay and the duration of hospital stay. As two trials11,12 account for more than 90% of the total weight in the fixed-effects meta-analysis, we reported the fixed-effects result as the main result. The pooled results showed that transfusion of fresher RBCs was not associated with a reduction in the mean log duration of ICU stay (mean log 0.01, 95% CI: −0.03 to 0.05, P=0.638; I2=81.5%), corresponding to 1.0% (95% CI: −3.0 to 5.1%) increase in the geometric mean, or the mean log duration of hospital stay (mean log 0.00, 95% CI: −0.04 to 0.04, P=0.957; I2=7.4%), corresponding to 0.0% (95% CI: −3.9 to 4.1%) increase in the geometric mean, compared with transfusion of older RBCs (supplementary Figure 1 and supplementary Figure 2).
Sensitivity analysisAs shown in supplementary Figure 3, sensitivity analysis suggested that the overall effect of storage age of transfused RBCs on short-term mortality was not changed after sequentially removing one study at a time.
DiscussionThis meta-analysis of six RCTs was conducted to detect the effect of storage time of RBCs on clinical prognosis in transfused critically ill patients. The results demonstrated that transfusion of fresher RBCs was not associated with a reduction in short-term mortality regardless of whether the trials were multi-center or single-center or with a low risk of bias. Additionally, the durations of ICU stay and hospital stay were not reduced with the use of fresher RBCs compared with that of older RBCs. Overall, the storage age of transfused RBCs did not affect the clinical outcomes of critically ill adult patients.
The “storage lesion” will inevitably affect RBCs during their storage in the blood bank; these lesions may result in impaired deformability and decreased oxygen delivery capacity,3–5 which is the potential explanation for the possible adverse effects associated with transfusion of older RBCs. Several studies have been conducted to support this view. One study by Marik et al.24 found an inverse association between the change in gastric intramucosal pH and the age of the transfused blood (r=−0.71, P<0.001), and the results indicated that transfusion of older RBCs was associated with splanchnic ischemia. Additionally, an observational study conducted by Leal-Noval et al.25 demonstrated no significant increase in cerebral oxygenation after transfusion of RBCs stored more than 19 days. Furthermore, a multi-center observational study suggested10 that transfusion of older RBCs was associated with a higher risk of death in critically ill patients. Therefore, considering that critically ill patients are more sensitive to tissue hypoxia, many clinicians, particularly intensivists, prefer to request fresher RBCs from the blood bank. However, blood banks typically provide the oldest compatible RBCs for the potential recipients.26 The conflicting theories on the transfusion of RBCs demand that researchers provide more compelling evidence with regard to the effects of storage age of transfused RBCs in critically ill patients.
Fortunately, two multi-center RCTs with high quality, namely, the TRANSFUSE trial11 and the ABLE trial,12 were successively published in recent years, both of which showed a neutral effect of transfusion of fresher RBCs on short-term or long-term mortality in critically ill patients compared with that of older RBCs. In the ABLE trial, 2430 critically ill patients were included in the intention-to-treat analysis; 1211 patients were assigned to receive fresh RBCs, and 1219 patients were assigned to receive standard-issue RBCs (older RBCs). They found no significant differences in the 90-day, 28-day, in-hospital or ICU mortality between patients who received fresher RBCs and those who received standard-issue RBCs; moreover, the differences in the length of ICU stay and hospital stay were not significant between the groups. Similar results were observed in the TRANSFUSE trial,11 which focused on the same topic in a similar population. The results from this study indicated that the 28-day mortality (19.4% vs. 18.8%, P=0.61), 180-day mortality (28.5% vs. 28.1%, P=0.75) and length of ICU stay and hospital stay were similar in the short-term storage group and long-term storage group. Recently, another multi-center RCT, namely, the INFORM trial,18 was conducted to detect the association between the duration of blood storage and mortality; however, the subjects in this study constituted a population of general hospitalized patients. Fortunately, the INFORM trial investigators conducted a stratified analysis for patients in the ICU, and the results revealed no significant difference in the in-hospital mortality between the short-term storage group and the long-term storage group (13.3% vs. 12.8%, P=0.54). However, this study did not provide more information about the duration of ICU stay or hospital stay. Interestingly, we found that all mortality rates in the above-mentioned three studies were slightly higher in patients who received fresher RBCs than in patients who received older RBCs, even though no statistical significance was found. These valid results lead us to hypothesize that transfusion of fresher RBCs may adversely affect the prognosis in critically ill patients. Hence, we performed this meta-analysis of RCTs with high quality to assess the real effect of the storage age of transfused RBCs on short-term mortality in the special population of critically ill patients. Finally, the results from our meta-analysis demonstrated that transfusion of fresher RBCs had no beneficial effect on short-term mortality compared with that of older RBCs; on the contrary, the former was associated with a slightly higher risk of death in the short term, even though there was no statistical significance (OR=1.04, 95% CI: 0.96–1.13). Additionally, there was no beneficial effect of the transfusion of fresher RBCs in reducing the length of ICU stay or hospital stay.
Strengths of our meta-analysis include the specificity of the population studied, the larger sample size and the high-quality RCTs included. To date, there are rare meta-analyses that report the relationship between the storage age of transfused RBCs and clinical outcomes in the particular population of critically ill patients, although several meta-analyses concerning the same topic on general hospitalized patients have been published. Six RCTs, five of them considered to be of high quality, were included and more than 18000 critically ill patients were enrolled in our meta-analysis; moreover, the trial sequential analysis demonstrated that the sample size in our meta-analysis was sufficient to detect a neutral effect of storage age of transfused RBCs on short-term mortality, and future studies concerning the same topic are futile. Undoubtedly, our meta-analysis of high-quality RCTs with a larger sample size would, to a large extent, decrease the sampling errors and selective bias and reveal the effect of storage time of RBCs on prognosis more objectively and thus guide clinical transfusion management decisions. However, our study has several limitations. First, the number of subjects in the six RCTs are differed substantially; it ranged from 51 to 10578, and the effect of some studies with a small sample size should be considered in interpreting the results.27 Second, we defined those patients admitted to the ICU as critically ill patients, however, the primary diseases that caused admission to the ICU were diverse and include cardiovascular disease, neurologic disease, trauma, and sepsis. Disappointingly, it is hard to conduct a subgroup analysis based on the primary disease type due to the limited trials included. In addition, the duration of ICU stay and hospital stay in some studies were presented as the median (IQR); then, the SD was estimated using the formula SD=IQR/1.35, and this approximate calculation should be taken into account when interpreting the results. Lastly, the definition of fresher RBCs differed in some studies; e.g., fresher RBCs in the study by Kor et al.22 were defined as a storage duration less than 5 days, and the storage age of older RBCs ranged from 7 to 42 days, whereas Lacroix et al.12 defined fresher RBCs as RBCs stored for less than 8 days in their study. However, in the trials conducted by Schreiber et al.,21 14 days was the threshold value for distinguishing the fresher RBCs from older RBCs. Therefore, there was substantial overlap in the storage age of RBCs in both groups in our meta-analysis, and the overlapping data will inevitably influence the real effect of storage age of transfused RBCs on prognosis.
ConclusionThis meta-analysis suggested that transfusion of fresher RBCs was not associated with a reduction in short-term mortality or the length of ICU stay or hospital stay in critically ill adults compared with that of older RBCs. In brief, transfusion of fresher RBCs was not associated with better clinical outcomes in critically ill adults. Our results support the practice of transfusing patients with the older RBCs in critically ill adults.
Contribution of the authorsXiaoyang Zhou have made contributions for the conception and design of this study, acquisition of data and writing of the paper. Zhaojun Xu have made contributions for the conception and design of this study and review of this paper. Yang Wang, Lingling Sun, Wenwei Zhou and Xianzhong Liu have made contributions for acquisition, analysis and interpretation of data. All the authors have read and approved the data presented in this manuscript and have contributed significantly to the content of the article.
Funding statementThis work was supported by the grants from the Natural Science Foundation of Ningbo (No. 2016A610139). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interestNone.
None.