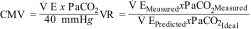To assess the correlation of dead space fraction (VD/VT) measured through time capnography, corrected minute volume (CMV) and ventilation ratio (VR) with clinical outcomes in COVID-19 patients requiring invasive mechanical ventilation.
DesignObservational study of a historical cohort.
SettingUniversity hospital in Medellin, Colombia.
ParticipantsPatients aged 15 and above with a confirmed COVID-19 diagnosis admitted to the ICU and requiring mechanical ventilation.
InterventionsMeasurement of VD/VT, CMV, and VR in COVID-19 patients.
Main variables of interestVD/VT, CMV, VR, demographic data, oxygenation indices and ventilatory parameters.
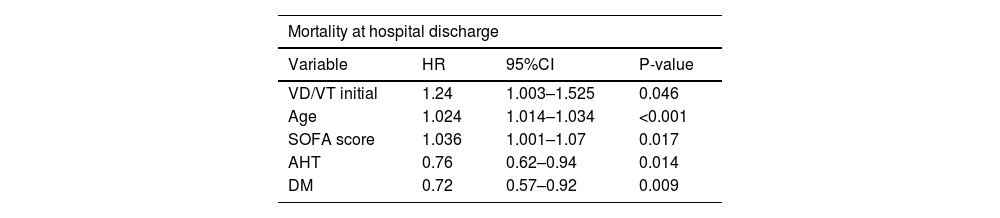
ResultsDuring the study period, 1047 COVID-19 patients on mechanical ventilation were analyzed, of whom 446 (42%) died. Deceased patients exhibited a higher prevalence of advanced age and obesity, elevated Charlson index, higher APACHE II and SOFA scores, as well as an increase in VD/VT ratio (0.27 in survivors and 0.31 in deceased) and minute ventilation volume on the first day of mechanical ventilation. The multivariate analysis revealed independent associations to in-hospital mortality, higher VD/VT (HR 1.24; 95%CI 1.003–1.525; p = 0.046), age (HR 1.024; 95%CI 1.014–1.034; p < 0.001), and SOFA score at onset (HR: 1.036; 95%CI: 1.001–1.07; p = 0.017).
ConclusionsVD/VT demonstrated an association with mortality in COVID-19 patients with ARDS on mechanical ventilation. These findings suggest that VD/VT measurement may serve as a severity marker for the disease.
Evaluar la asociación entre la fracción del espacio muerto (VD/VT) medida mediante capnografía de tiempo, el volumen minuto corregido (VMC) y la razón ventilatoria (RV) con los desenlaces clínicos en pacientes con COVID-19 que requirieron ventilación mecánica invasiva (VMI).
DiseñoEstudio observacional de una cohorte histórica.
ÁmbitoHospital universitario en Medellín, Colombia.
ParticipantesPacientes mayores de 15 años con diagnóstico confirmado de COVID-19 que fueron admitidos en la UCI y necesitaron VMI.
IntervencionesMedición de VD/VT, VMC y RV en pacientes con COVID-19.
Variables de interés principalesVD/VT, VMC, RV, datos demográficos, índices de oxigenación y parámetros ventilatorios.
ResultadosDurante el período de estudio se analizaron 1.047 pacientes con COVID-19 en VMI, de los cuales fallecieron 446 (42%). En los fallecidos se observó una mayor prevalencia de edad avanzada y obesidad, un índice de Charlson más elevado, mayor APACHE II y SOFA, así como un aumento en el VD/VT (0,27 en vivos y 0,31 en fallecidos) y del volumen minuto en el primer día de VMI. El análisis multivariado mostró una asociación independiente con mortalidad hospitalaria, mayor VD/VT (HR 1,24; IC 95% 1,003-1,525; p = 0,046), edad (HR 1,024; IC 95% 1,014-1,034; p < 0,001) y SOFA al inicio (HR: 1,036; IC 95%: 1,001-1,07; p = 0,017).
ConclusionesLa VD/VT mostró una asociación con la mortalidad en pacientes con SDRA por COVID-19 en VMI. Estos hallazgos sugieren que la medición de la VD/VT puede servir como marcador de gravedad de la enfermedad.
Infection due to SARS-CoV-2 was first described in Wuhan (China) in December 2019, and was declared a pandemic in March 2020. Although most affected patients are asymptomatic or present only mild symptoms, up to 5% may suffer serious illness and require admission to the Intensive Care Unit (ICU) – generally due to progressive respiratory failure secondary to acute respiratory distress syndrome (ARDS), which is associated with a high mortality rate.1–3
Acute respiratory distress syndrome is characterized by inflammation, increased vascular permeability and a loss of aerated lung tissue.4 In patients with ARDS, the alveolar damage and vascular alterations can increase the dead space (VD) (well-ventilated but poorly perfused alveoli),2 which has been associated with a poor prognosis.5,6
The dead space can be estimated by measuring the dead space fraction (VD/VT) using the modified Bohr equation or Enghoff formula (VD/VT physiological = PaCO2–PECO2/PaCO2),7 where VT is the tidal volume, PaCO2 is the CO2 pressure obtained from arterial blood gas measurements, and PECO2 is an estimate of exhaled CO2 pressure recorded by volumetric capnography. However, the latter technique is not always available in ICUs.8 Fortunately, some studies have demonstrated a significant correlation between VD/VT recorded by volumetric capnography and time capnography, which is more commonly available in the clinical setting.9
In addition to VD/VT, there are other surrogate measures of dead space, such as the corrected minute volume (CMV)10 and the ventilatory ratio (VR),11 which have also been associated with mortality in ARDS.10
In CMV, V E measures minute ventilation (Vm) in liters/minute, and PaCO2 is obtained from arterial blood gas measurements; the ideal PaCO2 is 40 mmHg. In VR,
predicted V E is obtained by multiplying Vm in milliliters/minute by 100, the predicted body weight, and the predicted PaCO2, which is 37.5 mmHg.
The present study involving a cohort of COVID-19 patients requiring invasive mechanical ventilation (IMV) was conducted to evaluate the correlation of VD/VT measured with time capnography, CMV and VR, and clinical outcomes.
Material and methodsThe present historical cohort observational study was conducted at a University Hospital in Medellín (Colombia) between March 2020 and June 2021. It included patients over 15 years of age with a firm diagnosis of COVID-19, admitted to the ICU and subjected to IMV. Patients requiring less than 48 hours of IMV were excluded, as were those without at least one arterial CO2 pressure measurement (PaCO2) and expired CO2 recording (PECO2); patients referred to other institutions; and patients rejecting the use of their data according to regulation 1581 of 17 October 2012 referred to personal data protection.
The patient cohort underwent measurements of VD/VT, CMV and VR. The study hypothesis was that the VD/VT ratio is related to mortality. In considering the sample size, we included all subjects that met the inclusion criteria during the period of the study. Sampling was not performed, since all eligible patients were included; however, a post hoc estimation was made of the statistical power for a sample of 1047 patients, with alpha 0.05 and a hazard ratio (HR) of 1.24. The estimated value was 0.93.
The data were obtained from the electronic case histories, using a form designed in the REDCAP database of the hospital. The demographic and clinical data included age, gender, weight, and the APACHE II score upon admission. We also recorded ventilatory parameters such as the initial positive end-expiratory pressure (PEEP), plateau pressure, conduction pressure, tidal volume (VT), the need for ventilation in the prone position, CMV and VR measured at the start of mechanical ventilation, and VD/VT defined as ventilation not participating in gas exchange, and which was measured on the first day of IMV using the Enghoff formula7 based on the recorded time capnography variables. Although this approach does not directly quantify the total amount of CO2 exhaled, and thus does not offer an absolute measure of CO2 production, it is considerably more accessible and directly applicable than volumetric capnography - even though the latter offers more precise information on total CO2 production.
In addition, we recorded outcome variables such as in-hospital mortality, and days without ICU stay and ventilation at 28 days.
Use was made of a time capnography adaptor (Nihon Kohden®) and CO2 sensor (Mainstream Capnomed; Nihon Kohden®, Ref.: CMZ70; EtCO2 sensor and airway adaptor).
The measurement of VD/VT was standardized in order to control for biases.
Statistical analysisA descriptive analysis was made of the categorical variables, with the calculation of absolute frequencies and percentages. The quantitative variables were reported as the mean and standard deviation (SD) or as the median (P50) and interquartile range (IQR)(P25-75), depending on whether the data showed a normal distribution or not, respectively, as determined by the Shapiro-Wilk test.
La continuous variable VD/VT was not categorized, in order not to lose discriminative capacity.
The descriptive data were reported according to patient vital status at hospital discharge (survivor/deceased), and the two variables were compared using the Mann-Whitney U-test or Student t-test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables.
The difference of medians was estimated using the Wilcoxon signed-rank test, given a non-normal distribution for the independent variables (age, APACHE-II, SOFA, Charlson index, PAFI [PaO2/FiO2 oxygenation index] before intubation, VT, respiratory frequency, Vm, PEEP and days of stay) between the patients with an initial low and high VD/VT.
Cox multivariate regression analysis was performed, in which the dependent variable was patient vital status at hospital discharge (survivor / deceased), failure was defined as in-hospital death and left censoring was described as survival at hospital discharge. The independent variables were the exposure risk factor and VD/VT. The other variables included in the model were those previously identified in the literature.1,6,12,13
There were no missing data, and so data imputation did not prove necessary. On an exploratory basis, the calculation was made of the discriminative capacity of the model with the corresponding receiver operating characteristic (ROC) curve to predict the outcome.
A post hoc estimate was likewise made of the statistical power of the study in identifying a difference in means of the dead space and survival.
All analyses were made using the Stata/IC 16™ statistical package (Stata Co., College Station, TX, USA), in its version for Microsoft Windows®.
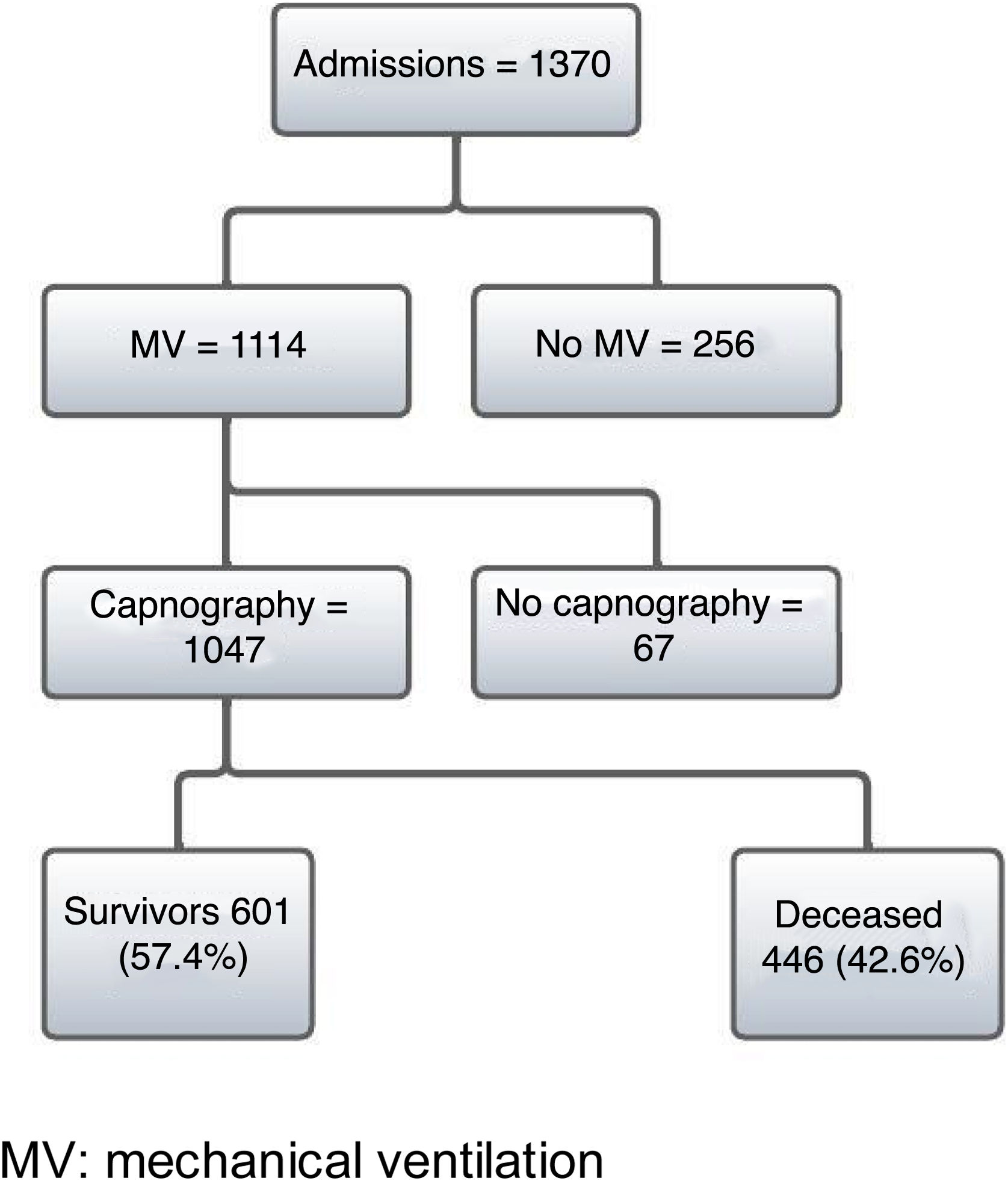
ResultsDuring the study period from 7 March 2020 to 19 August 2021, a total of 1370 patients with a firm diagnosis of COVID-19 were admitted to the ICU. Of these patients, 1114 required IMV and 1047 were subjected to PECO2 measurement using time capnography. Of the 1047 patients, a total of 446 (42%) died in hospital (Fig. 1).
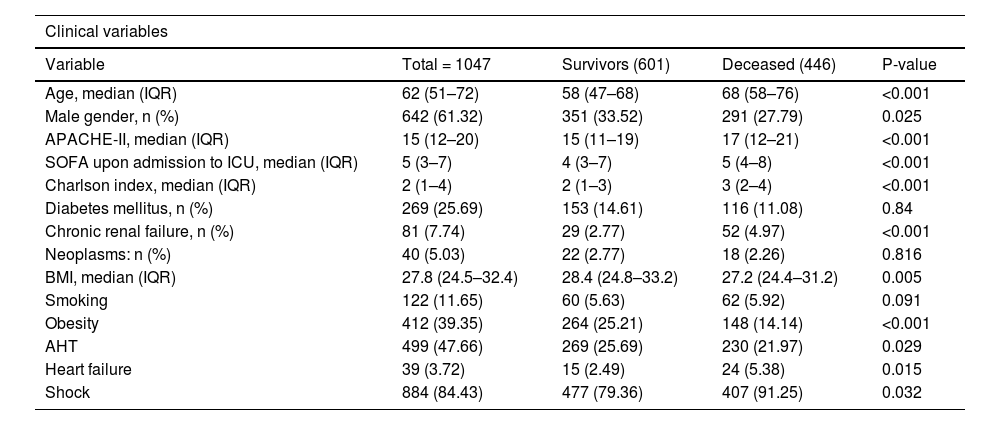
The results of the bivariate analysis are shown in Table 1. Exploration of VD/VT as a numerical variable and survival yielded a non-normal distribution. Among the survivors, the distribution (median and IQR P25-75) was 0.27 (0.2–0.35), while among the deceased it was found to be 0.31 (0.21–0.4)(p = 0.0003). In turn, a greater Vm measured on the first day of IMV was observed among the deceased, as can be seen in Table 2.
Demographic, clinical and ventilatory characteristics of the COVID-19 patients with ARDS on the first day of mechanical ventilation.
| Clinical variables | ||||
|---|---|---|---|---|
| Variable | Total = 1047 | Survivors (601) | Deceased (446) | P-value |
| Age, median (IQR) | 62 (51–72) | 58 (47–68) | 68 (58–76) | <0.001 |
| Male gender, n (%) | 642 (61.32) | 351 (33.52) | 291 (27.79) | 0.025 |
| APACHE-II, median (IQR) | 15 (12–20) | 15 (11–19) | 17 (12–21) | <0.001 |
| SOFA upon admission to ICU, median (IQR) | 5 (3–7) | 4 (3–7) | 5 (4–8) | <0.001 |
| Charlson index, median (IQR) | 2 (1–4) | 2 (1–3) | 3 (2–4) | <0.001 |
| Diabetes mellitus, n (%) | 269 (25.69) | 153 (14.61) | 116 (11.08) | 0.84 |
| Chronic renal failure, n (%) | 81 (7.74) | 29 (2.77) | 52 (4.97) | <0.001 |
| Neoplasms: n (%) | 40 (5.03) | 22 (2.77) | 18 (2.26) | 0.816 |
| BMI, median (IQR) | 27.8 (24.5–32.4) | 28.4 (24.8–33.2) | 27.2 (24.4–31.2) | 0.005 |
| Smoking | 122 (11.65) | 60 (5.63) | 62 (5.92) | 0.091 |
| Obesity | 412 (39.35) | 264 (25.21) | 148 (14.14) | <0.001 |
| AHT | 499 (47.66) | 269 (25.69) | 230 (21.97) | 0.029 |
| Heart failure | 39 (3.72) | 15 (2.49) | 24 (5.38) | 0.015 |
| Shock | 884 (84.43) | 477 (79.36) | 407 (91.25) | 0.032 |
| Ventilatory parameters | ||||
|---|---|---|---|---|
| Variable | Total = 1047 | Survivors (601) | Deceased (446) | P-value |
| VD/VT initial, median (IQR) | 0.28 (0.21–0.37) | 0.27 (0.2–0.35) | 0.31 (0.21–0.4) | <0.001 |
| CMV, median (IQR) | 11.7 (9.4–15) | 11.5 (9.4–14.7) | 11.9 (9.4–15.3) | 0.218 |
| VR, median (IQR) | 2.1 (1.7–2.7) | 2 (1.7–2.6) | 2.1 (1.7–2.8) | 0.532 |
| RF initial, median (IQR) | 27 (22–28) | 25 (22–28) | 25 (22–28) | 0.592 |
| MV initial, median (IQR) | 11.7 (9.4–15) | 10.95 (9–13.5) | 12.7 (9.9–16.5) | <0.001 |
| Pplat initial, median (IQR) | 27 (25–29 | 27 (25–28) | 27 (25–29) | 0.077 |
| PEEP initial, median (IQR) | 12 (12–14) | 12 (12–14) | 12 (12–14) | 0.02 |
| Conduction pressure initial, median (IQR) | 14 (12–16) | 14 (12–16) | 14 (12–16) | 0.002 |
| PAFI before intubation, median (IQR) | 78 (65–100) | 78 (65–106) | 77.5 (63–98) | 0.099 |
| PCO2 | 45 (38–53) | 44 (37–52) | 45 (38–55) | 0.14 |
| Prone position, n (%) | 871 (83.19) | 490 (81.5) | 381 (85.42) | 0.096 |
RF: respiratory frequency; AHT: arterial hypertension; BMI: body mass index; PAFI: PaO2/FiO2 oxygenation index; PEEP: positive end-expiratory pressure; Pplat: plateau pressure; IQR: interquartile range; VR: ventilatory ratio; VD/VT: dead space fraction; CMV: corrected minute volume; VT: tidal volume.
Multivariate Cox regression analysis.
| Mortality at hospital discharge | |||
|---|---|---|---|
| Variable | HR | 95%CI | P-value |
| VD/VT initial | 1.24 | 1.003–1.525 | 0.046 |
| Age | 1.024 | 1.014–1.034 | <0.001 |
| SOFA score | 1.036 | 1.001–1.07 | 0.017 |
| AHT | 0.76 | 0.62–0.94 | 0.014 |
| DM | 0.72 | 0.57–0.92 | 0.009 |
The variables of the model are those considered to be clinically relevant according to the literature and present an association with survival (p ≤ 0.1).
DM: diabetes mellitus; AHT: arterial hypertension; VD/VT: dead space fraction.
With regard to the clinical outcomes, the days without mechanical ventilation and ICU stay at day 28 were 12 (p < 0.001) and 7 (p < 0.001), respectively.
The Cox multivariate regression analysis included the following variables: initial VD/VT, age, gender, body mass index (BMI), APACHE II score, Charlson index, SOFA score, chronic renal failure, shock, smoking, obesity, arterial hypertension, diabetes mellitus, PAFI before intubation, initial plateau pressure, and initial PEEP. The variables showing statistically significant associations are reported in Table 2.
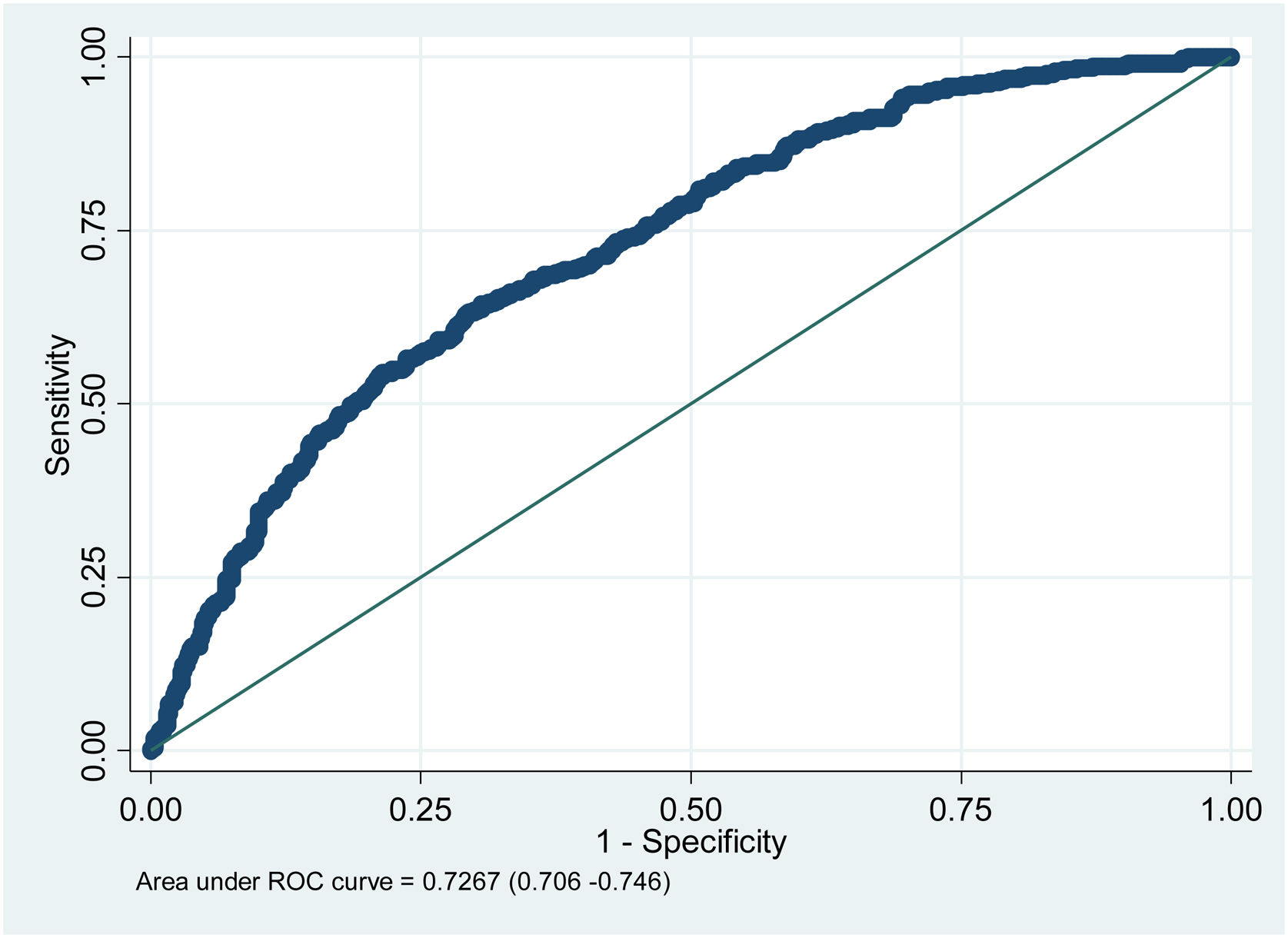
The ROC curve regarding the prognostic performance of the model in relation to its capacity to discriminate mortality risk yielded a value of 0.72 (Fig. 2).
DiscussionThe initial VD/VT, as well as older age and the initial SOFA score, were associated with increased in-hospital mortality among the patients with ARDS due to COVID-19 requiring IMV. In this regard, VD/VT measured at the start of IMV differed significantly between the two groups: 0.27 versus 0.31 (p < 0.001)(HR: 1.24; 95%CI: 1.003–1.525; p = 0.046), suggesting that VD/VT may be an important indicator of severity and of the clinical outcome in patients with ARDS due to COVID-19 subjected to IMV.
The patients with the highest mortality were older individuals, with greater severity according to the APACHE II score, with more organ dysfunction and with higher Charlson index scores, in concordance with the observations of different studies in the United States.14–16 Likewise, the group of deceased subjects presented a greater VD/VT and initial minute volume values, in line with the findings of Xia et al.,5 who investigated the presence of pathophysiological changes in the late stages of severe ARDS among patients with COVID-19. These results have been related to regional ventilation/perfusion heterogeneity,7 loss of lung perfusion regulation and hypoxic vasoconstriction,17 pulmonary microthrombosis18 or an increase in anatomical dead space due to pulmonary structural changes.5
Kallet et al.,19 in a cohort of 59 patients with ARDS of different causes, found VD/VT > 0.6 to be present in all the patients that died, while among the survivors the VD/VT ratio was generally between 0.46 and 0.53. Accordingly, they established a VD/VT cut-off value of 0.55 as being of prognostic value.
Although this study recorded a mean VD/VT of 0.27 among the survivors and of 0.31 among the deceased patients, in concordance with other studies,18,19 the values were comparatively much lower. This may be because VD/VT was recorded at the start of IMV, and no successive recordings were obtained as in the study carried out by Kallet,19 which may evidence greater ventilatory impairment over time.
In a meta-analysis, Jayasimhan et al.12 found the dead space to be autonomously linked to mortality in patients with ARDS, independently of the etiology of the latter. Nuckton et al.,18 in a prospective observational study involving 179 intubated patients with ARDS, found VD/VT, calculated with the Enghoff adaption of the Bohr equation, to be significantly higher among the patients that died (0.63 ± 0.10 versus 0.54 ± 0.09; p < 0.001), in line with the observations of the present investigation. Similarly, Kallet et al.,20 in a retrospective study based on volumetric capnography at the time of diagnosis of ARDS, recorded an association between VD/VT and mortality in patients with moderate to severe ARDS, with a 22% increase in mortality for every 0.05 increment in VD/VT ratio.
In this study, no significant differences were found in comparing VR and CMV between the two groups, which could be attributed to the possible time discrepancy between the gas recordings and the recording of the ventilatory data.
In the multivariate analysis, patient age, the initial SOFA score, and a greater VD/VT ratio were independently associated with increased mortality in this group of patients, in coincidence with the existing literature.14,15 Although arterial hypertension was seen to be associated with mortality in the univariate analysis, in the multivariate analysis it was identified as a protective factor. This may be due to the participation of the renin-angiotensin enzyme system (RAS) with endothelial dysfunction, inflammatory cytokine storm, and the prothrombotic state observed in patients with severe COVID-19, and which could be modulated through the chronic use of RAS inhibitors.21
Although the evidence warrants dead space measurement in critical care, its application at the patient bedside is limited by the lack of measuring instruments such as capnograph monitors or volumetric capnography in many Units,22 as well as by the difficulty of physiologically interpreting the information and the lack of methodologically solid studies evidencing improved patient outcomes with therapeutic strategies seeking to reduce the dead space.23 In heart surgery,24 measurement of the dead space offers valuable information about lung function and facilitates the monitoring of lung recruitment and PEEP adjustment, avoiding regional overdistension.22,25 It can also help to identify patients who might benefit from specific interventions such as the extracorporeal removal of CO2.25
The present study has several strengths, such as the inclusion of a large number of subjects; although the main diagnosis in these patients was ARDS due to COVID-19, the evidence12,19 warrants the association between dead space and mortality in different types of ARDS, including ARDS due to COVID-19. Furthermore, use was made of a measurement strategy that is widely available in most Units, thereby facilitating its application in routine practice.
Our study also has several limitations. In effect, its single-center design could complicate the generalization of the results obtained, though the latter were consistent with the data found in the literature.18–20,26 Although the ideal way to calculate dead space is through volumetric capnography, it is little used in actual practice – a fact that makes our results more applicable on a day-to-day basis. While the Enghoff approach to the calculation of VD/VT may be biased by large shunt fractions present in ARDS, this variable does allow global assessment of the efficiency of lung gas exchange,8 and there is evidence of this calculation with outcomes. Lastly, due to the retrospective nature of the study, there may be problems related to data quality and precision, such as time discrepancies between the gas recordings and ventilatory data registries. Factors such as nursing interventions, physiotherapy and ventilation adjustments may affect data precision by increasing CO2 production by more than 20% during up to 45 minutes.27 The lack of control of confounders is minimized through multivariate analysis, and by including the full patient cohort we were able to minimize selection bias. Considering the mentioned limitations, prospective studies are needed to improve our knowledge of the variables related to ventilation in ARDS.
Despite this evidence, the question of whether guiding the management of ARDS to reduce dead space ventilation improves the clinical outcomes remains the subject of research and could not be addressed by our study.
In conclusion, VD/VT measured from the data provided by time capnography was independently correlated to mortality among the patients with ARDS due to COVID-19. This could offer additional tools for physicians caring for patients of this kind and establish new research hypotheses.
Author contributionsStudy concept and design: Carlos Carvajal, Nelson Giraldo, Andrés David De La Hoz, Carlos Vidal, Hemel Pacheco, David Fernández, Laura González, Silvia Romero, Silvia Vergara, Juan Bolívar, Juliana Correa, Jorge Donado, Alejandro Narváez, Carlos Cadavid, Gisela de La Rosa; Methodology: Carlos Carvajal, Nelson Giraldo, Andrés David De La Hoz, Gisela de La Rosa; Collection of information and formal analysis: Carlos Carvajal, Nelson Giraldo, Carlos Vidal, Hemel Pacheco, David Fernández, Laura González, Silvia Romero, Silvia Vergara, Juan Bolívar, Juliana Correa, Alejandro Narváez, Carlos Cadavid; Writing of the original manuscript: Carlos Carvajal, Nelson Giraldo, Andrés David De La Hoz, Carlos Vidal, Hemel Pacheco, David Fernández, Laura González, Silvia Romero, Silvia Vergara, Juan Bolívar, Juliana Correa, Jorge Donado, Alejandro Narváez, Carlos Cadavid, Gisela de La Rosa; Manuscript review and edition: Carlos Carvajal, Nelson Giraldo, Andrés David De La Hoz, Carlos Vidal, Hemel Pacheco, David Fernández, Laura González, Silvia Romero, Silvia Vergara, Juan Bolívar, Juliana Correa, Jorge Donado, Alejandro Narváez, Carlos Cadavid, Gisela de La Rosa; Supervision: Carlos Carvajal, Nelson Giraldo, Jorge Donado, Alejandro Narváez, Gisela de La Rosa.
All the authors have read and accepted the final version of the manuscript submitted for publication.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Financial supportNone.