We maintain a dynamic position on extracorporeal blood purification therapies (EBPT). Continuous therapies are of choice in the hemodynamically unstable patient. We recommend their early introduction in the course of the disease, and starting with a dose of 30–35mL/kg/h. Above all, however, daily re-evaluation is required of the hemodynamic and metabolic situation and water balance of our patients in order to allow dynamic dose adjustment. Some data suggest that continuous EBPT can favorably influence the clinical course of our patients, even in the absence of acute kidney injury. The potential usefulness of hemofiltration at doses higher than the conventional doses (continuous ultrafiltration >50mL/kg/h or pulses of at least 4h a day to more than 100dosesmL/kg/h) for achieving blood purification has also been commented. We review the possible indications of this technique, together with the peculiarities of implementing these therapies in children.
Creemos que las técnicas de depuración extracorpórea deben seguir un planteamiento dinámico. Las técnicas continuas son de elección en los pacientes hemodinámicamente inestables. Recomendamos un inicio precoz en el curso de la enfermedad y comenzar con una dosis de 30-35ml/kg/h. Pero, sobre todo, deberemos hacer una reevaluación diaria de la situación del paciente (hemodinámica, metabólica y del estado hidroelectrolítico) para ajustar la dosis de forma dinámica. Algunos datos evidencian que las técnicas de depuración extracorpórea continuas pueden influir favorablemente en la evolución del paciente crítico, independientemente de su función renal. Se comenta también la potencial utilidad de usar dosis de depuración superiores a las convencionales (hemofiltración superior a 50ml/kg/h o pulsos de al menos 4h diarias de más de 100ml/kg/h). Revisamos, asimismo, otras posibles indicaciones de las técnicas de depuración extracorpórea, así como las peculiaridades de su aplicación en pediatría.
Acute kidney injury (AKI) is a common and serious problem for the intensive care unit (ICU) patient. In a study undertaken by the Nephrointensive Care Working Group of the Spanish society of intensive care (SEMICYUC) that covered 43 ICUs,1 the reported incidence for AKI was 5.6% (a figure that increased up to 8.6% when coronary patients were excluded) and in a high percentage of patients, AKI developed as a component of the multiorgan dysfunction syndrome (MODS) (up to 93% in this study).
For the last 25 years we have witnessed dramatic changes in the way we manage extracorporeal blood purification therapies (EBPT) in the critically ill. Probably the key of these changes remains in a change in the goals pursued with the treatment and is reflected mainly by a shift from the classic view of purification2 to a more preemptive role in the critically ill patient (CIP), when we aim now to minimize the negative impact of AKI in the evolution of MODS. This new focus (that we prefer to refer to as “renal support” instead of the more limited concept of “renal purification”) explains also why continuous extracorporeal blood purification therapies (CEBPT) have positioned in the last years as a cornerstone in the field of EBPT in the ICU. In the above mentioned Spanish study1 38% of the patients with AKI received EBPT and 84% continuous modalities were used.
This preference can be explained by different circumstances3,4: there is a good hemodynamic tolerance when a CEBPT is used (being the best alternative when hemodynamic instability precludes the use of an intermittent (IHD) modality); it is “slow”, progressive and continuous and therefore avoids the abrupt changes in intravascular volume and electrolyte concentrations that take place during IHD; since it contributes to a lower but continuous elimination of fluid it gives us more room for the administration of parenteral nutrition and intravenous medication, besides providing for a selective removal from the interstitial space; the circuits have a small extracorporeal priming volume and show a lower activation of the complement system (mainly because the use of more biocompatible membranes); and finally because of the low rate of complications reported with its use. And besides all these facts, CEBPT can be safely applied by nursing staff with regular ICU training without requiring specialized staff for IHD.
In this review we will develop an update of the different scenarios where EBPT can be indicated and will distinguish between pure renal indications from other possible “non renal” scenarios for its use.
Indications and timing of renal EBPT (Table 1)The classic scenario for initiation of an EBPT, as collected in the Kidney Disease Outcomes Quality Initiative guidelines (KDIGO)5 and the European Renal Best Practice (ERBP) position statement on the KDIGO,6 makes reference to the urgent indication when the electrolyte abnormalities, acid–base balance, azotemia and fluid overload compromise life.
- -
Initiate EBPT when life-threatening fluid overload, electrolyte and acid–base misbalances are present that cannot be corrected in a conservative way. (Not Graded)
- -
When deciding on the initiation of an EBPT, the clinical context for each individual case must be taken into consideration as well as lab-test trends and how these can be modified by the EBPT, instead of a fixed value for a specific blood marker (i.e. urea or creatinine). (Not Graded)
| Decalogue for EBPT in AKI |
|---|
| 1. AKI is a dynamic process that requires a dynamic approach. |
| 2. Initiation of EBPT in AKI should not be delayed but should start early. |
| 3. We propose to begin the EBPT if any of the following criteria are met: |
| I. Oliguria <200mL/12h. |
| II. Urea >120mg/dL and/or CrCl >25% and/or Cr >1.5x. |
| III. K+ >6mEq/L. |
| IV. Life-threatening situation: APE; Uremia; severe acidosis. |
| 4. Studies on dose have not taken into consideration the dynamic nature of AKI. |
| 5. At least 10% of the scheduled dose is not delivered. |
| 6. We suggest starting with a dose of at least 30mL/kg/h. |
| 7. Regardless of the chosen dose, we must monitor its effect and adjust accordingly. |
| 8. The choice of modality (CEBPT vs IHD) depends on the type of patient, the available infrastructure and our own experience. |
| 9. In the critically ill patient (especially if hemodynamically unstable) CEBPT seems the ideal modality. |
| 10. For a due dose, CVVHDF and CVVH are comparable so, in general, we should start with CVVHDF in order to optimize filters life. |
AKI: acute kidney injury. EBPT: extracorporeal blood purification therapies. CrCl: creatinine clearance. Cr: creatinine. K+: potassium. APE: acute pulmonary edema. CEBPT: continuous extracorporeal blood purification therapies. IHD: intermittent dialysis. CVVHDF: continuous venovenous hemodiafiltration. CVVH: continuous venovenous hemofiltration.
In any case, the ideal timeframe to initiate EBPT in the critically ill AKI patient is still undecided and a matter of continuous debate. It still remains controversial whether a “precocious vs late” indication could impact mortality or renal recovery in our patients. Furthermore, the terms precocious and late are subjective and as such are defined in different ways in the published studies. Right now a recommendation can not be made because we lack a clear reference, however, there is a trend to initiate them early based on several studies with methodology limitations and seems more clear that avoiding its use or delaying its initiation has some impact on mortality and can increase ICU stay.7–10
An additional problem remains in the impossibility to estimate the chances for AKI recovery regardless of the EBPT use and this makes harder the decision about when (or if) initiate the treatment.11 Several renal biomarkers might be useful if prove to be able to detect which patients will most probably recover before12 or after13 the initiation of the EBPT. In this context, it is possible that a furosemide test could be useful in predicting which patients will advance to a more severe stage of AKI.14 Consequently, until high quality evidence (i.e. RCT)9,15,16 is at our disposal a recommendation of early vs late initiation of the EBPT cannot be made.
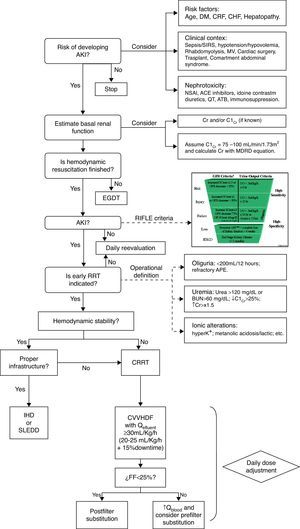
Therefore, if we take into consideration the evidence already published, we can define two approaches to the initiation of EBPT in AKI: (1) when there is an “absolute indication” as acute refractory cardiogenic pulmonary edema, toxic hyperkalemia or uremic symptoms and (2) when we consider a “precocious indication” as have been indicated by a urea over 120mg/dL, BUN over 60mg/dL, creatinine over 1.5 times the baseline, decrease of the creatinine clearance below 25%, diuresis less than 200mL/12h, metabolic acidosis, lactic acidosis or moderate-severe hyperkalemia (Fig. 1).17We have even less information about the timing for withdrawal of EBPT. Of course it is sensible withdrawing the treatment once the kidneys show enough improvement in function, however, the key question is how define improvement in function while EBPT is running. Observational studies have shown that the most important predictor for a successful withdrawal of EBPT is a sufficient production of urine. An urine output over 400mL/day without diuretics administration is a reasonable parameter, resulting in a correct decision in over 79% of cases.18 Another approach can be the estimation of glomerular filtration rate but the precise level of endogenous creatinine clearance necessary for a successful withdrawal of renal support has not yet been established but it is assumed to be between 15 and 20mL/min.19
Algorithm for initiation of CEBPT in critically ill patients. AKI: Acute kidney injury. DM: Mellitus diabetes. CRF: Chronic renal failure. CHF: Congestive heart failure. MV: Mechanical ventilation. SIRS: systemic inflammatory response syndrome. NSAI: nonsteroidal anti-inflammatory. ACE: converting enzyme inhibitors. ATB: antibiotics. CrCl: Creatinine clearance. Cr. Creatinine. EGDT: Early goal-directed therapy. RRT: extracorporeal blood purification therapies. K+: Potassium. APE: Acute pulmonary edema. CRRT: Continuous extracorporeal blood purification therapies. IHD: Intermittent dialysis. SLEDD: Sustained low-efficiency daily diafiltration. CVVHDF: Continuous venovenous hemodiafiltration. Q: Flow. FF: Filtration fraction.
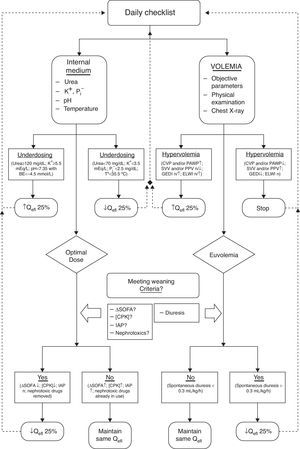
We, therefore, advocate for a dynamic approach when prescribing a EBPT, similar to the way we work with, for example, mechanical ventilation.17,20 For this approach to be successful we must count on a (daily) “check-list” of therapeutic objectives (addressing internal environment and volume status, either) for guidance in tapering the delivered dose until we can confidently suspend the EBPT21 (Fig. 2). Once the patient is capable by itself to maintain the internal milieu homeostasis and fluid balance without EBPT, we can definitively finish the treatment.
Algorithm for maintenance and withdrawal of CEBPT in critically ill patients. K+: Potassium. P: Phosphorus. BE: base excess. T°: Temperature. CVP: central venous pressure. PAWP: pulmonary wedge pressure. SVV: stroke volume variation. PPV: Variation in pulmonary pressure. GEDI: Overall volume index at the end of diastole. ELWI: Indexed extravascular lung water. Qefl: Effluent flow. ΔSOFA: SOFA Score increase. CPK: Creatine fosfoquinase. IAP: Acute pulmonary edema.
A significant number of CIP with AKI will require the implementation of an intermittent or continuous EBPT5 and the first step will be precisely the choice between one of them (intermittent vs continuous, in their different modalities). This decision will be based on the availability and the experience of the medical team, the hemodynamic status and characteristics and underlying pathology of the patient. It seems clear nowadays that peritoneal dialysis has been superseded in critically ill adult patients and it will only be used when other methods are not available. As discussed earlier when addressing the dynamic approach for the indication and management of EBPT, another aspect to be considered is the shifting between modalities to fit them to the clinical (and changing) situation of each individual patient (hemodynamic status, blood clotting problems, etc.).5,6
Over 20 clinical trials and more than 5 meta-analyses have been published analyzing differences between IHD and various modalities of CEBPT in the CIP, looking after mortality or renal recovery as main outcome22,23 but besides the fact that CEBPT facilitates the management of the instable patients none of those modalities has proven superiority over the others.
We can argue that, due to the sudden changes in the distribution of fluids between body compartments following the fast removal of liquids propitiated by IHD, CEBPT seem to be the best option for the hemodynamically unstable patient.3,6 This is because the osmolar disequilibrium that IHD induces (with the shift of water that ensues), continuous modalities must be used in patients with brain injury,3,6 as a matter of fact a slower change in solutes and water avoids negative effects on intracranial pressure. On the other side, the rapid elimination of toxins occurring in the IHD, makes this procedure the suitable choice in cases of toxic hyperkalemia, poisoning or tumor lysis syndrome (recommendation not graded). Furthermore, a lower requirement for anticoagulation on the extracorporeal circuit during IHD, and the short period of time during which it is running confreres certain advantages for the intermittent over the continuous modalities. For examples, IHD seems more suited for bleeding patients with coagulopathy or patients subjected to frequent diagnostic and/or therapeutic interventions, involving the need for hospital transfers (not graded).
But as a whole, despite the fact that some studies have shown a tendency toward a better evolution of the patients treated with some of the continuous techniques, and taking into consideration the methodological flaws detected in all the RCT referred previously, we must conclude that both (intermittent and continuous) are equivalent therapies and that, as a result, there is no ideal universal method for all our patients and, in addition to this, that there is no ideal method for a single patient during the entire process of the disease. So, once more, the choice between different modalities of EBPT must be continuously adapted to the clinical condition of our patients.20
As for the “hybrid therapies”, the fact is that there are no studies comparing them with IHD and the few that compare them with CEBPT do not show homogeneous results. Moreover, there is fewer experience reported with this modality than with intermittent or continuous modalities.
Dosing the extracorporeal purification therapyGiven the complexity of the CIP that develops AKI, it has been argued the possibility that implementing a EBPT and adjusting the delivered dose may have a low impact on the outcome for those patients that show the lesser and highest severity (i.e. those that will probably recover or die whatever we try) but could make a difference for the group of intermediate severity.5,6 It is for this group that a dynamic tailoring of the dose to the patient's clinical condition seems more reasonable.17,20,21 In addition, it is possible that this group would benefit the most of a timely initiation of the therapy (before the patient becomes too ill). We believe that EBPT dose should be decided on the basis of the clinical condition (before actually initiating the therapy) and should be frequently reevaluated to adjust continuously the prescription.6
When prescribing a CEBPT, the dose must be calculated in mL/kg/h of effluent obtained (not graded) starting with a recommended dose of at least 30mL/kg/h.5,17 We must take into consideration that the delivered dose is more often than not below the proposed initially (unavoidable down runtime because different causes) and hence we recommend to begin with a dose between 30 and 35mL/kg/h. After this initial prescription is ongoing, we suggest to re-evaluate daily the hemodynamic, metabolic and water status in order to adjust the dose consequently (Fig. 1).17,20,21 Likewise, it is necessary to readjust the dosage of those prescribed drugs that are cleared by the EBPT according to the changes in its dosage.5,6,17
When prescribing an IHD or hybrid therapy, some disputes persist regarding dosage. According to the KDIGO guidelines and its adaptation by the Scientific Society of Oceania the recommended prescription amounts to a Kt/Vd (K: urea clearance of the hemofilter; t: duration of the session; Vd: volume of distribution of urea) of 3.9 per week5,22 but in regard that Kt/Vd has only been validated for chronic renal patients (rather different from critically ill patients with AKI developing often as part of a MODS) and that this estimate monitors the efficiency of the therapy only based on urea kinetics, the European Renal Best Practices – ERBP – does not recommended Kt/Vd but instead to adjust the dose to the metabolic and body water requirements of the patient as proposed in different studies.6
Other “non renal” possible indications for EBPTSince the beginning of the use of these techniques, some additional benefits apart from those directly derived from the normalization of the internal milieu have been considered in the CIP setting. In this regard, some data point to the possibility that CEBPT could influence favorably the clinical course and, possibly, the outcome of these patients, even in absence of AKI (Table 2).
| Other possible indications for EBPT | Grade |
|---|---|
| MODS: | |
| Hemodynamic | A |
| Respiratory | B |
| Cardiac failure | B |
| Fulminant hepatic failure | B |
| Crush syndrome | C |
| Intoxications | A |
| Brain edema | B |
| Metabolic acidosis | A |
| Electrolyte abnormality | A |
| Hypotermia/hypertermia | A |
MODS: multiple organ dysfunction syndrome. GRADE: methodology for formulating and grading recommendations in clinical practice.
Managing fluid overload is difficult in unstable patients. It is a real challenge to regulate intravascular and extravascular volume, especially in patients in septic shock. An early and adequate resuscitation followed by a subsequent conservative fluid strategy has been shown to be associated with significant improvement in outcome23 and fluid overload has been proven an independent predictor of mortality in CIP and is now considered an undesirable effect of resuscitation strategies. However on the other side, aggressive removal of excess fluid can prove a difficult task and even contribute to hypovolemia, increasing vasopressors needs and exposing the patients to an unnecessary risk resultant from beta and alpha receptor stimulation.24
Fluid removal by ultrafiltration, unlike the effect of diuretics, has the capability to extract volume both from the intravascular and the interstitial compartment at a similar rate due to an isotonic “dehydration” that promotes a continuous intravascular refilling from the interstitial compartment at the same rate as the fluid is extracted from the body. As long as this refilling capability is maintained the fluid extraction will be well tolerated.Slow continuous ultrafiltration (SCUF) is a CEBPT modality that aims to remove excess fluid and for its use requires a low convective and blood flows (50–100mL/min) by means of a highly permeable filter and without need for replacement fluid. SCUF has shown some utility in patients with acute cardio-renal syndrome once tubular drug-resistance ensues.25 A “classic” study26 has shown how daily sessions of fixed dosed SCUF have a positive impact on survival of CIP with congestive heart failure and, not less important, on their perception of quality of life. Bart et al.27 carried out a controlled pilot study (RAPID-CHF), including 40 patients with congestive heart failure (20 patients treated conventionally vs 20 implementing conventional treatment plus ultrafiltration) that proved that SCUF provides significantly more fluid removal than the conventional treatment and higher relief of the cardiac failure symptomatology. In this same line, Constanzo et al.,28 in a controlled study including 200 patients with congestive heart failure (UNLOAD), compared the clinical evolution between patients receiving conventional treatment with others that were managed exclusively with SCUF. The main result was a superior fluid removal in the SCUF group, with a longer symptom-free and hospitalization period. More recently, the ULTRADISCO study, published in 2011,29 demonstrated in 30 decompensated heart failure patients that SCUF, compared with intravenous diuretics, didn’t only achieve a greater clinical improvement, but also showed a significant improvement in a number of hemodynamic parameters.
Nevertheless, in 2012 the continuation of the pilot experience by Bart et al., known as the CARRESS-HF study, was published,30 this time with negative results. The study has been criticized due to several methodological problems: i.e. fixed SCUF dosage (200mL/h), authors do not use on line hematocrit neither hemodynamic parameters for guiding dosage, patients with Cr>3.5 and use of vasodilators and inotropic agents are excluded (those who would have benefited more from SCUF), the definition of overload is not very reliable (CVP>10, leg edema, etc.), and introduced combined end points.
Waiting for new studies that clarify the role of SCUF and from the point of view of a dynamic approach in the management of the critically ill patient, to include parameters of hemodynamic tolerance and refilling capability in order to adjust the “dehydration rate” of our patients can make of SCUF an emerging, effective and safe option for the handling of serious fluid overload, especially in hemodynamically unstable patients.
Acute hepatic failureThere are different case series in the literature reporting the use of CEBPT as a component in the management of hepatic encephalopathy in the context of fulminant hepatic failure.31 Nowadays specific techniques of extracorporeal depuration for liver failure could increase the apparent benefits of these techniques.
IntoxicationsEBPT is the treatment of choice when an intoxication is accompanied by electrolyte imbalances, AKI or hemodynamic instability. The use of a continuous technique supposes an additional benefit preventing the rebound effects of some intoxications as lithium, valproic acid, N-acetil procainamide, methotrexate, teophylline and metformin.32 Experts recommend EBPT at lactate concentrations >20mmol/L or pH ≤7.2, in case of shock or decreased level of consciousness, and when standard supportive measures fail.33 CEBPT is thought to be physiologically more appropriate than IHD, mainly because its large volume of distribution within a two-compartment pharmacokinetic model that implies that metformine may be more effectively cleared by prolonged EBPT. This was corroborated by Keller et al.34 who recently showed a dramatic reduction of metabolic acidosis and decrease of metformin plasma concentration within the first 24h after initiating CEBPT.
Metabolic acidosisDue to the already referred capability of CEBPT for removal of high amounts of fluids, these modalities enable the administration of important amounts of bicarbonate to patients with severe acidosis, diminishing the risk of hypernatremia or fluid overload. Severe lactic acidosis is now a generally accepted indication for CEBPT. The alkalosis secondary to the use of high doses of citrate as anticoagulant has been employed in some isolated reports as an adjuvant in the management of patients with acidosis.35
Hyperthermia/hypothermiaExtracorporeal circuits allow for cooling or heating the patient's blood through the control of the temperature of the fluids used or the blood inside the circuit, and could be used as hyperthermia treatment or in severe and refractory to conventional treatment hypothermia. Accidental hypothermia is a problem of public health not negligible, both for its intrinsic importance, and its prognostic influence in other pathologies as severe trauma (as part of the “triangle of death” of hypothermia, coagulopathy and bleeding). While cardiopulmonary bypass is considered the most effective method for warming on severe hypothermia (central temperature <28°C), the invasiveness of this technique makes it suitable in most cases. Another simpler option is the venovenous rewarming, which is significantly faster than conventional methods, and may be useful in certain cases.3
CEBPT could be also used to facilitate the normo-hypothermia patient to certain diseases, such as septic shock, Out-of-Hospital Cardiac Arrest, severe brain trauma, etc.; control that could have a prognostic significance. Several authors have introduced temperature control between the criteria to indicate a CEBPT in critically ill patients.36
Electrolyte abnormalityAlmost any electrolyte abnormality can be successfully treated with a suitable technique combined with adequate amendments in the fluids for dialysis/replacement. CEBPT have been successfully used to treat patients with hyperkalemia, dysnatremia or hypercalcemia refractory to standard measures.3,36
Crush syndromeCrush injury is a serious medical condition characterized for hyperkalemia, myoglobinuria and acute renal failure. The reported experience shows that with the use of adequate prophylactic measures the impact of a EBPT is minimal in the management of these patients37 but when in need for its use, CEBPT based on convection can show some advantages due to its capability for removal of myoglobin (not cleared by diffusion because its molecular weight) and furthermore a more accurate control of fluid than for intermittent techniques.
BurnsAcute renal failure is a common condition in patients with major burn injuries. CEBPT is generally used in patients who cannot tolerate intermittent therapies, allowing optimum control of the catabolic state and fluid handling.38
Brain edemaCEBPT should be the first option for these patients since it is associated with less increase in ICP compared with intermittent therapies and is also associated with better maintenance of the auto-regulation of cerebral blood flow after traumatic brain injury. This advantage is due to a slower modification in solute concentration, avoiding large osmotic fluctuations and fluid shifts.39
Cardiac surgerySome groups have demonstrated the utility of CEBPT in patients subjected to cardiopulmonary by-pass during a cardiac surgery.40,41 Indeed, this technique reduces the hemodilution that takes place during the intervention and mitigates the secondary inflammatory response, resulting in a positive effect on the hemodynamics of our patients, an effect detected in adults as much as in children, but being especially useful in the latter.
High volume hemofiltration (HVHF)The use of high doses of a convective therapy regardless of the renal function of the CIP, high volume hemofiltration (HVHF), has been advised as a mean for the elimination of inflammatory mediators and or other toxic substances. The problem for this modality is a lack of agreement over what “high volume” means. In the consensus conference of “Acute Dialysis Quality Initiative” (ADQI) held in 200242 HVHF was defined as a volume of effluent over 35mL/kg/h but even this threshold has been challenged and 35mL/kg/h is not to be considered high enough to CEBPT “high volume” according to current practices.
In 2007, at the Pardubice Consensus Conference,43 HVHF was defined as a dose greater than 50mL/kg/h in a continuous basis or a prescription over 100mL/kg/h as a pulse of at least 4h/day, followed by a more conventional dose for the rest of the day (Table 3).
In severe systemic inflammatory conditions, such as sepsis or acute pancreatitis, organ dysfunction (MODS) is a common occurrence and the pathophysiological alterations involved rest in the release of various inflammatory mediators that exert a modulatory function on the response system. CEBPT and specifically HVHF can have a role as a rescue therapy and even play a central role on the resuscitation management of MODS. Different mechanisms have been proposed to explain the beneficial effect of HVHF in severe inflammatory conditions.44,45 The clearance of inflammatory mediators from the circulatory system in periods in which they show a high concentration can be effective in reducing their plasmatic peak and its derived harmful effects. Moreover, the elimination of these molecules from the blood decreases their concentration in tissues as well (where they produce tissue damage) by means of a balance between these two compartments; this effect can explain why some studies show beneficial effects without detecting any changes in the plasma concentrations of these mediators. Furthermore, an increased lymph flow induced by high fluid shifts during HVHF exerts a flushing effect at the tissue level. Finally, it has been suggested recently that HVHF can act directly on the cellular level, restoring the immune function of monocytes and neutrophils.
It is important to note that along these potential beneficial effects, HVHF can also show serious side effects. It increases the losses of valuable molecules (drugs, electrolytes, vitamins or trace elements),20 forcing a close monitoring of their clearance, a control difficult to perform accurately in clinical practice. Other known risks after CEBPT (anticoagulant-related hemorrhage, infection, embolism, hemodynamic intolerance) have also been reported. Complex techniques such as HVHF can compromise patient safety by multiplying the risk of errors, that may also have amplified consequences (important even in small time periods). It is therefore essential the use of these therapies with a rigorous quality and safety control.46
Potential indicationsHVHF has been proposed as a mean for organ support in CIP with high risk of death, regardless of renal function, as can be (for instance) septic shock, post-resuscitation syndrome, post-surgery cardiac shock, acute pancreatitis or acute liver failure, especially when a severe hemodynamic compromise and dependence on high doses of vasoactive drugs are present, the rational resting in the clearance of circulating inflammatory mediators already discussed.
Preclinical studies and pilot studiesVarious animal studies47,48 have shown that HVHF decreases the plasma concentration of inflammatory mediators and improves hemodynamics and survival in sepsis and pancreatitis. In some of them a dose–response relationship was found (greater effectiveness at higher doses and frequent changes of filter) and also a relationship with the membrane used (polyacrylonitrile being found more effective than polysulfone). Nonetheless these studies should be interpreted cautiously because of the difficulty in translating results of animal studies to the clinical practice. Many preliminary clinical studies have shown potential benefits with different techniques when comparing high versus conventional dose hemofiltration; however, these studies have considerable methodological problems.
EvidenceIn line with the initial animal studies on the usefulness of HVHF for severe systemic inflammation,47,48 Journois et al. demonstrated the beneficial effect of HVHF (100mL/kg/h) in the management of children with SIRS in postoperative cardiac surgery with cardiopulmonary bypass49 but after these experiences, the use of HVHF has not demonstrated clear clinical benefits in large clinical trials. Nonetheless, experimental and preliminary clinical studies suggest that this technique can improve organ dysfunction and hemodynamics in septic shock and other clinical situations.
Severe sepsis and septic shockCole and colleagues50 demonstrated in a randomized, controlled study, with a crossover design, carried out in 11 patients, that HVFV (6L/h for 8h) provided a clear hemodynamic benefit compared with conservative CEBP (1L/h for 8h). These findings were subsequently confirmed in a pilot study comparing different doses of purification (65 vs 35 vs 20mL/kg/h) as hemodynamic optimization therapy.51
There are several other available studies that show positive results with the use of HVHF as a rescue therapy in severe sepsis and septic shock:
Oudemans et al.52 evaluated in a prospective cohort study whether HVHF had any impact on mortality of CIP and found a significantly lower mortality for these patients based on APACHE II, SAPS II and Liaño index. Honoré et al.53 in a group of 20 patients with severe circulatory failure secondary to septic shock despite conventional treatment, evaluated the response to a HVHF round (35L in 4h). In the group of 11 “responders”, mortality was 18%, much lower than expected (p<0.05). Furthermore, they noted that the group of “responders” was initiated with the EBP in an earlier stage and the delivered dose was significantly higher than for the “non-responders” group.
In a later study from Joannes-Boyau et al.,54 the use of HVHF 40–60mL/kg/h for 96h in 24 patients with septic shock was associated with significant hemodynamic improvement, as well as in the mortality observed at 28 days (compared with expected mortality).
Ratanarat et al.55 analyzed the effect of high volume “pulses” of 6–8h of 85mL/kg/h, followed by 16–18h of 35mL/kg/h in 15 patients with severe sepsis and found a positive effect on hemodynamics and expected survival.
More recently, Piccinni et al.56 analyzed the effect of an early pulse of 6h HVHF (45mL/kg/h) (within the first 12h after admission) in septic shock patients, followed by conventional dose CEBPT. In this retrospective study of 80 CIP they found a significant hemodynamic and respiratory improvement after the initiation of HVHF.
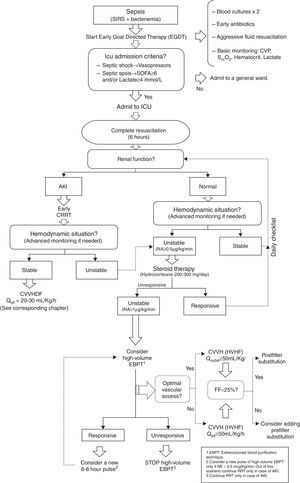
Some other authors have proposed including HVHF in the management of patients in refractory septic shock, in order to stabilize the hemodynamic status and our group has proposed a similar algorithm57 (Fig. 3).
HVHF management algorithm in unstable critically ill patients. SIRS: systemic inflammatory response syndrome. CVP: central venous pressure. ScvO2: Venous oxygen saturation. ICU: Intensive Care Unit. SOFA: SOFA Score. AKI: Acute kidney injury. CRRT: Continuous extracorporeal blood purification therapies. NA/NE: Noradrenaline. CVVHDF: Continuous venovenous hemodiafiltration. Qefl: Effluent flow. EBPT: extracorporeal blood purification therapies. CVVH: Continuous venovenous hemofiltration. HVHF: high volume hemofiltration. Qsubst: Effluent flow. EBPT: Extracorporeal blood purification therapies.
In an attempt to strengthen the role of HVHF, the IVOIRE European study58 was launched in 2005. It was a multicenter, randomized, controlled trial in which they use a fixed-dose of CEBPT (35mL/kg/h compared with 75mL/kg/h in septic patients with AKI). The study was conducted in 18 ICUs in France, Belgium and the Netherlands, looking for the impact of such therapy on mortality at 28 days. 140 patients (137 analyzed) were recruited but the study stopped early because of difficulties with the recruitment. The results of this study, and a recent meta-analysis based primarily on this study59 show a lower than expected mortality in both groups (37.9% vs 40.8%), but similar between the two treatment groups (as expected because the study design).
Severe acute pancreatitis (SAP)Several authors have published studies in humans, with different designs, that seem to support the usefulness of HVHF on MODS after SAP. In a study by Jiang et al.,60 with 37 patients comparing 1L/h vs 4L/h, as well as early (48h) vs late (96h) onset, survival at 14 days was significantly better in the higher dose group (94.4% vs 68.4%; p<0.01) and a more noticeable hemodynamic improvement was also detected. A similar benefit was found in the early treatment group. Meanwhile, the group of Zhu et al. published in 2011 a study on 63 patients61 in which the role of early beginning of HVHF (75mL/kg/h on first 24h of ICU admission) was evaluated in patients with SAP. The main result of the study was that early use of HVHF (for an average of 5 days) in patients with SAP improved survival at 28 days (81% vs 57.6%; p=0.026). Oddly enough, no hemodynamic benefit was demonstrated.
HVHF after post-resuscitation syndromeThe similarities detected in MODS after recovered cardiac arrest with that of the sepsis patient motivated a randomized clinical trial to assess the usefulness of HVHF62 in this setting. In this study, three groups were defined: one group was treated with a pulse of 8h of HVHF (200mL/kg/h); in a second group, a moderate therapeutic hypothermia was added, and the third group it was managed conservatively without EBPT or hypothermia. The results in mortality at six months showed a statistically significant improvement in patients receiving HVHF alone against HVHF plus hypothermia (45% vs 21%; p=0.026), and then both groups against the conventional medical management.
HVHF in other types of SIRSSome promising studies have been conducted on the role of HVHF on patients with severe trauma or major burns63,64 an also on patients in shock after cardiac surgery, but in this last population, the HEROICS study65 have shown negatives results. This was a randomized, multicenter clinical trial involving 224 patients randomized to receive early HVHF (80mL/kg/h for 48h), followed by a standard dose EBPT until the resolution of shock and acute renal dysfunction, or a standard dose EBPT (only in cases on AKI). Although no differences in mortality or duration of mechanical ventilation were detected, patients under HVHF showed a faster correction of metabolic acidosis and a tendency toward fastest reversal of shock, but also had more often hypophosphatemia, thrombocytopenia and metabolic alkalosis.
Alternative therapiesIn addition to HVHF, other forms of blood purification can be helpful: coupled filtration–adsorption, obtained by modifications on the structure and composition of the membranes; hemoperfusion with polymyxin-B or the use of membranes with a high sieving coefficient. These techniques are promising, but nowadays they are still experimental. Future research should address both the understanding of the pathophysiology of severe inflammatory conditions along the effect of the different modalities of blood purification, besides the development and application of technical improvements and a greater attention to their safety.
Differential features in CEBPT application in pediatricsIn children, the most common indication for CEBPT is fluid overload resistant to diuretics (early indication, anticipatory) and more specifically, although uncommon, a blood purification indication in the context of inborn errors of metabolism (hyperammonemia and organic acidemia),66–68 which are more efficiently purified by CEBPT than by peritoneal dialysis.
EBPT in pediatrics: key pointsThe use of CEBPT in younger children differs significantly from adults because of the amount of blood that remains in the extracorporeal circuit (designed for adult size).69
- 1.
Vascular access and blood flows. Blood flow usually ranges between 2 and10mL/kg/min69 with a minimum of 30–50mL/min, although flows above 80mL/min are advisable to prevent extracorporeal circuit coagulation. These flows demand the use of a vascular access size of at least 6.5–8 Fr that can not be anatomically possible to insert in newborns and small infants.70 These catheters occupy a larger proportion of the vessel diameter, so they are more prone to wall troubles, problems of venous return and thrombosis. When using regional anticoagulation strategies it is possible to maintain flows in the lower range without these concerns.71
- 2.
Selection of the filter in relation to weight (Table 4). Although the extracorporeal circuits and filters try to adapt to pediatric size, they remain inadequate for the smallest patients. They might represent a volume of more than 10% in children under 10kg and develop therapy overdose, despite prescribing in normal ranges (10–40mL/kg/h).
Table 4.CEBPT filters used in pediatrics.
Monitor-filter Surface (m2) Blood volume (filter+circuit) Patient weight PRISMA M10 (Gambro) 0.042m2 75mL* (*25mL hotline) 2–15kg* (*discouraged by multiple coagulation system) Prismaflex HF20 (Gambro) 0.2m2 60mL 3–15kg* (*according to data sheet >8kg) Aquarius-Aquamax HF03 (Baxter) 0.3m2 96mL* (*circuit aqualine S, pediatric: 64) 5–20kg Prismaflex M60 (Gambro) 0.6m2 93mL 10–30kg* (*according to data sheet >11kg) Aquarius-Aquamax HF07 (Baxter) 0.7m2 118mL* (*circuit aqualine S, pediatric: 64) 20–40kg Prismaflex M100 (Gambro) 0.9m2 152mL ≥30kg Aquarius-Aquamax HF12 1.2m2 178* (*normal circuit aqualine N, 105mL) >40kg Prismaflex M150 (Gambro) 1.5m2 189mL ≥50kg Aquarius-Aquamax HF19 1.9m2 214* (*normal circuit aqualine N, 105mL) >60kg CEBPT: continuous extracorporeal blood purification therapies.
- 3.
Blood heating. Children lose heat more easily due to their greater body surface area in relation to their weight. This loss is increased by their diminished ability to compensate for it and is markedly enlarged by the oversized extracorporeal circuit when they are on CEBPT, so that the use of the heater is mandatory. It is usually placed in the return line but sometimes it is necessary to place another one in the input line. It is also possible to heat the fluids, but it is only effective for higher flows.
- 4.
Patient connection. The amount of blood flow that remains in the extracorporeal circuit predisposes children to hypotension, hemodilution, and a high risk of cardiac arrest, especially in newborns and unstable infants at the time of connection, and principally when the priming volume of the circuit is discarded.72 Therefore, we recommend administering fluids to the patient while the priming volume is being discarded, or to perform a second heparin-free priming that allows its introduction into the patient. Second priming with packed red blood cells is not recommended because of the risk of bradykinin release syndrome.
- 5.
Drugs dosage. The volume of distribution of water-soluble drugs is higher in children than in adults because of significant changes in extracellular volume occurring in the first years of life.73 Additionally, we should take into account the significant increase in volume that represents the extracorporeal circuit, which is proportionally greater in the smallest patients. There are also differences in plasma protein binding and renal clearance of different drugs by age.74
- 6.
Dialytrauma. Hypophosphatemia occurs despite “adequate” phosphates prescription in relation to patient weight. Nevertheless, it may be corrected using phosphorus-enriched solutions. Blood transfusion is usually needed in newborns and small infants whenever blood clots in the system.72
A study recently conducted in Italy has reported the successfully clinical use of a neonate CEBPT miniaturized machine especially designed for newborns and small infants (CARPEDIEM).75 Its main characteristics are a lower priming volume (<30mL), miniaturized roller pumps and precise control of ultrafiltration using calibrated scales to the nearest gram. This miniaturized machine could represent a significant improvement for CEBPT in neonates and young infants, although more studies are needed to validate it.
Funding sourceNone.
ContributionAll authors have actively participated in the preparation of the article. Correspondence author has made the final editing of the manuscript. All authors have approved the final version of the article.
Conflict of interestsNone, for all authors. Possible sources of funding have not been involved in the study design, data collection and in drafting the manuscript. It is a unique idea and work of the Nephrointensive Care Working Group of the Spanish society of intensive care (SEMICYUC).
Our acknowledgment to all members of the working group who contributed to this work: Catalán Ibars R, Cremades Navalón I, Peña López Y, Herrera Rojas D, Alcalá Llorente MA, Gómes González C, Roglán Piqueras A, Sánchez Morán F y Maynar Moliner FJ.
We also thank the people who have helped us in translating the manuscript.