Heart failure is a worldwide issue due to its prevalence and because it takes over a huge number of resources. Also, population aging and the improved prognosis of other acute cardiovascular processes anticipate higher incidence rates within the next few decades.1 Acute heart failure (AHF) is a life-threatening disease characterized by a lack of balanced between the supply and demand of oxygen due to heart damage. In addition, it has a rapid onset that requires emergency assessment and treatment.2 Cardiogenic shock (CS)—where cellular hypoxia can trigger multi-organ failure—is the most serious type of AHF. A total of 5 different stages have been easily established through physical examination, biochemical markers (lactate and degree of metabolic acidosis), and hemodynamic parameters associated with prognosis: A (patients at risk of CS), B (beginning CS), C (classic CS), D (deteriorating/doom CS), and E (extremis CS).3 The process of CS deterioration can be reversible if identified early and the proper measures are implemented to control the triggering causes, establish mechanical circulatory support (MCS) that restores tissue perfusion, and replace failed organs.
The management of CS is complex and has different stages: identification and classification, hemodynamic assessment and early stabilization, cardiac procedures (whether coronary or structural), if necessary, indication of early implantation of circulatory support in cases of refractory shock, specialized intensive care focused on multi-organ support, and finally, long-term outflow tracts. Therefore, throughout 2022, different scientific societies collaborated to draft an expert consensus document to propose a multidisciplinary organization to allow rapid and proper care in the form of a code.4
JustificationAlthough the prevalence of CS varies depending on the definition, clinical care setting, and the era of data mining, it represents 14%–16% of the patients admitted to the intensive care unit (UCI) due to AHF.5 Despite the advances made on its management, the in-hospital mortality rate is high (somewhere between 30% and 60% depending on the underlying etiology), which amounts to over half of the deaths reported within the first 24 h after admission. This high mortality rate is determined by both non-modifiable factors like the patient’s age or his underlying disease, and modifiable factors like precocity in case identification, recovery of tissue perfusion, and access to MCS.2
The use of MCS is a highly specialized process that requires resources in critical care units not available in all the centers assisting acute patients, thus making it necessary to establish in-hospital coordination and coordination criteria to facilitate proper patient referral and improve the patient’s prognosis.6–10
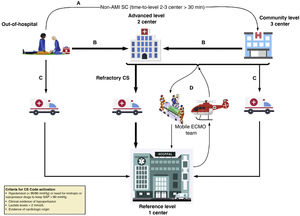
Therefore, the management of CS meets the code criteria based on its incidence rate, severity, and need for standardization both regarding in-hospital management and coordination (Fig. 1).
Flow of patients from the Cardiogenic Shock Care Network.
Adapted from M. Martínez-Sellés et al.4A: to achieve the early stabilization of a patient with CS not associated with an acute myocardial infarction (AMI) diagnosed outside the hospital, the patient can be transferred to the closest level 3 hospital available if transfer to a level 1–2 center exceeds 30 min compared to the transfer to such level 3. B: a patient diagnosed with CS outside the hospital setting or who remains at a level 3 center should be transferred to a level 1 or 2 center depending on transfer times, especially in the acute coronary syndrome setting. C: a patient with CS diagnosed outside the hospital setting or who remains at a level 3 hospital can be transferred to a level 1 center if the need for high-complexity care is anticipated. D: activation of the ECMO team. A mobile unit can be activated from the level 1 center towards the different reference centers available (level 2 and 3 centers) if high-complexity mechanical circulatory assist device implantation is required to secure a safe transfer. CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenation; SAP, systolic arterial pressure.
The CS code is born to facilitate the proper multidisciplinary care and promote standardized and ongoing healthcare by using proper resources early in a context that assigns a certain level of care to a patient’s clinical situation while taking into consideration the time factor. Therefore, a network care model known as “Hub and Spoke” has been proposed to administer treatment based on the patients’ needs in a timely manner and in the most adequate center while taking into consideration the geographic characteristics of each center and health region.
Therefore, it is essential to identify the characteristics of the hospitals based on their level of care (Table 1). Level 3 hospitals play an essential role regarding identification where index assessments by a doctor specialized in critical care facilitate the activation of the CS Code. Level 2 PCI-capable hospitals also capable of implanting venoarterial extracorporeal membrane oxygenation (VA-ECMO)-type short-term MCS—whether surgical or percutaneous based on the availability of heart surgery—will be receiving patients transferred from level 3 centers. Finally, level 1 hospitals have a heart team coordinating the entire process with accredited experience in the use of short-, mid-, and long-term MCS and/or heart transplant (Table 2).
Characteristics of the hospitals from the Cardiogenic Shock Care Network based on their level of care.
| Function | Equipment | |
|---|---|---|
| Level 3 or community hospital | Detection of CS | Vasoactive drugs |
| Early care | Hemodynamic monitorization | |
| Diagnosis | ||
| Advanced life support | ||
| Level 2 or advanced hospital | Detection of CS | Level 3 |
| Early care | Primary PCI | |
| Advanced CS care | Short-term MCS (VA-ECMO, IABP, Impella®) | |
| Cardiac surgery* | ||
| Level 1 or reference hospital | Detection of CS | Level 2 |
| Early care | Transportation equipment | |
| Advanced CS care | Mid-long-term duration VAD | |
| CS team | Heart transplant | |
| Transportation | ||
| Definitive treatment |
Adapted from M. Martínez-Sellés et al.4 CS, cardiogenic shock; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; PCI, percutaneous coronary intervention; VA-ECMO, venoarterial extracorporeal membrane oxygenation; VAD, ventricular assist device.
Composition of the heart team managing cardiogenic shocks, functions, and targets.
| Members | Functions | Common targets |
|---|---|---|
| Emergency medical services | First contact with the patient if he/she is still not hospitalized | 1. Guarantee fast diagnosis |
| Emergency doctors and nurses | Risk stratification and early approach | 2. Identify specific phenotype |
| In-hospital intensivists | Decision regarding the receiving center | 3. Assignment to the appropriate care level |
| Center transfer with level 1 or 2 support | 4. Decision-making on interventions and MCS | |
| Specialist savvy in coronary care (shock doc) and critical care nursing staff | Process coordination | 5. Recognize uselessness and adopt palliative measures |
| Identification, stratification, and diagnosis | 6. Identify patients eligible for clinical trials | |
| Medical therapy | ||
| Invasive hemodynamic monitorization | ||
| Follow-up, planning, and early decision of MCS | ||
| Percutaneous implantation of short-term MCS | ||
| Multi-organ failure support | ||
| Follow-up after intervention and postoperative | ||
| Neurological assessment | ||
| Rehabilitation and nutrition | ||
| Life support adequation | ||
| End-of-life/Palliative care | ||
| Organ donation | ||
| Expert cardiologist in HF and heart transplants | Medical therapy | |
| Decision to proceed with long-term MCS | ||
| Indications and contraindications of heart transplant | ||
| Interventional cardiologist and nurse | Structural heart procedure | |
| Early decision of MCS | ||
| Short-term MCS percutaneous implantation | ||
| Surgical block (cardiac surgery, vascular, anesthesiology, perfusionist, surgical nursing team) | Short-and-mid-term MCS percutaneous implantation | |
| Heart transplant/long-term VAD | ||
| MCS device control during implantation, exchange or transfer |
Adapted from M. Martínez-Sellés et al.4 MCS, mechanical circulatory support; VAD, ventricular assist device.
A key element of this process is inter-center transfers. Therefore, the need for transferring different teams and equipment to implant MCSs in level 3 or 2 centers should be taken into consideration, as well as the need for further transfers to higher level hospitals.11 These cannulation and transfer teams should become adapted to the regional needs, be available on a 24/7 basis, and experienced and skilled enough transferring and managing the possible complications than can occur.
The CS Code should be a program focused on continuous improvement. Therefore, easy-to-measure process indicators like the in-hospital mortality rate, the number of patients with CS due to acute coronary syndrome on the emergency coronary angiography performed (<120 min), and participation in the RENACER registry of MCS in Spain should be established.4
ReflectionThe CS Code poses an organizational challenge for the entire healthcare system opening a new care circuit that will require changes in both the flow of patients and funding. Its implementation can find obstacles in all the aforementioned steps. Placing the patient at the center of care for the development of the code, the benefit of multidisciplinary care grouped into expert centers exceeds by far the reservations this proposal can trigger like incomprehension from unselected hospital to host coordination teams or the need for funding, structural, and human resources.
The development of regional protocols with institutional support and ongoing training with hospital participation across all levels of the transportation and system network will be the key for success, and the efficient and responsible use of this resource.
Conflicts of interestNone reported.
FundingNone whatsoever.