Since the first cases of pneumonia due to a new betacoronavirus discovered in Wuhan, province of Hubei (China) were reported back in December 2019,1 the spread of the infection has been growing nonstop worldwide. The World Health Organization (WHO) declared the situation of pandemic back in March 11, 2020 and now we are facing the biggest challenge in the history of intensive medicine. The correct characterization of the patients and the risk factors involved in the serious clinical signs seen should help at the present time and in the coming years to provide better healthcare and keep the infection under control.
This is a retrospective, cross-sectional study of 59 cases of severe pneumonia due to COVID-19 admitted to an intensive care unit (ICU) from a total of 525 hospitalized patients (11.24%). Patients were recruited from March 12 through May 1, 2020. Three of them still remained at the ICU when the study was submitted with a mean stay of 21 days (median of 19). A statistical analysis was conducted of the patients’ clinical and demographic characteristics and of the data derived from the management of respiratory failure, use of mechanical ventilation, complications, and mortality. Fisher’s exact test was used to compare the categorical variables and Mann-Whitney U test was used to compare the continuous ones. This study has been approved by our center ethical committee of clinical research.
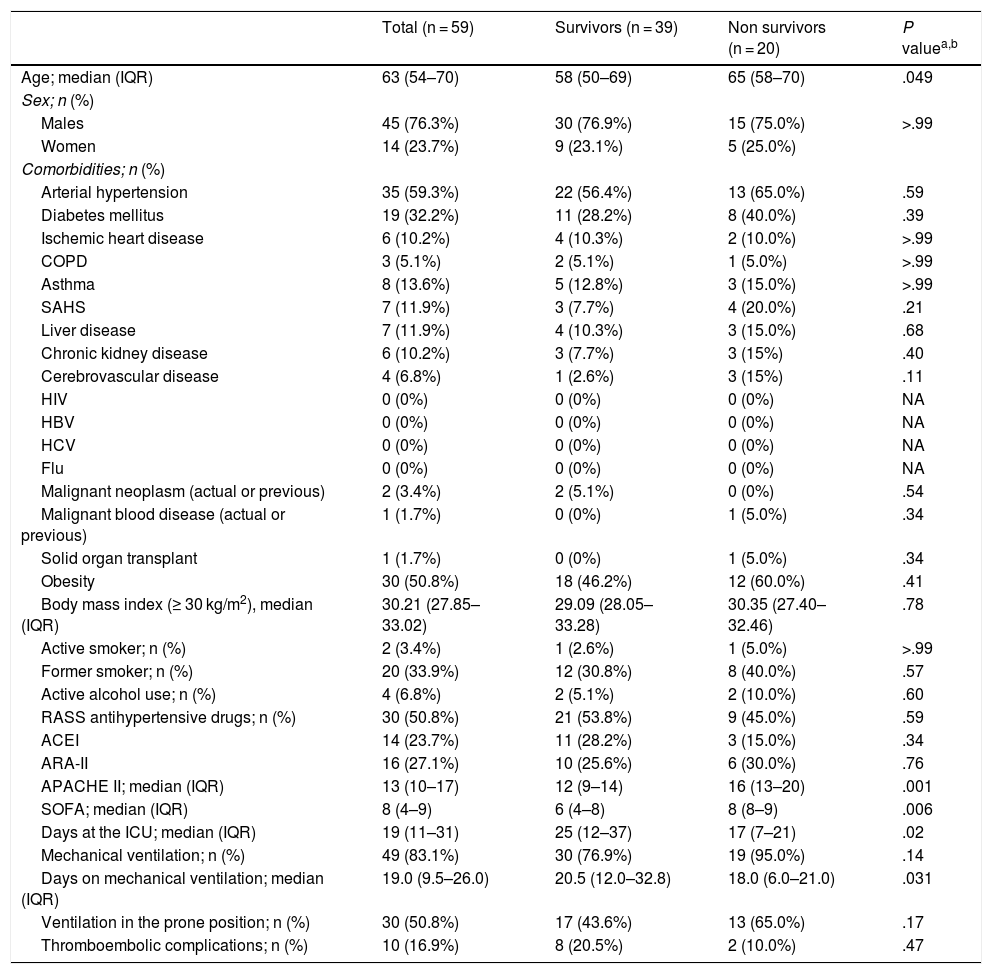
The median of age of the patients included in the study was 63.0 years (standard deviation [SD] 11.2), 45 of them were males (76.3%), and arterial hypertension was the most prevalent comorbidity (n = 35 [59.3%]). Table 1 shows the patients’ clinical and demographic characteristics.
Clinical and demographic characteristics of mechanical ventilation and related complications.
| Total (n = 59) | Survivors (n = 39) | Non survivors (n = 20) | P valuea,b | |
|---|---|---|---|---|
| Age; median (IQR) | 63 (54–70) | 58 (50–69) | 65 (58–70) | .049 |
| Sex; n (%) | ||||
| Males | 45 (76.3%) | 30 (76.9%) | 15 (75.0%) | >.99 |
| Women | 14 (23.7%) | 9 (23.1%) | 5 (25.0%) | |
| Comorbidities; n (%) | ||||
| Arterial hypertension | 35 (59.3%) | 22 (56.4%) | 13 (65.0%) | .59 |
| Diabetes mellitus | 19 (32.2%) | 11 (28.2%) | 8 (40.0%) | .39 |
| Ischemic heart disease | 6 (10.2%) | 4 (10.3%) | 2 (10.0%) | >.99 |
| COPD | 3 (5.1%) | 2 (5.1%) | 1 (5.0%) | >.99 |
| Asthma | 8 (13.6%) | 5 (12.8%) | 3 (15.0%) | >.99 |
| SAHS | 7 (11.9%) | 3 (7.7%) | 4 (20.0%) | .21 |
| Liver disease | 7 (11.9%) | 4 (10.3%) | 3 (15.0%) | .68 |
| Chronic kidney disease | 6 (10.2%) | 3 (7.7%) | 3 (15%) | .40 |
| Cerebrovascular disease | 4 (6.8%) | 1 (2.6%) | 3 (15%) | .11 |
| HIV | 0 (0%) | 0 (0%) | 0 (0%) | NA |
| HBV | 0 (0%) | 0 (0%) | 0 (0%) | NA |
| HCV | 0 (0%) | 0 (0%) | 0 (0%) | NA |
| Flu | 0 (0%) | 0 (0%) | 0 (0%) | NA |
| Malignant neoplasm (actual or previous) | 2 (3.4%) | 2 (5.1%) | 0 (0%) | .54 |
| Malignant blood disease (actual or previous) | 1 (1.7%) | 0 (0%) | 1 (5.0%) | .34 |
| Solid organ transplant | 1 (1.7%) | 0 (0%) | 1 (5.0%) | .34 |
| Obesity | 30 (50.8%) | 18 (46.2%) | 12 (60.0%) | .41 |
| Body mass index (≥ 30 kg/m2), median (IQR) | 30.21 (27.85–33.02) | 29.09 (28.05–33.28) | 30.35 (27.40–32.46) | .78 |
| Active smoker; n (%) | 2 (3.4%) | 1 (2.6%) | 1 (5.0%) | >.99 |
| Former smoker; n (%) | 20 (33.9%) | 12 (30.8%) | 8 (40.0%) | .57 |
| Active alcohol use; n (%) | 4 (6.8%) | 2 (5.1%) | 2 (10.0%) | .60 |
| RASS antihypertensive drugs; n (%) | 30 (50.8%) | 21 (53.8%) | 9 (45.0%) | .59 |
| ACEI | 14 (23.7%) | 11 (28.2%) | 3 (15.0%) | .34 |
| ARA-II | 16 (27.1%) | 10 (25.6%) | 6 (30.0%) | .76 |
| APACHE II; median (IQR) | 13 (10–17) | 12 (9–14) | 16 (13–20) | .001 |
| SOFA; median (IQR) | 8 (4–9) | 6 (4–8) | 8 (8–9) | .006 |
| Days at the ICU; median (IQR) | 19 (11–31) | 25 (12–37) | 17 (7–21) | .02 |
| Mechanical ventilation; n (%) | 49 (83.1%) | 30 (76.9%) | 19 (95.0%) | .14 |
| Days on mechanical ventilation; median (IQR) | 19.0 (9.5–26.0) | 20.5 (12.0–32.8) | 18.0 (6.0–21.0) | .031 |
| Ventilation in the prone position; n (%) | 30 (50.8%) | 17 (43.6%) | 13 (65.0%) | .17 |
| Thromboembolic complications; n (%) | 10 (16.9%) | 8 (20.5%) | 2 (10.0%) | .47 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; ARA-II, angiotensin-2 receptor antagonists; COPD, chronic obstructive pulmonary disease; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NA, non-applicable; RASS, renin-angiotensin-aldosterone system; SAHS, sleep apnea-hypopnea syndrome.
P values ≤.05 were considered statistically significant.
Diagnosis was established using the reverse transcriptase polymerase chain reaction test (RT-PCR) that tested positive in 56 (94.9%) of the patients while in the remaining 3 (5.1%) it was only serological. In 33 cases (55.9%), the RT-PCR only tested positive in the nasopharyngeal exudate, in 18 (30.5%) both in the exudate and lower respiratory tract samples, in 3 (5.1%) it tested positive in the tracheal aspirate or brochoalveolar lavage and in 2 (3.4%) it tested positive in sputum. Since some of the patients had been transferred from the hospital conventional wards, a lower respiratory tract sample was collected if the previous RT-PCR in exudate had tested negative. The later application of the ELISA technique for serological diagnosis tested 35 patients only (59.3%), 27 (77.1%) of whom tested positive for both IgM and IgG. From the very moment it became available, both the patients admitted to the ICU and those already hospitalized were tested during their disease progression.
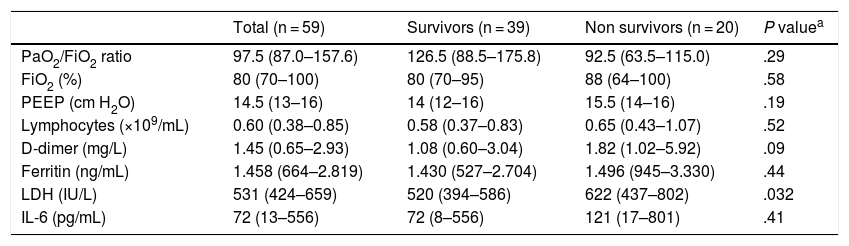
Of the total number of cases, 49 (83.1%) received invasive mechanical ventilation (IMV) at some point, 30 (61.2%) exclusively and 19 (38.8%) following high-flow nasal cannula (HFNC) oxygen therapy failure. The median of days on IMV was 19 (IQR, 9.5–26), 61.2% of the patients required ventilation in the prone position with a median of 2 cycles (IQR, 1–3.25). Twenty-nine patients underwent a percutaneous tracheostomy, 59.2% of those ventilated with a median of days from intubation until the procedure was performed of 11 days (IQR, 9.5–15). Tracheostomies were performed early on given the beneficial effects of this procedure in ventilated patients with long hospital stays and the fact that it is an already established procedure for the management of COVID-19 related pneumonia. The remaining cases, 9 (15.3%) and 1 (1.7%) received HFNC and conventional oxygen therapy, respectively, with no need for IMV. Regarding other life-support therapies, 8 patients (13.6%) required extra-renal depuration therapy and 4 (6.8%) received inhaled nitric oxide. Table 2 shows the ventilatory and analytical parameters of patients at admission.
Ventilatory and analytical parameters at admission; median (IQR).
| Total (n = 59) | Survivors (n = 39) | Non survivors (n = 20) | P valuea | |
|---|---|---|---|---|
| PaO2/FiO2 ratio | 97.5 (87.0–157.6) | 126.5 (88.5–175.8) | 92.5 (63.5–115.0) | .29 |
| FiO2 (%) | 80 (70–100) | 80 (70–95) | 88 (64–100) | .58 |
| PEEP (cm H2O) | 14.5 (13–16) | 14 (12–16) | 15.5 (14–16) | .19 |
| Lymphocytes (×109/mL) | 0.60 (0.38–0.85) | 0.58 (0.37–0.83) | 0.65 (0.43–1.07) | .52 |
| D-dimer (mg/L) | 1.45 (0.65–2.93) | 1.08 (0.60–3.04) | 1.82 (1.02–5.92) | .09 |
| Ferritin (ng/mL) | 1.458 (664–2.819) | 1.430 (527–2.704) | 1.496 (945–3.330) | .44 |
| LDH (IU/L) | 531 (424–659) | 520 (394–586) | 622 (437–802) | .032 |
| IL-6 (pg/mL) | 72 (13–556) | 72 (8–556) | 121 (17–801) | .41 |
P values ≤.05 were considered statistically significant.
We analyzed the thromboembolic complications that occurred during the ICU stay and found 10 events (16.9%) including pulmonary thromboembolism (n = 3 [5.1%]), deep venous thrombosis (n = 3 [5.1%]), ischemic stroke (n = 3 [5.1%]), and acute myocardial infarction (n = 1 [1.7%]). All patients received thromboembolic prophylaxis and only those with a confirmed diagnosis received therapeutic doses. The recommendations established by the Spanish Society of Thrombosis and Hemostasis (SETH)2 were followed.
The overall mortality at the ICU setting was 33.9% (20 patients) with a median of age that was significantly higher in non-survivors (P = .049). Studying the different age groups, a growing tendency with older age was confirmed. Patients were categorized into 5 different groups: <40 years (n = 0 [0%]), from 41 years to 50 years (n = 1 [10%]), from 51 years to 60 years (n = 5 [35.7%]), from 61 years to 70 years (n = 10 [43.5%]), and over 70 years (n = 4 [40%]). The mortality rate associated with ventilatory support of those who received IMV was only 46.7% while the mortality rate of those who were previously treated with HFNC was 26.3%. Both subgroups had a similar APACHE II score at admission, a median of 14 (IQR 11.5–17.5) for the group that received IMV only and a median of 13 (RIQ 11–15) for the group that received HFNC followed by IMV. This suggests that an initial conservative approach in certain patients may not lead to higher mortality rates. The APACHE II and SOFA scores at admission were higher in non-survivors (P = .001 and P = .006, respectively).
Although the study has some limitations, the results are consistent with other results published to this date regarding previous comorbidities3,4 and the possibility that the D-dimer is a risk factor for mortality,3 which shows a trend towards statistical significance but is not statistically significant per se. Our rate of thromboembolic complications was lower compared to the one obtained in the study conducted by Klok et al.5 (31%) and similar to that from other series of thromboembolic disease reported in critically ill patients.6,7 Similarly, the mortality rate seen in our patients (33.9%) was lower compared to the mortality rate of series previously published such as the study conducted by Yang et al.8 (61.5%) and other studies (49%–67%).9,10
FundingNone whatsoever.
Conflicts of interestNone reported.
Please cite this article as: Serrano-Martínez JL, Machado-Casas JF, Redondo-Orts M, Manzano-Manzano F, Castaño-Pérez J, Pérez-Villares JM. Características y resultados de una serie de 59 pacientes con neumonía grave por COVID-19 ingresados en UCI. Med Intensiva. 2020;155:580–583.