Optimal management of sedation, analgesia and delirium offers comfort and security for the critical care patient, allows support measures to be applied more easily and enables an integral approach of medical care, but at the same time lowers the incidence of complications, which translates in better patient outcomes.
ObjectiveTo update the Guía de práctica clínica basada en la evidencia para el manejo de la sedoanalgesia en el paciente adulto críticamente enfermo published in Medicina Intensiva in 2007, and give recommendations for the management of sedation, analgesia, and delirium.
MethodologyA group of 21 intensivists from 9 countries of the Federación Panamericana e Ibérica de Sociedades de Medicina Crítica y Terapia Intensiva, 3 of them also specialists in clinical epidemiology and methodology, gathered for the development of guidelines. Assessment of evidence quality and recommendations were made based on the Grading of Recommendations Assessment, Development and Evaluation system. Strength of recommendations was classified as 1=strong, or 2=weak, and quality of evidence as A=high, B=moderate, or C=low. Two authors searched the following databases: MEDLINE through PUBMED, The Cochrane Library and Literatura Latinoamericana y del Caribe en Ciencias de la Salud and retrieved pertinent information. Members assigned to the 11 sections of the guidelines, based on the literature review, formulated the recommendations that were discussed in plenary sessions. Only those recommendations that achieved more than 80% of consensus were approved for the final document. The Colombian Association of Critical Medicine and Intensive Care (AMCI) supported the elaboration of these guidelines.
ResultsFour hundred and sixty-seven articles were included for review. An increase in number and quality of publications was observed. This allowed to generate 64 strong recommendations with high and moderate quality of evidence in contrast to the 28 recommendations of the previous edition.
ConclusionsThis guidelines contains recommendations and suggestions based on the best evidence available for the management of sedation, analgesia and delirium of the critically ill patient, including a bundle of strategies that serves this purpose. We highlight the assessment of pain and agitation/sedation through validated scales, the use of opioids initially to appropriate analgesic control, associated with multimodal strategies in order to reduce opioide consumption; to promote the lowest level of sedation necessary avoiding over-sedation. Also, in case of the need of sedatives, choose the most appropriate for the patient needs, avoiding the use of benzodiazepines and identify risk factors for delirium, in order to prevent its occurrence, diagnose delirium and treat it with the most suitable pharmacological agent, whether it is haloperidol, atypical antipsychotics or dexmedetomidine, once again, avoiding the use of benzodiazepines and decreasing the use of opioids.
El óptimo manejo de la sedación, analgesia y delirium ofrece al paciente crítico comodidad y seguridad, facilita el buen desarrollo de medidas de soporte y manejo integral y disminuye complicaciones, impactando en un mejor desenlace.
ObjetivoActualizar la Guía de práctica clínica basada en la evidencia para el manejo de la sedoanalgesia en el paciente adulto críticamente enfermo publicada en Medicina Intensiva en el 2007 y dar recomendaciones para el manejo de la sedación, analgesia y delirium.
MetodologíaSe reunió un grupo de 21 intensivistas procedentes de 9 países de la Federación Panamericana e Ibérica de Sociedades de Medicina Crítica y Terapia Intensiva, 3 de ellos además especialistas en epidemiología clínica y metodología para elaboración de guías. Se acogió la propuesta del Grading of Recommendations Assessment, Development and Evaluation Working Group para emitir el grado de recomendación y evaluar la calidad de la evidencia. La fuerza de las recomendaciones fue calificada como 1=fuerte, o 2=débil, y la calidad de la evidencia como A=alta, B=moderada, o C=baja. Expertos en búsqueda de literatura apoyaron con esta estrategia de búsqueda: MEDLINE a través de PUBMED, bases de datos de la biblioteca Cochrane a través de The Cochrane Library y la base de datos Literatura Latinoamericana y del Caribe en Ciencias de la Salud. Los miembros asignados a las 11 secciones de la guía, basándose en la revisión de la literatura, presentaron las recomendaciones, sustentadas y discutidas en sesiones plenarias, aprobando aquellas que superaron el 80% del consenso. La elaboración de las guías contó con el soporte de la Asociación Colombiana de Medicina Crítica y Cuidado Intensivo.
ResultadosPara la elaboración de la guía fueron finalmente seleccionadas 467 referencias, observándose un importante aumento en el número y calidad de los estudios, permitiendo realizar 64 fuertes recomendaciones con evidencia alta y moderada, contrastando con las 28 de la edición anterior.
ConclusionesEsta guía contiene recomendaciones y sugerencias basadas en la mejor evidencia para el manejo de la sedación, analgesia y delirium del paciente crítico, incluyendo un paquete de medidas (bundle). Se destacan: evaluación del dolor y la agitación/sedación mediante escalas; usar inicialmente opioides para el control de la analgesia, adicionando técnicas multimodales para disminuir consumo de opioides; promover el menor nivel de sedación necesario, evitando la sobresedación; en caso de requerir medicamentos sedantes, escoger el más apropiado, evitando el uso rutinario de benzodiazepinas; por último, identificar factores de riesgo para delirium, prevenirlo, diagnosticarlo y manejarlo, con el medicamento más conveniente, ya sea haloperidol, antipsicóticos atípicos o dexmedetomidina, evitando el uso de benzodiazepinas y disminuyendo el uso de opioides.
Sedation and analgesia are an integral part of the management of critical patients in the Intensive Care Unit (ICU). The aim of sedoanalgesia is to offer patients maximum comfort with safety, reducing anxiety and disorientation, facilitating sleep, and ensuring adequate pain control. This likewise contributes to avoid interferences with the medical and nursing care received.1 Critically ill patients in the ICU are at risk of suffering anxiety, agitation, aggressivity, delirium and withdrawal syndrome (opioids, alcohol, nicotine, etc.). An accurate diagnosis of these clinical manifestations is crucial, since adequate management is dependent upon it.2
Objectives of the guideThe objectives of the present guide are to offer a series of recommendations on the use of sedation and the management of pain in adult patients admitted to the ICU, with or without tracheal intubation (nasal or orotracheal) (TI) and ventilatory support, and/or with certain conditions or diseases. The recommendations are based on a consensus of experts in Critical Care Medicine from different member countries of the Federación Panamericana e Ibérica de Sociedades de Medicina Crítica y Terapia Intensiva (FEPIMCTI). The guide is transparent as regards the literature supporting the level of evidence, the recommendations, and the methodology used in developing the guidelines, and can be adopted in any ICU.
In selecting the recommendations, the economic aspects (cost/effectiveness) addressed in global studies were not taken into account, since the concrete circumstances of each individual country can imply large variations in terms of applicability. The full technical report is available and can be requested from the guide coordinator via e-mail: edgarcelis.mdgmail.com.
Scope of the guideThe recommendations have been grouped into different sections, according to the specific conditions of the patients involved:
- A.
Patients requiring conscious or cooperative sedation
- B.
Monitorization of sedoanalgesia
- C.
Patients with delirium and withdrawal syndrome
- D.
Patients without endotracheal intubation or ventilatory support
- E.
Patients with endotracheal intubation and mechanical ventilation
- F.
Patients undergoing withdrawal of the endotracheal tube and mechanical ventilation
- G.
Special populations: trauma patients, elderly subjects, pregnant patients and burn victims
- H.
Sedoanalgesia in the immediate postoperative period of cardiovascular surgery
- I.
Neurological and neurocritical patients
- J.
Patients with kidney or liver failure
- K.
Patients requiring special procedures (tracheostomy, thoracic catheters or tubes, peritoneal lavage, wound or burn lavage and debridement)
- L.
Non-pharmacological strategies or complementary treatments
This guide does not cover the pediatric population or adults with conditions different from those cited above, such as transplant recipients, patients with brain death in the context of organ donation, or psychiatric patients.
UsersThe guide has been developed for use by physicians, nurses and physiotherapists (therapists) involved in the management of critically ill adult patients, though it can also prove useful in teaching activities involving residents and students.
Methodology for developing the guideCreation of the consensus groupA total of 21 people from 9 countries were invited to participate in the creation of the guide. The participants were selected by the Societies of Critical Care Medicine of each intervening country, based on criteria such as personal experience in the field and in the methodology used for developing guides (Appendix A).
All the participants are specialists in Critical Care Medicine, and three of them are moreover specialized in clinical epidemiology and in the methodology used for developing guides. Eighteen of the participants had already contributed to development of the 2007 guide. The role of the methodologists was to orientate and support the specialists in the literature search, and in the development of the methodology used to produce the guide.
Development of the guideThe 21 experts defined the scope of the guide, the topics to be evaluated, and the relevant questions requiring answers. Two experts were assigned per topic.
The group of experts decided to take the conclusions of the 2007 guide on the management of sedation and analgesia in adult critical patients of the FEPIMCTI as being valid, and used them as a starting point.3 The experts were instructed on the methodology to be used, and the proposal of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group4 was employed to establish the grade of recommendation and evaluate the quality of the evidence, based on the criteria found in Table 1.
Grading of recommendations.
| Description of the grade of recommendation | Risk/benefit and barriers | Methodological quality of the evidence | Implications |
| 1A. Strong recommendation. High quality of evidence | The benefit is greater than the risk and the barriers, or vice versa | RCT without important limitations or observational studies with very strong evidence | Strong recommendation. Applicable to most patients in most circumstances, without limitations |
| 1B. Strong recommendation. Moderate quality of evidence | The benefit is greater than the risk and the barriers, or vice versa | RCT with important limitations (inconsistent or imprecise results, methodological weaknesses, indirect evidence) or, exceptionally, observational studies with strong evidence | Strong recommendation. Applicable to most patients in most circumstances, without limitations |
| 1C. Strong recommendation. Low or very low quality of evidence | The benefit is greater than the risk and the barriers, or vice versa | Observational studies or series of cases | Strong recommendation, but can change when greater quality evidence is obtained |
| 2A. Weak recommendation. High quality of evidence | The benefit is almost balanced with the risk | RCT without important limitations or observational studies with very strong evidence | Weak recommendation. The best action depends on the patient circumstances or social values |
| 2B. Weak recommendation. Moderate quality of evidence | The benefit is almost balanced with the risk | RCT with important limitations (inconsistent or imprecise results, methodological weaknesses, indirect evidence) or, exceptionally, observational studies with strong evidence | Weak recommendation. The best action depends on the patient circumstances or social values |
| 2C. Weak recommendation. Low or very low quality of evidence | Uncertainty in the estimation of risk, benefit and barriers, or may be balanced | Observational studies or series of cases | Very weak recommendation. Other alternatives may be equally reasonable |
RCT: randomized clinical trial.
The search strategy was designed by experts in literature searches and biomedical information and systematic reviews.
The following article inclusion criteria were applied:
- 1.
Types of studies. Randomized clinical trials, systematic reviews, cohort studies, case–control studies, descriptive studies and case series.
- 2.
Types of patients. Adults in critical condition or admitted to the ICU under some of the following circumstances: without TI; with TI and MV; undergoing weaning from MV and from the endotracheal tube; in the immediate postoperative period of heart surgery; chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome (ARDS), elderly patients, pregnant or nursing women; polytraumatized patients; neurocritical cases; renal failure; liver failure; agitation and/or delirium and/or withdrawal syndrome.
- 3.
Types of interventions. Monitorization of sedation and conscious sedation (with the inclusion of lorazepam, midazolam, propofol, diazepam, dexmedetomidine, thiopental sodium, haloperidol, clozapine, methadone, ketamine, non-pharmacological strategies or complementary treatments); analgesia (with the inclusion of morphine, fentanyl, remifentanil, sufentanil, clonidine, nonsteroidal antiinflammatory drugs [NSAIDs], hydromorphone, regional anesthesia methods, patient-controlled analgesia [PCA]); immobilization procedures; and frequent surgical operations in the ICU (tracheostomy, thoracic catheters or tubes, peritoneal lavage, cures and debridement of wounds or burns).
Identification of the relevant studies was carried out by an electronic search of all studies related to the proposed topics, covering the period from 1 January 2007 onwards.
A search was made of the MEDLINE database through PUBMED (1 January 2007–31 July 2012) and the following databases of the Cochrane Library: Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), National Health Service Economic Evaluation Database (NHS EED) through the Cochrane Library number 2, of 2012, and the Literatura Latinoamericana y del Caribe en Ciencias de la Salud (LILACS) database (31 July 2012). The Appendix Banexo 2 details the different search strategies in PUBMED. The literature search identified 1101 references in the different databases mentioned. The project coordinator and a methodologist selected the studies considered to be of relevance for developing the guide, and discarded those studies that did not meet the inclusion criteria or which corresponded to references already identified in another database. Then, an intensivist with training in clinical epidemiology evaluated the full texts of the remaining articles, with a final selection of 201 studies for the guide. In addition, the experts included 266 secondary references identified from the studies found by the electronic literature search, with inclusion of some of them in the references. The studies on which the recommendations were based were evaluated according to the standards of the GRADE Working Group4 by the participating experts, supported by the three intensivists with expertise in epidemiology. This evaluation was carried out using standardized instruments. The proposals for the recommendations were presented by an expert in a plenary session, along with the literature references supporting the recommendation. Following joint discussion, the final recommendations were issued. All proposals exceeding a consensus of over 80% were included as recommendations, while those proposals rejected by over 80% of the votes were excluded.
Final recommendations of the guideThe distribution of the final recommendations according to the grade of recommendation is shown in Table 2.
Distribution of the final recommendations according to grade.
| Grade of recommendation | Number |
| 1A. Strong recommendation. High quality of evidence | 4 |
| 1B. Strong recommendation. Moderate quality of evidence | 60 |
| 1C. Strong recommendation. Low or very low quality of evidence | 50 |
| 2A. Weak recommendation. High quality of evidence | 0 |
| 2B. Weak recommendation. Moderate quality of evidence | 10 |
| 2C. Weak recommendation. Low or very low quality of evidence | 13 |
| Total | 137 |
It is proposed that the guide be updated two years after its date of publication.
ExonerationIt is important to bear in mind that guides are simply a useful tool designed to improve medical decision making, and must be used taking a number of elements into account, including medical criterion, the patient needs and preferences, and the availability of resources. Likewise, it must be remembered that clinical research can produce new evidence that might require a change in current practices even before the guides are updated.
Recommendations, level of evidence and justificationThis guide is presented as a list of recommendations referred to each question of the selected topic.
General recommendationAll critical patients have the right to receive adequate pain management where required
Grade of recommendation: strong. Level of evidence: low (1C).
A. Patients requiring conscious or cooperative sedationWhich are the most sensitive scales and elements for the monitorization and diagnosis of agitation?
A1. Objective evaluation is recommended of the presence and magnitude of agitation in all patients at risk of developing the condition in the ICU, based on a validated measurement scale (Richmond Agitation Sedation Scale [RASS] or Sedation-Agitation Scale [SAS]). This should be done systematically by trained personnel.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Agitation is defined as frequent movements of the head, arms or legs, and/or disadaptation from the ventilator, persisting despite attempts by the supervising medical team to calm the patient.5,6 Agitation can occur due to toxicity of the central nervous system (CNS) secondary to drugs or other common critical patient conditions.7,8 Agitation exhibits a vicious circle resulting from a feedback mechanism in which the defensive reaction of the supervising medical team induces further agitation in the patient, with the risk of physical aggression and the tearing out of tubes, catheters and the endotracheal tube.9,10 The increase in oxygen demand in turn can lead to myocardial ischemia or other forms of organ failure in the seriously ill patient–thus justifying the need for quick and effective treatment.2,11,12
The Ramsay sedation scale (Table 3)13 was specifically validated more than 30 years ago for assessing the level of sedation. It contemplates only one agitation category, however, and so is of very limited use in quantifying the level of agitation.
Ramsay sedation scale.
| Level | Description |
| Awake | |
| 1 | With anxiety and agitation or restless |
| 2 | Cooperative, oriented and calm |
| 3 | Drowsy. Responds to normal verbal stimuli |
| Sleeping | |
| 1 | Rapid response to loud noise or light percussion on the brow |
| 2 | Lazy response to loud noise or light percussion on the brow |
| 3 | No response to loud noise or light percussion on the brow |
In recent years more effective scales have been developed for evaluating agitation. Those offering the greatest validity and reliability include the Motor Activity Assessment Scale (MAAS),14 the SAS (Table 4)15,16 and the RASS (Table 5).17 The RASS and SAS scales are easy to use and remember, and this in turn favors their acceptance among the ICU personnel.17,18
Sedation-Agitation Scale (SAS).
| Score | Level of sedation | Response |
| 7 | Dangerous agitation | Attempts withdrawal of the endotracheal tube and catheters; attempts to get out of bed, aggressive with personnel |
| 6 | Very agitated | Does not calm down when spoken to, bites the tube, requires physical restraint |
| 5 | Agitated | Anxious or with moderate agitation, tries to sit up but calms with verbal stimuli |
| 4 | Calm and cooperative | Calm or easily awoken, follows instructions |
| 3 | Sedated | Difficult to wake up, wakes up with verbal stimuli or gentle movements, but quickly falls asleep again. Follows simple instructions |
| 2 | Very sedated | Can be awoken with physical stimulus, but does not communicate or follow instructions. Can move spontaneously |
| 1 | Cannot be awoken | Can move or gesticulate slightly with painful stimuli, but does not communicate or follow instructions |
Richmond Agitation Sedation Scale (RASS).
| Score | Designation | Description | Exploration |
| +4 | Combative | Combative, violent, with immediate danger for personnel | Observe the patient |
| +3 | Very agitated | Aggressive, attempts to remove tubes or catheters | |
| +2 | Agitated | Frequent and purposeless movements; struggles with the ventilator | |
| +1 | Restless | Anxious, but without aggressive or vigorous movements | |
| 0 | Alert and calm | ||
| −1 | Drowsy | Not fully alert, but stays awake (≥10s) (eye opening and following with gaze) when called | Call the patient by name and say: “open your eyes and look at me” |
| −2 | Mild sedation | Briefly awakens (<10s) when called, and follows with gaze | |
| −3 | Moderate sedation | Eye movement or opening when called (but no following with gaze) | |
| −4 | Deep sedation | No response when called, but with eye movement or opening in response to physical stimuli | Stimulate by shoulder shaking or rubbing upon sternal region |
| −5 | Without response | No response to voice of physical stimulation |
If RASS equals −4 or −5, stop and re-evaluate the patient later on.
If RASS>−4 (−3 to +4), proceed, if indicated, to evaluation of delirium.
Actigraphy, which measures movements recorded by an accelerometer affixed to a body extremity, shows good correlation to changes in neurological state as evaluated by sedation and pain scales, and could be useful for the early identification of agitation and its management.19
What are the factors that contribute to the development of agitation?
A2. It is advisable for the personnel attending the patient to assess and quantify the presence of agitation risk factors, with the aim of ensuring early treatment of such factors.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The factors contributing to the development of agitation can be classified according to origin as follows5,6,20:
- 1.
Exogenous (external) or toxic-organic origin. Agitation is produced by the action of toxic agents or in the course of medical diseases. These episodes are of sudden onset. In the case of drugs or substances of abuse, agitation results from overdose, adverse reactions or privation.
The substances of abuse capable of causing agitation include alcohol (delirium tremens and hallucinations), smoking (privation),21 stimulants, marihuana and hallucinogens. The causal drug substances in turn include atropine, corticosteroids, phenytoin, barbiturates, phenothiazines, tricyclic antidepressants and disulfiram. The toxic-organic causes include epilepsy, subdural hematoma, cerebrovascular events, hypertensive encephalopathy, subarachnoid hemorrhage, intracranial tumors, sepsis, human immunodeficiency virus (HIV) infection with involvement of the CNS, hypothyroidism, puerperal psychosis, fever22 and hypoglycemia. Agitation also can manifest in encephalopathy associated to liver failure and renal failure.
- 2.
Psychogenic origin. Agitation can result from situations of stress in patients with a susceptible personality and who easily suffer decompensation.
- 3.
Endogenous origin. Agitation results from schizophrenic psychosis or maniac-depressive psychosis.
¿In which situations can conscious or cooperative sedation be considered indicated?
A3. Conscious or cooperative sedation is recommended in those patients who do not need deep sedation, and particularly in those who require periodic evaluation of consciousness due to some critical condition or complex procedure such as the introduction of noninvasive MV, the adaptation to spontaneous invasive MV modes, or during the endotracheal tube withdrawal process, especially in patients at risk of suffering serious neurological complications.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Conscious or cooperative sedation can be defined as the minimum lowering of consciousness allowing the patient to maintain a permeable airway. From a more operative perspective (that of the patient bedside), it can be regarded as sedation in which the patient maintains an appropriate response to verbal or tactile stimulation, with maintenance of the airway reflexes, and with adequate spontaneous ventilation. The cardiovascular situation usually remains stable.23–25
Conscious or cooperative sedation has been used to shorten the duration of MV26–31 and the time from the start of weaning to extubation28; to shorten the stay in the ICU26,28,31,32 and in hospital30; to reduce the frequency of tracheostomy33; and to reduce the incidence of psychological disturbances during hospitalization or after discharge,32 including delirium29 and post-traumatic stress syndrome.27 In this context, a lesser incidence of delirium is associated to increased survival.29,30
Sedation can be provided during different therapeutic, diagnostic or surgical procedures; when frequent neurological evaluation is required; during the introduction of noninvasive MV; for adapting to spontaneous invasive MV modes; or during the TI removal process. Sedation is to be administered carefully, since it can be associated to adverse effects such as agitation, particularly in groups of patients subjected to MV and with disorders related to alcohol or drug abuse.34
Different methods for providing conscious sedation have been described, including the use of sedation protocols and algorithms, with or without daily interruption of the sedatives; patient wakening each day with or without spontaneous ventilation tests; the use of analgesia-sedation instead of hypnotic sedation; or the administration of new drugs with a lesser depressive effect upon the respiratory center.26–31,33 “Non-sedation” with the associated use of opioid analgesics could be regarded as a variant of conscious sedation.30
A4. The use of dexmedetomidine, fentanyl, remifentanil, propofol (boluses or infusion), or midazolam (only rescue boluses), in doses titered according to response, is recommended for conscious sedation in minor therapeutic, diagnostic or surgical situations in the ICU.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Dexmedetomidine, a highly specific, short-acting α2-agonist, produces analgesia, anxiolysis and sedation that has been described as conscious sedation, reducing mental state disorders such as delirium.35,36 On the other hand, dexmedetomidine does not produce clinically significant respiratory depression–a fact that facilitates the management of these patients from the respiratory perspective and as regards the maintenance of airway permeability.37–43
Fentanyl, remifentanil and propofol can afford conscious sedation at variable doses and times, conditioned to the pharmacokinetic characteristics of each drug.44 Metabolite accumulation must be taken into account when using continuous infusions. On the other hand, it always must be remembered that midazolam and propofol do not produce analgesia.20,23–25,37,39–43,45–54
A5. The use of droperidol combined with opioids for neuroleptoanalgesia requires caution, with evaluation of the risk–benefit ratio for each patient, due to the appearance of extrapyramidal symptoms and the possible risk of torsades de pointes tachycardia.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Droperidol, a fast- and short-acting butyrophenone, is useful for the treatment of psychomotor agitation and aggressivity. The doses are highly variable (from 0.625 to 1.25mg in bolus form, and from 5 to 25mg in infusion over 24h). Its adverse hemodynamic effects must be taken into account: vasodilatation, hypotension and tachycardia. It has no significant analgesic effect, and so is usually combined with an opiate.
However, its reported adverse effects include extrapyramidal symptoms and possible lengthening of the QT-interval, with the consequent risk of torsades de pointes tachycardia–though this effect has not been clearly demonstrated at low doses.55–57
Remifentanil has been used in anesthesia. Despite its brief effect and negligible accumulation, the use of this drug for the conscious sedation of critical patients requires very close monitorization and caution, since small dose increments can cause a loss of airway control.
B. Monitorization of sedoanalgesiaWhat are the benefits of systematically evaluating sedoanalgesia in the critical patient?
B1. Protocolization of a systematic evaluation of pain and analgesia is recommended.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Monitorization improves the effective management of pain, allowing better adjustment of the sedating and analgesic medication.58 Many studies have shown that adequate monitorization of sedoanalgesia allows us to reduce the time on MV, the duration of stay in the ICU, and the number of nosocomial infectious complications, particularly ventilator-associated pneumonia (VAP).59–65 Some authors have even reported a decrease in mortality following the introduction of systematic sedoanalgesia monitorization.66
What are the best tools for identifying pain in critical patients?
B2. The use of a validated scale is recommended, based on patient personal scoring of pain, whenever possible.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Pain remains a frequent problem in critical patients, and can even be caused by routine techniques and care such as postural changes.67 A number of approaches can be used to identify pain68: we can try to have the patient report pain personally; assume that pain is felt when maneuvers potentially capable of causing pain are produced; we can use a validated behavior indicator scale; ask the patient relatives whether they believe that the patient suffers pain; and we can assess the response to analgesia.
Since pain is a subjective experience, the best evaluation of pain is that made by the patient in person.69 Different scales have been developed to quantify pain in critical patients. The most widely used instruments are based on a numerical or dimensional (length) scoring system (visual numerical scale, visual analog scale) displayed in a horizontal or vertical direction, in which the patient scores the intensity of the experienced pain.69–71 It is important to make the patient understand what kind of information we are seeking, and to use instruments of sufficient size, particularly in patients with sensory difficulties. A comparative study of these scales showed the maximum sensitivity and the greatest negative predictive value in discriminating pain to be obtained with the expanded-size visual numerical scale.71 On the other hand, most failures with these scales were observed in the more seriously ill patients or in individuals suffering delirium.
B3. The use of a validated scale is recommended, based on pain-related behavior indicators in patients who are unable to communicate.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: When the patient is unable to explain or express his or her pain, identification of the latter becomes complicated and requires the use of specific tools, generally based on physiological changes or behaviors associated to pain. The lack of an established gold standard has caused the development of many of these pain measuring instruments to be based on clinimetrics. Their application is therefore strongly conditioned to adequate validation (i.e., ensuring that they effectively measure what they are intended to measure) and reproducibility (i.e., affording the same results over time and with different observers).
The Behavioral Pain Score (BPS) (Table 6) uses a scale from 1 to 4 to score the facial expression of the patient, the posture of the upper extremities, and synchronization with MV. Higher scores imply increased intensity of pain.72 This scale has been validated by groups independent of the group that developed the instrument,73 and has demonstrated adequate correlation to the subjective scales.74 A modified version has even been developed for use in non-intubated patients, replacing the item adaptation to MV with the item vocalization.75
Behavioral Pain Scale (BPS).
| Item | Description | Points |
| Facial expression | Relaxed | 1 |
| Partially tense | 2 | |
| Completely tense | 3 | |
| Grimacing | 4 | |
| Upper extremities | No movements | 1 |
| Partially flexed | 2 | |
| Fully flexed, with flexing of fingers | 3 | |
| Permanently retracted | 4 | |
| Adaptation to ventilator | Tolerates movement | 1 |
| Coughs but tolerates ventilation most of the time | 2 | |
| Struggles with the ventilator | 3 | |
| Impossible to control ventilation | 4 | |
Other groups have developed different instruments for the identification of pain, based on behavior indicators. In this context, particular mention can be made of the Critical-Care Pain Observation Tool (CPOT)76,77; the Face, Legs, Activity, Cry, Consolability scale, developed from the pediatric COMFORT scale, and also applicable to children78; the Campbell scale69,79; or the recent modification of the latter scale designed for better adaptation to ventilated patients (the Scale of Behavior Indicators of Pain [ESCID]).80 Although the mentioned instruments have been developed with a process for the validation of adequate content, construction and criteria, the limited experience with their use to date means that there is not enough published evidence to recommend any concrete scale.
B4. It is recommended that physiological parameters isolatedly should not be used to identify pain, since they are nonspecific.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Pain is associated with physiological changes. In this sense, we can observe an increase in heart rate, increased blood pressure, or dilation of the pupils, among other phenomena. When these changes occur suddenly and are intense, we can suspect that the patient is experiencing pain. However, these alterations often manifest irregularly, and the characteristics inherent to critical patients cause them to be nonspecific.81,82 In the development stage of the CPOT scale, the authors included a series of physiological indicators that were subsequently removed, since they were unable to improve the discriminating capacity of the behavioral components of the instrument.82
What are the best tools for controlling the level of sedation (and for evaluating the degree of agitation) in the critical patient?
B5. A validated scale measuring the depth of sedation based on patient capacity to respond to stimuli is recommended. The selected scale should quantify both the level of sedation and the degree of agitation.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: A number of clinical scales have been developed for documenting the depth of sedation according to the type and intensity of the physical stimulus needed to elicit a patient response. In this context, the Ramsay scale,13 developed over 35 years ago, remains the most widely used instrument. The Ramsay scale is very easy to use and is well accepted by the nursing personnel.83 It scores the patient condition according to 6 levels – only one of which corresponds to agitation. Among other instruments developed to evaluate sedation, mention can be made of the Vancouver Interaction and Calmness Scale,84 the Observer's Assessment of Alertness/Sedation Scale,85 the Adaptation to the Intensive Care Environment (ATICE),86 or the MAAS14–though currently the most widely used instruments are the SAS,15 the RASS18 or the ATICE.
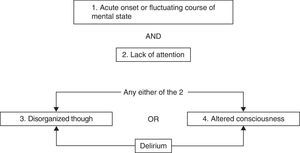
The SAS scale comprises 7 categories, ranging from the absence of patient reactivity or responsiveness to dangerous agitation. It has been validated by several groups and is well accepted by the nursing personnel87,88 for documenting both the degree of sedation and the degree of agitation. The ATICE scale86 comprises 5 categories, of which two correspond to the consciousness domain and three to the tolerance domain. Its use within a sedation management algorithm in critical patients without brain injury has been associated to a shortening of the duration of MV and ICU stay.60 The RASS scale in turn starts from level zero in an alert and calm patient, and quantifies agitation in terms of four positive degrees and the depth of sedation in terms of 5 negative degrees. This instrument has been adequately validated and is well accepted,17 and moreover shows good correlation to the Ramsay scale.89 It is easy to use, and offers the advantage of constituting a component for the identification of delirium by means of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU)90 (Table 7). The RASS scale is regarded by the authors of the present recommendations as the instrument of choice.
Confusion Assessment Method for the Intensive Care Unit (CAM-ICU).
| Criteria and description of the CAM–ICU | |||
| 1. Acute onset or fluctuating course | Absent | Present | |
| Positive if answer is “yes” to 1A or 1B | |||
| 1A. Is there evidence of an acute change in mental state with respect to the basal condition? or | |||
| 1B. Has behavior (abnormal) fluctuated in the last 24h? In other words, does it tend to appear and disappear, or increase and decrease in severity, as evidenced by the fluctuation of a sedation scale (e.g., RASS), or GCS, or in the previous evaluation of delirium? | |||
| 2. Lack of attention | Absent | Present | |
| Did the patient have difficulty in focusing attention, as evidenced by scores of <8 in any of the visual or auditory components of the ASE? | |||
| 2A. Start with the ASE of letters. If the patient is able to do this test and the score is clear, register it and move on to point 3 | |||
| 2B. If the patient is not able to do this test or the score is not clear, do the ASE of figures. If both tests are made, use the result of the ASE of figures for scoring | |||
| 3. Disorganized thought | Absent | Present | |
| Is there evidence of disorganized or incoherent thought as evidenced by incorrect answers to 2 or more of the 4 questions, and/or incapacity to follow instructions? | |||
| 3A. “Yes” or “no” questions (alternate group A and group B): | Absent | Present | |
| Group ACan a stone float on water?Are there fish in the sea?Do you weigh 1kg or more than 2kg?Can a hammer be used to insert a nail? | Group BCan a leaf float on water?Are there elephants in the sea?Do 2kg weigh more than 1kg?Can a hammer be used to cut wood? | ||
| 4. Altered consciousness | Absent | Present | |
| Positive if the RASS score is different from 0 | |||
| Global score | Yes | No | |
| If 1 and 2, and any of criteria 3 or 4 are present, the patient suffers delirium | |||
ASE: Attention Screening Examination; CAM-ICU: Confusion Assessment Method for the Intensive Care Unit; GCS: Glasgow Coma Score; RASS: Richmond Agitation Sedation Scale.
What is the role of monitorization of the depth of sedation based on the bispectral index (BIS) in the critical patient?
B6. Use of the BIS is recommended only to avoid under- and over-sedation in patients requiring neuromuscular block, or in which utilization of the clinical scales is not possible.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The use of techniques based on the electroencephalogram (EEG) for confirming unconsciousness in the operating room during surgery has led to the evaluation of these systems in the management of sedation in other settings, including the ICU. Among these systems, mention must be made of entropy and particularly of the bispectral index (BIS), which is the most widely used and evaluated of all the techniques.91,92 The BIS is an adimensional parameter derived from the EEG tracing and which ranges from 0 (absence of brain activity) to 100 (fully alert). A rating of between 40 and 60 is considered appropriate for surgical anesthesia.91
Different studies have examined the correlation between BIS and the clinical scales, with varied results, though some authors consider performance of the index to be acceptable.93–98 In general, the results have not been considered adequate for adjusting sedoanalgesia.99–101 The main cause of such poor correlation is the artifacts produced by the electromyogram,102–105 and which logically disappear when neuromuscular blockers are used. Profound depression of brain activity is reflected by the BIS, but not by the clinical scales.106
Due to the above considerations, the prevalent opinion is that the BIS should not be used when the clinical scales can be applied.107–110 In this context, the main advantage of BIS would be to allow the control of sedation in patients under neuromuscular block,111 where the recommendation of BIS 40–60 for surgical anesthesia would be appropriate. It is important to underscore that sedation below BIS 40 and an increase in suppression rate (percentage of isoelectric ECG recordings) have been associated to increased patient mortality.112
The BIS has also been used as a prognostic tool after cardiorespiratory arrest, as a marker of brain death, for the monitorization of neurocritical patients, and as an indicator of the degree of hepatic encephalopathy. Such indications fall beyond the scope of these recommendations, however, and in general little supporting evidence can be found in the literature.
B7. The use of BIS is recommended for the evaluation of consciousness in patients with fulminant liver failure and encephalopathy on the active waiting list for liver transplantation, for follow-up before and after organ grafting.
Grade of recommendation: strong. Level of evidence: weak (1C).
Justification: The use of BIS in anesthesia has been validated for assessing alertness or awakening. It has been recommended particularly in total intravenous anesthesia, since it is noninvasive and easy to interpret. In patients with fulminant liver failure, increases in BIS were seen to occur shortly before recovery was observed on the Glasgow coma scale in the postoperative period of liver transplantation.113
C. Patients with delirium and withdrawal syndromeDeliriumWhat are the factors contributing to the appearance of delirium?
C1. Identification is recommended of the risk factors for the development of delirium in the critical patient.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Delirium has a high incidence in the seriously ill patient, and is an independent risk factor for mortality and prolonged admission to the ICU.
In 1996, Inouye and Charpentier114 identified the risk factors for delirium in a population of 160 individuals over 70 years of age admitted to hospital. The incidence of delirium was 18%. The risk factors associated to its appearance were found to be physical limitation (odds ratio [OR] 4.4; 95% confidence interval [95%CI]: 2.5–7.9), denutrition (OR 4.0; 95%CI: 2.2–7.4), the administration of ≥3 drugs (OR 2.9; 95%CI: 1.6–5.4), bladder catheterization (OR 2.4; 95%CI: 1.2–4.7), and an iatrogenic event (OR 1.9; 95%CI: 1.1–3.2). Based on these data, the authors developed and validated a predictive model, assigning one point per factor (Table 8). The delirium rate per person in the low, intermediate and high risk groups was 3%, 20% and 59%, respectively (p<0.001).
Stratification of delirium risk in hospitalized patients ≥70 years of age.
| Risk factors |
| Use of physical restraint |
| Malnutritiona |
| Administration of >3 drugs |
| Urinary catheter |
| Some iatrogenic episode |
| Risk group | Probability of delirium (%) | Number of risk factors |
| Low | 3 | 0 |
| Intermediate | 20 | 1–2 |
| High | 59 | ≥3 |
Van Rompaey et al.115 evaluated the factors risk for the development of delirium in a series of 523 patients admitted to the ICU, without intubation at the time of inclusion in the study. Four domains were established: characteristics of the patient, chronic disease, acute disease and environmental factors (Table 9). The incidence of delirium was 30%, presenting in up to 75% within the first day and in over 90% from the third day of inclusion in the study.
Risk factors for delirium.
| Risk factors | Univariate analysis (OR) | Multivariate analysis (OR) |
| Non-modifiable | ||
| Patient characteristics | ||
| - Lives alone at home | 1.94 (1.06–3.57) | |
| - Alcohol (>3units/day) | 3.23 (1.29–4.80) | 3.23 (1.30–7.98) |
| - Smoking (≥10cigarettes/day) | 2.04 (1.05–3.95) | |
| - Age>65 years and gender | NS | |
| Chronic disease | ||
| - Dementia: OR 2.41 | 2.18 (1.14–4.14) | 2.41 (1.21–4.79) |
| - Heart failure and lung disease | NS | |
| Modifiable | ||
| Acute disease | ||
| - Stay in ICU before inclusion | 1.26 (1.17–1.35) | |
| - Stay in ICU >1day | 2.78 (1.89–4.09) | |
| - Stay in ICU >2days | 5.77 (3.71–8.97) | |
| - Disease of medical origin | 1.57 (1.07–2.29) | |
| - High risk of death: SAPS II>40, APACHE II>24 | 2.5 (1.31–4.66) | |
| - TISS-28≥30 | 2.81 (1.60–5.05) | |
| - Psychoactive drugs | 3.34 (1.99–4.99) | 3.34 (1.50–11.23) |
| - Sedation | 13.66 (7.15–26.1) | |
| - Use of benzodiazepines | 2.89 (1.44–5.69) | |
| - Presence of endotracheal or tracheal cannula | 7.04 (4.30–14.16) | 8.07 (1.18–55.06) |
| - Presence of gastric tube | 7.80 (4.30–14.16) | |
| - Presence of bladder catheter | 5.37 (2.09–13.80) | |
| - Number of infusions | 1.35 (1.20–1.52) | |
| - ≥3 infusions | 2.87 (1.85–4.47) | 2.74 (1.07–7.05) |
| - Unable to eat regularly | 3.83 (2.36–6.22) | |
| - Use of morphine, presence of fever and arterial catheter | NS | |
| Environmental factors | ||
| - Patient isolation | 3.74 (1.69–8.25) | 2.89 (1.0–8.36) |
| - Does not see light of day | 1.75 (1.19–2.56) | 2.39 (1.28–4.45) |
| - Receives no visits | 2.83 (1.5–5.36) | 3.73 (1.75–7.93) |
| - Admission from another area (no emergencies) | 1.98 (1.20–3.28) | |
| - Physical restraint | 33.8 (11.1–102.3) | |
| - Admission through emergencies, open room, absence of visible clock and number of visits | NS | |
NS: nonsignificant; OR: odds ratio; ICU: Intensive Care Unit.
Is it possible to predict the appearance of delirium in the critical patient?
C2. Use of the PREdiction of DELIRium in ICu patients (PRE-DELIRIC) model is recommended for the early prediction of delirium and the adoption of preventive measures in the critical patient.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The PRE-DELIRIC (Table 10)116 is a model that has been developed and validated for predicting the risk of delirium in critical patients. It contemplates 10 risk factors: age, the Acute Physiology and Chronic Health Evaluation (APACHE II)score, neurological impairment, the type of patient (surgical, clinical or trauma), infection, metabolic acidosis, the use of opioids and the use of sedatives (benzodiazepines or propofol), uremia and emergency admission. The model has an area under the receiver operating characteristic (ROC) curve of 0.87, while the area under the ROC curve corresponding to assessment on the part of physicians and nurses was 0.59. The model allows the identification of patients at high risk and the early introduction of guided preventive measures.116 The online version can be consulted and downloaded from: www.umcn.nl/Research/Departments/intensive%20care/Pages/vandenBoogaard.aspx.
Formula of the PREdiction of DELIRium in ICu patients (PRE-DELIRIC) model.
| The risk of delirium is calculated with the risk of delirium formula=1/(1+exp−[−6.31])a |
| +0.04×age |
| +0.06×APACHE II |
| 0 no coma |
| +0.55 drug-induced coma |
| +2.70 other types of coma |
| +2.82 coma of combined origin |
| 0 surgical patients |
| +0.31 clinical patients |
| +1.13 trauma patients |
| +1.38 brain injury patients |
| +1.05 Infection |
| +0.29 metabolic acidosisb |
| 0 no use of morphine |
| +0.41 for morphine doses 0.01–0.71mg/24h |
| +0.13 for morphine doses 0.72–18.6mg/24h |
| +0.51 for morphine doses >18.6mg/24h |
| +1.39 for use of sedatives |
| +0.03×plasma urea (mmol/l) |
| +0.40 emergency admission |
Which are the scales and elements most widely used for the monitorization and diagnosis of delirium?
C3. Use of the scale CAM-ICU is recommended for the monitorization and diagnosis of delirium.
Grade of recommendation: strong. Level of evidence: moderate (1B).
C4. All patients with a RASS score of −3 to +4 should be evaluated with the CAM-ICU scale.
Grade of recommendation: strong. Level of evidence: moderate (1B).
C5. Caution is recommended when using the Intensive Care Delirium Screening Checklist (ICDSC) for the detection of delirium, due to the risk of false-positive cases.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: In clinical practice, assessment begins with the RASS sedation scale before applying the CAM-ICU90 (Fig. 1). Brain function is evaluated in a second step. The instrument that has been validated for the monitorization of delirium is the CAM-ICU. Wei et al.,117 in an evaluation of the CAM-ICU, recorded a sensitivity of 94% (95%CI: 91–97%) and a specificity of 89% (95%CI: 85–94%). In patients subjected to MV, the CAM-ICU affords a non-verbal evaluation of the CAM scale, with a sensitivity of 95–100% and a specificity of 93–98%.117
Flow chart of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU).
The ICDSC scale, developed for the detection of delirium in seriously ill patients, is also useful for detecting subclinical forms of delirium. It has an area under the ROC curve of 0.90. A cutoff point of ≥4 offers a sensitivity of 99% and a specificity of 64%. The false-positive rate is therefore 36%.118
Which are the best therapeutic options?
C6. A non-pharmacological approach to delirium is recommended, before resorting to drug treatment.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The first step in the management of delirium in the seriously ill patient is to secure an early diagnosis. Once the condition has been detected, the risk factors must be treated.119–121 The general recommendations to this effect are: (a) adjust sedation (avoid excessive sedation, monitor sedation, interrupt it daily, avoid neuromuscular relaxants, adjust the dosage and duration of the combinations of sedatives); (b) perform early tracheostomy (when indicated, it reduces the need for sedation and improves patient communication capacity and mobility); (c) optimize the management of pain; and (d) establish an early diagnosis, with prevention and treatment of withdrawal syndrome.
The non-pharmacological strategies include reorientation, cognitive stimulation several times a day, adjustment of the sleeping–waking ratio, early mobilization, early catheter withdrawal, visual and auditory stimulation, adequate management of the pain, and minimization of noise and artificial illumination. With these interventions it is possible to reduce the incidence of delirium up to 40%.
C7. Antipsychotics and/or dexmedetomidine are recommended for the drug treatment of delirium.
Grade of recommendation: strong. Level of evidence: moderate (1B).
C8. Haloperidol is the drug recommended for the management of delirium in the seriously ill patient, starting with a dose of 2.5–5mg via the intravenous route, at intervals of 20–30min, until the symptoms are controlled.
Grade of recommendation: strong. Level of evidence: moderate (1B).
C9. The atypical antipsychotics (olanzapine, risperidone, quetiapine) are recommended as an alternative in the management of delirium.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Campbell et al.122 evaluated the drug interventions for the prevention and treatment of delirium. Regarding the preventive measures, single-dose risperidone after cardiovascular surgery resulted in a significant reduction of delirium versus placebo. There was no decrease in the incidence of delirium with the use of haloperidol, donepezil or citicholine. Sedation with dexmedetomidine or lorazepam in patients subjected to MV, and the use of anesthetic agents in the intraoperative phase of non-heart surgery, failed to reduce the incidence of delirium.
Haloperidol is the drug of choice, affording a decrease in the severity of the symptoms and in the duration of delirium. The second-generation antipsychotics are an alternative in patients not amenable to or intolerant of the first-generation drugs. Lonergan et al.123 compared haloperidol versus risperidone, olanzapine and quetiapine in the treatment of delirium. The results showed no significant differences in the overall effects of the atypical antipsychotics in delirium in comparison with haloperidol (OR 0.63; 95%CI: 0.29–1.38). The incidence of adverse effects with low-dose haloperidol was no higher than that recorded with the atypical antipsychotics. Haloperidol at high doses (>4.5mg/day) was associated with an increased incidence of extrapyramidal effects, compared with olanzapine.
Devlin et al.124 compared quetiapine versus placebo for the treatment of delirium in the ICU with the need for haloperidol. The time to resolution of delirium was shorter in the quetiapine group (median 1day [interquartile range (IQR) 0.5–3days]) than in the placebo group (median 4.5days [IQR 2–7days]) (p=0.001). The quetiapine group received haloperidol during a shorter period of time (median 3days [IQR 2–4days]) than the placebo group (median 4days [IQR 3–8days]) (p=0.05). The duration of the episodes of delirium was shorter in the quetiapine group (median 36=h [IQR 12–87h]) than in the placebo group (median 120h [IQR 60–195h]) (p=0.006).
C10. Dexmedetomidine is recommended as an alternative in the management of delirium.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Reade et al.125 evaluated the use of dexmedetomidine versus haloperidol in 20 patients with failed weaning because of agitation. The patients assigned to the dexmedetomidine group were extubated sooner than those in the haloperidol group (median 20h [IQR 7–24h] versus median 42.5h [IQR 23–119h]) (p=0.021h). Dexmedetomidine significantly shortened the stay in the ICU (1.5 versus 6.5) (p=0.004).
C11. Benzodiazepines are not indicated for the management of delirium, since they can produce excessive sedation, respiratory depression and a worsening of the cognitive dysfunction.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Lonergan et al.,126 in a systematic review, evaluated benzodiazepines for the treatment of delirium. The mean number of days without delirium in the patients treated with lorazepam was 7days (range 5–10), versus 10days (9–12) in the dexmedetomidine group (p=0.09). The mean number of days without coma in the patients treated with lorazepam was 8 (5–10), versus 9 days (9–12) in the dexmedetomidine group (p=0.001). The prevalence of coma was 92% in the lorazepam group and 63% in the dexmedetomidine group (p=0.001). The prevalence of delirium or coma was 98% in the patients administered lorazepam versus 87% in the patients receiving dexmedetomidine (p=0.003). However, in view of the lack of quality studies on this subject, the authors considered that additional controlled studies are needed in order to define the role of the benzodiazepines in the control of delirium not related to alcohol among hospitalized patients.
C12. Choline esterase inhibitors are not recommended in the management of delirium.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Overshott et al.,127 in a systematic review, concluded that only one clinical trial compared donepezil (5mg/day) versus placebo (one tablet/day) for the prevention and treatment of delirium in the postoperative period of 80 patients. Fifteen subjects developed delirium: 8 (20.5%) in the donepezil group and 7 (17.1%) in the placebo group (relative risk [RR] 1.20; 95%CI: 0.48–3.00). There was no significant difference between the treatment group and the placebo group in the duration of delirium (mean difference −0.3%; 90%CI: −7.8 to 7.2).
Withdrawal syndrome in the Intensive Care UnitWhat are the factors that contribute to the development of withdrawal syndrome?
C13. Evaluation of the development of tolerance and withdrawal syndrome is recommended in all seriously ill patients that have been treated with sedatives and opioids, particularly when used at high doses and in combined form during more than 48h.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Tolerance, which may be metabolic or functional, is a frequent complication of sedation when used for more than one week and at high doses. The incidence of withdrawal syndrome in pediatric and adult ICUs can reach 62%.128
The risk factors associated with the development of withdrawal syndrome are: (a) high doses of benzodiazepines, opioids or propofol; (b) infusion during more than three days; (c) abrupt drug suspension; (d) the use of drug combinations; and (e) the administration of barbiturates.129,130
Withdrawal syndrome due to benzodiazepines manifests with agitation, delirium, seizures, hallucinations, cognitive alterations, insomnia, trembling, fever, nausea, vomiting and sympathetic hyperactivity (tachycardia, hypertension, tachypnea).129,131–139
Withdrawal syndrome due to propofol is associated to infusions during more than 24h and high drug doses. It is characterized by confusion, trembling, hallucinations, tonic–clonic seizures, tachycardia, tachypnea and fever. The development of tolerance to propofol is subject to controversy.51,140–150
Withdrawal syndrome due to opioids is characterized by irritability, trembling, clonus, delirium, hypertonicity, choreoathetosic movements, hallucinations, vomiting, stridor, diarrhea, arterial hypertension, tachycardia, diaphoresis and fever. A total fentanyl dose of over 1.5mg/kg or a duration of infusion of more than 5days is associated to a 50% incidence of withdrawal syndrome, while a total dose of over 2.5mg/kg during more than 9days is associated to an incidence of 100% in children.132,151–155
What are the best treatment options?
C14. The use of protocols involving gradual sedative and opioid dose reduction is recommended in order to avoid withdrawal syndrome.
Grade of recommendation: strong. Level of evidence: low (1C).
C15. The use of lorazepam is recommended during the withdrawal of high-dose and prolonged infusions of midazolam.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: The strategies for reducing the incidence of withdrawal syndrome when administering sedatives and opioids in the seriously ill patient include the following: (a) the use of scales for adjusting dosage to the therapeutic objectives of sedation; (b) avoidance of excessive sedation; (c) limiting the days of treatment as far as possible; (d) definition of the method of administration (boluses or infusion) for each concrete case and sedative drug; (e) progressive and gradual reduction of sedatives and analgesics; (f) avoidance as far as possible of sedative drug combinations, particularly at high doses; and (g) evaluation of the use of dexmedetomidine to facilitate opioid and sedative dose reduction.
Different schemes have been proposed for the withdrawal of drugs:
- 1.
For sedation lasting less than 5days, the reduction should be 10–15% of the dose every 6–8h until suspension.156
- 2.
The oral or subcutaneous administration of low doses is recommended for sedation lasting 7 days or more, especially when using drugs characterized by slow elimination.130
- 3.
Following prolonged midazolam infusions, it is advisable to switch to oral lorazepam, taking into account that the midazolam/lorazepam potency–half-life ratio is 1.2 and 1.6, respectively. After the second dose of oral lorazepam, the midazolam dose should be lowered 50%, followed by another 50% after each dose via the oral route.156,157
- 4.
For the reduction of opioids, it is advisable to initially reduce the dose 20–40%, followed by 10% reductions every 12–24h.157
C16. Oral methadone is recommended during the withdrawal of opioids administered at high doses and for prolonged periods of time.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: The starting dose of methadone should be the same as the total dose of intravenous fentanyl. After the second oral dose of methadone, the fentanyl infusion should be reduced 50%, and so on until the fourth dose. The manifestations of withdrawal are to be treated with rescue doses of morphine. The total morphine dose used for rescue purposes should be considered in calculating the methadone dose of the following day. If excessive sedation is observed, methadone can be lowered 10–20% until sedation is controlled. The methadone dose should be gradually lowered 20% every week. In this way the opioids can be suspended in 5–6 weeks.156
C17. The use of dexmedetomidine or clonidine is suggested to facilitate the withdrawal of sedatives and opioids and to treat withdrawal syndrome.
Grade of recommendation: low. Level of evidence: moderate (2B).
Justification: It has been suggested that clonidine or dexmedetomidine can be useful for the treatment of withdrawal syndrome due to sedatives and opioids.158–162
C18. Buprenorphine is recommended in the management of withdrawal syndrome due to opioids.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Gowing et al.,163 in a systematic review, showed that buprenorphine and progressive reduction of the methadone dose offer the same efficacy in the management of withdrawal syndrome due to opioids, though the symptoms are resolved faster with buprenorphine. Adherence to the treatment of withdrawal appears more likely with buprenorphine than with methadone (RR 1.18; 95%CI: 0.93–1.49) (p=0.18).
Withdrawal syndrome due to alcoholWhat are the first choice measures and treatment alternatives?
C19. The use of benzodiazepines is recommended as first line treatment for withdrawal syndrome due to alcohol, and for prevention and management of the seizure episodes and delirium tremens.
Grade of recommendation: strong. Level of evidence: moderate (1B).
C20. The use of dexmedetomidine is advised as a coadjuvant to treatment with benzodiazepines in the management of withdrawal syndrome due to alcohol.
Grade of recommendation: weak. Level of evidence: low (2C).
C21. The use of ethanol for the management of withdrawal syndrome due to alcohol is not recommended.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Alcohol abuse and the disorders secondary to alcohol intake are common in hospitalized patients. The symptoms of withdrawal due to alcohol include insomnia, trembling, mild anxiety, anorexia associated to nausea and vomiting, headache, diaphoresis and palpitations (first 6h), tonic–clonic seizure episodes (12–48h) and hallucinations (12–24h).164 Delirium tremens is the most serious manifestation, and can prove life-threatening. It is characterized by hallucinations, disorientation, fever, tachycardia, agitation and diaphoresis, and is associated to the acute suspension of intake or withdrawal due to alcohol.164
Benzodiazepines are the first line treatment for the management of withdrawal syndrome due to alcohol, and for prevention and treatment of the seizure episodes and delirium tremens.164
Amato et al.165 compared benzodiazepines with placebo or other drug treatments, and also such treatments among each other. The results showed benzodiazepines to be effective in controlling the symptoms of withdrawal syndrome due to alcohol versus placebo (RR 0.16; 95%CI: 0.04–0.69). There were no statistically significant differences between treatment with benzodiazepines and treatment with other drugs.
A metaanalysis showed benzodiazepines to be more effective than placebo in reducing the signs and symptoms of withdrawal syndrome due to alcohol. On the other hand, a significant reduction was observed in the number of crises (−7.7 seizures per 100 patients; 95%CI: −12.0 to −3.59) (p=0.003), and in the incidence of delirium (−4.9 cases per 100 patients; 95%CI: −9.0 to 0.7) (p=0.04).166
In a retrospective study of 17 patients with alcohol withdrawal, the addition of dexmedetomidine was associated with a decrease of 32mg/day in the dose of benzodiazepines (61.5%) (95%CI: 16.7–48.1) (p<0.001), and of 5.6mg/day in the dose of haloperidol (46.7%) (95%CI: −0.03 to 11.23) (p=0.05), while the severity of withdrawal syndrome decreased 1.9 points (21%) (95%CI: 0.44–3.36) (p<0.015) in the first 24h after administration. Regarding the hemodynamic parameters, the heart rate dropped an average of 23bpm (22.8%) (95%CI: 18.4–28.4) (p<0.001), while the systolic blood pressure decreased 13.5mmHg (9.6%) (95%CI, 3.8–15.4%) (p=0.002).167
Weinberg et al.,168 in a randomized clinical trial of 50 patients, observed no advantage with the administration of ethanol versus the administration of benzodiazepines during four days in the prevention of withdrawal syndrome due to alcohol.
Withdrawal syndrome due to stimulants (cocaine and methamphetamines)What are the treatment measures?
C22. The current scientific evidence does not allow recommendations on the management of withdrawal syndrome due to stimulants. However, in view of the frequency of this syndrome, the conduction of randomized clinical trials is advised in order to define the integral management of withdrawal syndrome due to cocaine and amphetamines.
Justification: Withdrawal syndrome due to stimulants produces dysphoria with sleepiness, appetite and motor alterations. Depressive symptoms can develop in the first 8–48h, and can persist for as long as two weeks.169
The treatment of withdrawal syndrome due to stimulants using indirect dopamine agonists (methylphenidate, amantadine) or antidepressants (desipramine, bupropion) has not been found to be effective in reducing the intensity of the symptoms.169
Kampman et al.170 evaluated the administration of adrenergic antagonists (propranolol) versus placebo in patients with withdrawal syndrome due to cocaine. No superiority on the part of propranolol was demonstrated, except in those patients with severe cocaine withdrawal syndrome.
In a systematic review, treatments for withdrawal syndrome due to amphetamines using amineptine or mirtazapine showed no differences versus placebo.171
Delirium and persistent cognitive deficitWhat are the risk factors associated with persistent cognitive deficit after admission to the ICU?
C23. An evaluation is recommended of the risk factors associated to the appearance of persistent cognitive deficit in patients admitted to the ICU.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Few studies have evaluated the risk factors underlying persistent cognitive deficit. Delirium has been found to be a triggering element of cognitive dysfunction in two studies that identified a positive correlation between delirium during the stay in the ICU and cognitive deficit persisting beyond hospital discharge.172,173 In a multicenter observational study, Iwashyna et al.174 found severe sepsis to be a risk factor for persistent cognitive deficit (OR: 3:34; 95%CI: 1.53–7.25). Hyperactive and mixed delirium have been the subtypes most closely associated with the development of such cognitive dysfunction, in comparison with the hypoactive subtype.175 Acute critical illness, even in the absence of delirium, can also be a risk factor for persistent cognitive dysfunction two months after discharge from the ICU.176 A retrospective study of 74 ARDS survivors found hyperglycemia to be associated to cognitive dysfunction.177 However, this study did not adjust risk to certain covariables such as the severity of disease.
In a case–control study of 37 pairs of critical patients, hypoglycemia was associated with cognitive dysfunction referred to visuospatial skills one year after discharge from the ICU. These observations require confirmation by other studies, however.178 Failure to remember events occurring during the stay in the ICU also appears to be associated to cognitive dysfunction persisting at least one year after hospital discharge.179 Chronic critical disease also seems to behave as a risk factor for persistent cognitive deficit.180
Which are the most frequently used scales for the diagnosis and monitorization of cognitive dysfunction?
C24. The use of validated scales is advised as instruments for the identification of persistent cognitive deficit.
Grade of recommendation: weak. Level of evidence: low (2C).
Justification: Many scales have been used to diagnose and quantify cognitive dysfunction. The most common instruments are standardized tests, including the Wechsler Adult Intelligence Test Revised,181 the Wechsler Memory Scale-Revised,181 the Rey Auditory-Verbal Learning Test, the Rey Osterrieth Complex Figure Test,182 the Trail Making Test Parts A and B,183 and the Verbal Fluency Test.184 There is considerable experience with the use of these neurocognitive tests, and they have been validated in different settings for the assessment of persistent cognitive deficit.
Other tests that can also be used are the Mini-Mental State, which has been validated and is easier to use in the ICU185; the Cambridge Neuropsychological Test Automated Battery, which can be used in patients who are unable to speak186; and the Questionnaire on Cognitive line in the Elderly, which can be administered to the patient relatives or visitors.187 To the best of our knowledge, no studies have compared the accuracy of these scales.
What are the best management options?
C25. The adoption of preventive measures against persistent cognitive deficit is advised, in view of the lack of clinical trials evaluating possible treatment options once the condition has become manifest.
Grade of recommendation: weak. Level of evidence: low (2C).
Justification: To date, no studies have evaluated persistent cognitive deficit as a primary outcome of any therapeutic intervention. It has been assessed as a secondary outcome, however.188 Early mobilization and occupational therapy have been associated with a decreased incidence of delirium in the ICU and in hospital, as well as with better functional outcomes over the long term. Given the direct relationship between certain modifiable risk factors and the occurrence of persistent cognitive deficit, it could be inferred that the modification of such factors may have a significant impact upon the incidence of the disorder. There is not enough information to support this affirmation, however.
Non-pharmacological prevention of delirium in the Intensive Care UnitWhat non-pharmacological interventions are recommended for preventing the development of delirium in the ICU?
C26. The use of an early mobilization protocol is recommended as a major component of strategies for the prevention of delirium in patients admitted to the ICU.
Grade of recommendation: strong. Level of evidence: moderate (1B).
C27. The joint use of multiple interventions is advised, together with the use of earplugs to prevent delirium.
Grade of recommendation: weak. Level of evidence: low (2C).
Justification: The aim of non-pharmacological interventions is to avoid or revert potential risk factors. To date, only a few studies have evaluated these non-pharmacological strategies for the prevention of delirium, and most of them have been conducted outside the critical care setting. Schweickert et al.188 found that early mobilization and occupational therapy can shorten the duration of delirium in patients subjected to MV. A before-and-after study was made, evaluating an intervention based on patient reorientation, music therapy and the use of a sedation and analgesia protocol, in which the incidence of delirium did not change–though the incidence of subclinical delirium was reduced.189 Needham and Korupolu evaluated early mobilization in the context of a quality improvement program. Following implementation of the early mobilization protocol, they found the incidence of delirium to decrease (days in the ICU without delirium: 53% versus 21%, p=0.003).190 In a recent study evaluating the use of earplugs for improving sleep quality and reducing the incidence of delirium, the latter did not decrease–though the frequency of mild confusion was significantly lowered.191
What quality indicators should be used for evaluating the prevention measures?
C28. Interconsultation with physical and occupational therapy is advised for adequate mobilization of the patient in the ICU. Oversedation, the incidence of delirium, assessment of the pain, safety events and functional mobility should be used as quality indicators for the prevention of delirium–though further studies are needed in reference to this topic.
Grade of recommendation: weak. Level of evidence: low (2C).
Justification: Only one study evaluating quality indicators for the prevention of delirium has been published to date. In this study, Needham and Korupolu190 used the following quality indicators for the prevention of delirium: (1) interconsultation with physical and occupational therapy (proportion of patients); (2) oversedation alert (proportion of days in the ICU); (3) incidence of delirium (proportion of days in the ICU); (4) pain scales (mean daily scores); (5) physiological instability (measured as change in heart rate, diastolic blood pressure and oxygen saturation from the start to the end of the procedure); (6) unexpected events (proportion of treatments); and (7) functional mobility (proportion of treatments with the patient sitting on the edge of the bed or during standing attempts).
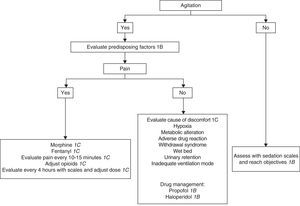
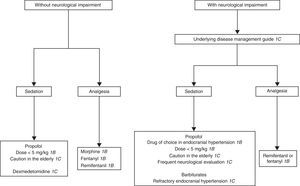
D. Patients without tracheal intubation or ventilatory supportWhat are the recommendations for the management of patients with anxiety and agitation admitted to the ICU?
D1. The start of sedation in the agitated critical patient is recommended only after affording adequate analgesia and treatment of the potentially reversible causes.
Grade of recommendation: strong. Level of evidence: low (1C).
D2. The objective of sedation in each patient should be established and redefined periodically. Treatment response should be evaluated on a continuous basis and systematically documented.
Grade of recommendation: strong. Level of evidence: low (1C).
D3. In patients without TI and/or without ventilatory support, it is advisable to use drugs with a low risk of producing respiratory depression and severe hemodynamic adverse effects, such as haloperidol and dexmedetomidine.
Grade of recommendation: strong. Level of evidence: low (1C).
Is drug treatment useful in uncooperative patients subjected to noninvasive MV?
D4. Sedation with drugs that do not cause respiratory depression in uncooperative patients subjected to noninvasive MV is recommended.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Drug treatment in the agitated critically ill patient without an artificial airway may lead to lesser cooperation on the part of the patient. Moreover, drugs in themselves can cause agitation or airway loss, giving rise to acute emergency situations. Consequently, monitorization of the level of sedation is more important than the chosen sedation technique.192
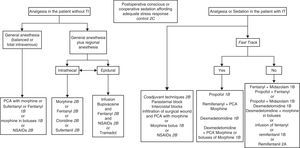
In patients without TI we use the same drugs as in intubated patients. Administration in bolus form is advisable at the start, followed by infusion which should be adjusted according to the response obtained. Very agitated patients can be immobilized while the appropriate drugs are administered via the intravenous route.192
The most important element in sedation of the agitated critical patient without MV is the physical presence of personnel adequately trained in airway management.192 The appropriate choice of sedating medication is often difficult, and depends on the individual needs. If quick awakening is required, as in neurological patients, propofol is the recommended drug. Haloperidol has been the preferred drug in delirium. Benzodiazepines are not advised, since they worsen agitation. The drug dosage required for adequate sedation also varies in the critical patient depending on the comorbidity, interaction of the sedative with other drugs, and the response to treatment193 (Fig. 2).
In recent years there has been an increase in the use of noninvasive MV in the ICU. This ventilation mode is mainly indicated in cases of acute respiratory failure (ARF), with the purpose of improving arterial oxygenation, increasing alveolar ventilation, reducing respiratory effort and avoiding TI. Noninvasive MV reduces mortality and the need for intubation in patients with COPD and respiratory failure when employed both in the ICU and in hospitalization. In the ICU, noninvasive MV also reduces the duration of stay.194 Patient tolerance is important to ensure the efficacy of noninvasive MV. However, this assisted ventilation mode often reduces patient comfort, causing anxiety and difficulty in synchronizing the patient with the ventilator.
The chosen treatment schemes vary, though drugs that cause respiratory depression should be avoided.195 Of the existing substances used for sedation, the only drug that does not produce respiratory depression is dexmedetomidine.195,196 Midazolam195 or remifentanil197,198 at low doses and under close medical monitorization can also be used.
In a clinical trial of 41 patients subjected to noninvasive MV, both dexmedetomidine and midazolam were found to be effective in reaching the desired RASS score, and maintained the same respiratory frequency and gas exchange parameters. In the dexmedetomidine group, two patients required dose adjustment, versus three in the midazolam group.195 Huang et al. also compared the effect of dexmedetomidine versus midazolam in 62 patients with cardiogenic lung edema that rejected noninvasive MV. The group treated with dexmedetomidine required less TI (21% versus 45%) (RR 0.47; 95%CI: 0.22–1.02) (p=0.06) and a shorter stay in the ICU (4.9 versus 8.5days) (p=0.04); mortality in the ICU was similar with both drugs (6% versus 10%).199
Should patient immobilization be carried out? When?
D5. The use of restraining measures is only recommended in clinically appropriate situations, not as a routine practice. When immobilization is used, its benefit must be clear and based on a Department protocol.
Grade of recommendation: strong. Level of evidence: low (1C).
D6. The immobilization measures should be as noninvasive as possible and capable of optimizing patient safety, comfort and dignity.
Grade of recommendation: strong. Level of evidence: low (1C).
D7. The rational use of immobilization and its duration should be documented in the clinical history. Written instructions are required.
Grade of recommendation: strong. Level of evidence: low (1C).
D8. Periodic evaluation of the possibility of suppressing the immobilization measures is recommended.
Grade of recommendation: weak. Level of evidence: low (2C).
D9. The use of analgesics, sedatives and neuroleptics is recommended for the treatment of pain, anxiety or psychiatric disorders in patients in the ICU, since they can lessen the need for immobilization. However, such drugs should not be administered in excess as a form of “chemical immobilization”.
Grade of recommendation: strong. Level of evidence: low (1C).
D10. The development of complications due to immobilization should be evaluated at least once every 4h.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Immobilization is commonly used in the ICU to protect the patient from the risks associated to the accidental tearing out or removal of devices such as endotracheal tubes, nasogastric tubes, venous catheters, arterial catheters, drains and urinary catheters. It is also used to protect the healthcare personnel from unintentional injury due to alterations in the cognitive condition of the patient.200,201 Restraint may also be needed to limit patient movements when the latter are contraindicated, for example in spinal fractures, for facilitating procedures in which the patient cannot collaborate, and for facilitating the management of certain psychiatric patients.202 The most commonly used method is the limitation of mobility of the upper extremities through binding at wrist level (87%).201
Immobilization only should be used when alternative measures prove ineffective or cannot be used without placing the patient at risk. The non-pharmacological alternative measures include the limitation of noise, the avoidance of unnecessary patient awakening, and family cooperation. Immobilization may be required if such measures fail.
On prescribing immobilization, due consideration is required of the potential conflict between the perceptions of the medical-nursing team and the best interest of the patient. On one hand, we should evaluate the need to protect the patient from his or her involuntary actions, though we also must take into account that immobilization can worsen the symptoms of delirium and can cause injuries. On the other hand, the healthcare team caring for the patient must be protected from unintentional aggression by the agitated patient.
Immobilization should be used as briefly as possible, and its indication must be reflected in the clinical history. According to the norms of the Joint Commission on Accreditation of Healthcare Organizations,203 once the alternatives to immobilization have failed and instructions are given to immobilize the patient, the decision must be stated and duly explained in the clinical history. In actual practice, the suggestion to immobilize the patient usually originates from the nursing personnel. Each institution should develop its own program to ensure the quality of care, in accordance with the principles and policies for the adequate use of physical immobilization201 (Fig. 3).
The policies referred to mechanical immobilization in the ICU vary greatly throughout the world, even in the presence of similar technologies or nursing-patient relationships,204 and the prevalence of such measures also varies greatly (from 0% to 100%).205 Some studies have found a certain relationship between non-scheduled tracheal extubation and mechanical restraint.206,207 Mechanical immobilization has also been associated to an increased risk of suffering delirium115 and post-traumatic stress syndrome in patients discharged from the UCI,208 though the methodological designs of the studies do not allow the drawing of firm conclusions.
E. Patients with mechanical ventilationAre there physiological benefits of using sedation and analgesia during MV?
E1. The routine use of sedoanalgesia is recommended in patients subjected to mechanical ventilation with TI.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: A lack of adaptation to the ventilator produces many complications that can further worsen the condition of the critical patient, including respiratory acidosis secondary to hypoventilation and increased CO2production; hypocapnia due to hyperventilation; hypoxemia as a result of dyssynchrony between the patient and the ventilator; increased intrathoracic pressure with a decrease in venous return, cardiac minute volume and arterial pressure; and increased O2 consumption due to the rise in muscle activity.3,59,209–211
Sedation and analgesia are commonly indicated in critically ill patients who at the same time require other priority medications or treatments. The indication of sedation and analgesia is established empirically, with a choice of drug and dosage that often proves inadequate.212
Not all ventilated patients require some or all of these medications, as in the case of the patients with neuromuscular problems (e.g., Guillain–Barré syndrome), who require mild daytime sedation and nocturnal sedation in order to sleep. A patient with severe ARDS will probably need maximum levels of analgesia, sedation and, sometimes, muscle relaxation.213,214
Analgesia implies the absence of sensitivity to pain or to aggressive stimuli such as the presence of an endotracheal tube or the aspiration of secretions. It is very common in MV to use sedatives that “sleep” the patient, but which do not protect him or her from pain or the systemic reactions caused by it–including tachycardia, increased myocardial oxygen consumption, etc. If the administration of analgesics results in disappearance of the physiological alterations, then pain can be confirmed as having been the cause of such alterations.3,61,64
Sedation in the critical patient is indicated as basic treatment for anxiety and agitation. These two sensations, combined with MV, imply that sedation in these patients is unavoidable, at least in the early stages.3,211 There are differences in the way sedating agents are used in acute or short-lasting situations versus the prolonged sedation that accompanies MV. Such differences refer not only to the drug used, but also to the method of administration involved.
What are the recommendations for the management of sedation and analgesia in patients subjected to MV?
E2. The use of a scale for the evaluation of pain and depth of sedation is recommended in patients subjected to MV.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: It is important to carry out a reproducible evaluation of whether the analgesia we seek has been satisfactorily obtained. Since pain is essentially subjective, we should try to know the opinion of the patient, if possible. Since these are ventilated patients, subjected to TI, we can use a graphic pain scale (visual analog scale) that proves easy to understand, for example a line extending from “no pain” at one end to “maximum pain” at the other. In the case of sedated patients, in whom it is particularly common to underuse analgesia, it is important to evaluate the somatic and physiological equivalents of pain. Among the former, patient facial expression, movements and body posture can be clear indicators of pain, as in the BPS scale.215,216 In turn, among the physiological signs, the presence of tachycardia, hypertension, tachypnea and ventilator disadaptation, included in the Non-Verbal Pain Scale,215,216 requires us to consider the administration of analgesics, if not already administered, or to increase their dose.3,61,64
Another problem is the difficulty of assessing the depth of sedation. The use of scales for evaluating the depth of sedation during MV is recommended by most consensus guides on the subject. Sedation scales are a fundamental part of the protocols referred to the adaptation of patients with MV60,61,64,106 (Table 3).
E3. Implementation of the sedation and analgesia protocols preferably should be carried out by the nursing personnel. It is not advisable to apply this recommendation when there are not enough nurses available.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: With some exceptions, different authors have reported better results with the application of strict sedation and analgesia protocols controlled and applied by the nursing personnel of the ICU. However, it is clear that when the number of available nurses is insufficient, they cannot be overburdened by this additional task.34,65,217–230
E4. The routine use of deep sedation (RASS 1 to −3) in patients subjected to MV is not recommended.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The levels of sedation differ from one type of patient to another. The adequate levels of sedation for ventilated patients are between 2 and 4 on the Ramsay scale or between 1 and −3 according to the RASS13,27,29,32,231–234 (Tables 3 and 5). In patients with MV due to complex situations (e.g., exacerbated COPD, severe asthma, ARDS), levels corresponding to Ramsay 3 or 4 or RASS −4 are recommended. With these levels of sedation we often observe anterograde amnesia–a situation which some authors associate to an increased incidence of delirium and post-traumatic stress.
It should be remembered that deep sedation corresponding to level 5 or 6 according to the Ramsay scale or RASS −5 may be useful only in sedation forming part of the treatment of intracranial hypertension, or in situations such as tetanus or malignant hyperthermia.235,236
E5. Whenever possible, it is advisable to use conscious or cooperative sedation with titrated doses of a continuous infusion of propofol or dexmedetomidine.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: A number of studies have shown this approach to shorten the duration of MV, the days of stay in the ICU, and the incidence and duration of delirium. Likewise, it has been reported that this strategy does not increase the incidence of myocardial ischemia.29,32,231–234,237,238
E6. Opioids are recommended as the analgesics of choice in the ventilated patient–the first line drugs being fentanyl and morphine–especially in patients requiring prolonged ventilation.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The most important side effects of the opioids, which are regarded as the drugs of choice for analgesia in the critical patient, are respiratory depression, arterial hypotension, gastric retention and ileus. Despite these adverse effects, correct analgesia is a primary objective in the critical patient subjected to MV.3,59,61,64,210,239
Morphine, in sulfate or hydrochloride form, is the analgesic drug of choice for ventilated patients. Its advantages include analgesic potency, low cost, and an euphorizing effect. Morphine is to be administered via the intravenous route, starting with a loading dose followed by continuous venous infusion. The recommended loading dose is 0.05mg/kg administered in 5–15min. Most adults require continuous infusion of between 2 and 3mg/h, with levels of up to 4–6mg/h in some patients. During the continuous infusion of morphine it is common to need one or more bolus doses at the same dosage as the initial loading dose, in order to secure an adequate analgesic effect. If administration in bolus form is decided, it should be scheduled as repeated doses, once every 3h, attempting to adjust the dosage according to the therapeutic response obtained.3
Fentanyl is the analgesic drug of choice for ventilated patients with hemodynamic instability, or in patients with symptoms of histaminic release or allergy with the use of morphine. Fentanyl does not cause histamine release–a fact which may explain its lesser effect upon arterial pressure and bronchial smooth muscle. It has a relatively short half-life (30–60min), due to its rapid distribution. However, the prolonged administration of fentanyl gives rise to accumulation of the drug in the peripheral compartments, and extends its half-life (contextual half-life) to 16h. The drug is to be administered in continuous infusion at a rate of 1–2μg/kg/h, following one or more loading doses of 1–2μg/kg. The routine use of fentanyl is not recommendable in all patients, since its analgesic effect is similar to that of morphine; the drug tends to accumulate as a result of prolongation of its half-life; and its cost is usually greater.3
E7. It is advisable not to use meperidine, nalbuphine or buprenorphine in the critical patient.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Meperidine has an active metabolite, normeperidine, which can accumulate and produce excitation of the central nervous system and seizures, particularly in patients with acute neurological damage.3
Nalbuphine and buprenorphine are usually prescribed to calm mild or moderate pain in the immediate postoperative period. It must be remembered that they can revert the effect of other opioids through interaction at receptor level. They can be used as an option when the traditional opioids are contraindicated.3
E8. Whenever possible, it is advisable to minimize or suppress the use of sedatives in prolonged MV, using the sedation scheme based on analgesia.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: A number of studies have reported a decrease in the number of days with MV and in the duration of ICU stay when the use of sedatives is suppressed or minimized by the administration of opioid analgesics in continuous infusion in patients with MV. The drug most widely used in the studies that propose this approach is remifentanil–its main advantage being rapid disappearance of the effect and independence of renal and/or liver function.31,36,240–243
Remifentanil is a synthetic opioid that exhibits practically no accumulation, since it is quickly metabolized by plasma esterases. It likewise does not accumulate in patients with kidney or liver failure. These properties imply that recovery from the effects of the drug takes place in only a few minutes, even after prolonged administration. The usual analgesic doses are 0.05–0.3μg/kg/min. Depending on the dose, remifentanil produces central depressor effects like other opioids. Some studies recommend its use in analgesia based sedation Schemes.36,240,241,242,243 The drug has also been used in the ventilator weaning process.244–246
E9. Midazolam, propofol or lorazepam are recommended as sedatives of choice in patients with MV requiring deep sedation corresponding to RASS −4 to −5 or Ramsay 4–6.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: There are differences in the use of sedating agents in acute or short-lasting situations and in deep sedation accompanying MV in patients with diseases such as those mentioned above (Fig. 4). These differences refer not only to the drug employed but also to the method of administration.247–251
All parenteral benzodiazepines cause anterograde amnesia, and it should be remembered that they do not have analgesic effects. Although the prototype drug for intravenous sedation is diazepam, it is no longer recommended, because: (a) it often causes pain and thrombophlebitis when administered through a peripheral vein; (b) administration in bolus form can lead to excessive sedation; (c) administration as a continuous intravenous infusion increases its half-life, reaching 7 days in some patients; and (d) the drug requires dilution in a large volume–which implies a risk of water overload in the context of prolonged administration. However, it is used in some centers because of its low cost and rapid mechanism of action when performing brief maneuvers (electrical cardioversion, TI), being indicated as a single bolus dose.3 Midazolam and propofol are the drugs of choice for brief sedation, as for example when maneuvering in TI at the start of MV. The usual midazolam dose for effective sedation during TI maneuvering or other brief procedures is 0.2mg/kg, which can be repeated in bolus doses of 0.07mg/kg until the desired sedation level is reached.3
Propofol is an intravenous anesthetic which at sub-anesthetic doses produces sedation and hypnotic effects, as well as a degree of anterograde amnesia. Propofol and midazolam have been shown to have the same sedative effect in comparative studies.3,252
Propofol offers fast action after the administration of an intravenous bolus (1–2min), due to its rapid penetration of the central nervous system, and its effect is short lasting (10–15min). When used in prolonged treatments, it should only be administered in continuous infusion with the precaution of using a central vein rather than a peripheral venous access. Propofol also significantly prolongs its half-life when administered for prolonged periods of time, due to accumulation of the drug in the lipid deposits–its half-life reaching 300–700min.252,253 In order to secure rapid sedation, as in TI maneuvering, bolus dosing at 2–2.5mg/kg is recommended. If we wish to keep the patient sedated, in the case of continuing with MV, a continuous infusion should be used at a starting dose of 0.5mg/kg/h, followed by stepwise increments of 0.5mg/kg every 5–10min, according to the clinical response obtained. A common maintenance dose is between 0.5 and 3mg/kg/h. The administration of propofol in bolus form usually produces a drop in arterial pressure that can reach 30% of the basal blood pressure value.254,255
In recent years, many studies have shown benzodiazepines (midazolam and lorazepam) to be associated with an increased incidence of coma and delirium in intubated and ventilated patients. This in turn results in longer periods of MV, a longer stay, and greater morbidity–mortality.29,32,35,36,231,233,234,256
Lorazepam is one of the drugs suited for prolonged sedation in the ventilated patient. This intermediate-acting benzodiazepine is less lipophilic than other drugs of the same group, and therefore undergoes less peripheral accumulation. In comparison with midazolam, lorazepam has a longer half-life and a similar capacity to produce anterograde amnesia. In view of its half-life, lorazepam is suitable for administration in intermittent bolus doses, though it can also be used in continuous infusion. Since the pharmacological latency period of lorazepam is longer than that of other benzodiazepines, it is advisable to start with a dose of diazepam or midazolam to induce rapid sedation. The lorazepam dose recommended as starting bolus or as “reinforcement” dose is 0.05mg/kg, to be repeated every 2–4h as required. In prolonged sedation, some authors advise the administration of a continuous infusion of lorazepam at doses of 0.025–0.05mg/kg/h. These doses are usually insufficient, however, and can be doubled or tripled in some patients. A number of authors have reported elevated propyleneglycol levels that could prove toxic under these circumstances, though no evident clinical effects have been described.251 The intravenous form of lorazepam is not available in all countries.3
E10. The use of a sedative with a shorter half-life, such as dexmedetomidine, is recommended for reducing the duration of MV and the incidence of delirium in patients that can tolerate mild sedation levels (RASS 1 to −3 or Ramsay 2–3).
Grade of recommendation: strong. Level of evidence: moderate (1B).
E11. Dexmedetomidine is recommended as a useful drug for postoperative sedation and analgesia in patients requiring MV for short periods of time, and particularly in septic patients.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Dexmedetomidine is an α2 agonist with greater affinity for α2 receptors than for α1 receptors. The drug inhibits the postsynaptic receptors, thereby inducing not only a drop in blood pressure and heart rate but also a clear anxiolytic and sedating effect. Its action at spinal receptor level also affords an analgesic effect. The initial loading dose in ventilated patients is 1μg/kg in 10min. The maintenance infusion rate is 0.2–1.4μg/kg/h, considering that the side effects are greater with doses above 0.7μg/kg/h. The maintenance dose often must be increased when using dexmedetomidine in prolonged treatments. The loading bolus can cause bradycardia accompanied by hypotension, which in some cases can be sustained over time, particularly in hypovolemic and elderly patients. Some authors recommend obviating the loading doses in order to avoid these side effects.
This drug does not produce respiratory depression or gas exchange alterations, and can be safely administered in patients with renal failure. Dexmedetomidine likewise does not cause alterations in adrenal cortical or inflammatory function. In view of these properties, some authors choose dexmedetomidine as the best sedative for weaning from MV, and for conscious sedation. Observational studies also show that dexmedetomidine appears to reduce the incidence of delirium and the mortality rate among septic patients (hazard ratio 0.3; 95%CI: 0.1–0.9).29,32,248,253,256–260 These findings must be confirmed by further studies, however. The dosage should be lowered in patients with liver failure.13,35,36,38,42,248,259,261–264
E12. It is advisable not to use etomidate in sedoanalgesia for patients subjected to MV.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Etomidate should be contraindicated in the critical patient, due to its capacity to produce adrenal gland insufficiency.265,266
E13. Daily interruption of the infusion of sedatives and analgesics is recommended in order to reduce the total administered dosage.
Grade of recommendation: strong. Level of evidence: moderate (1B).
E14. The daily interruption of sedoanalgesia is recommended, with performance of a spontaneous ventilation test (when allowed by the respiratory condition of the patient), with the purpose of reducing the development of complications and the duration of MV.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Kress et al.,267 in a clinical trial involving 128 patients treated with the same sedoanalgesic scheme (midazolam or propofol plus morphine) for maintaining a Ramsay score of 3 or 4, compared the effect of the daily interruption of sedoanalgesia from 48h after the start of treatment (experimental group) versus the interruption of sedoanalgesia according to physician criterion (control group). The mean duration of MV in the experimental group was 4.9days versus 7.3days in the control group of (p=0.004); the duration of stay in the ICU was 6.4 and 9.9days (p=0.02), respectively. The incidence of complications (e.g., accidental withdrawal of the endotracheal tube) was 4% and 7%, respectively. A decrease in the total dose of sedatives and morphine was observed among the patients treated with midazolam (40% lesser dosage in the experimental group), but not in those treated with propofol.
Similar results have been recorded in a retrospective study that likewise registered a lesser incidence of pneumonias, sinusitis, bacteremia and deep venous thrombosis in patients subjected to the daily interruption of sedoanalgesia.267,268 The daily suspension of sedoanalgesia should not be carried out on a routine basis in patients with intracranial hypertension, MV involving non-conventional ventilatory modes or with hemodynamic instability. Individual assessment is required in such cases.267,268
There have been reports of improved sleep performance with this practice.269 Some authors have not been able to reproduce these data, however.34 If the patient is disadapted to the ventilator, already has adequate analgesia and a Ramsay score of 4 or a RASS score of under −3, use can be made of neuromuscular relaxants. It is not advisable to progress to higher sedation levels to achieve adaptation to the ventilator. In patients receiving an infusion of muscle relaxants, we must wait for their effects to subside before deciding the daily suspension of sedoanalgesia. If the patient does not present disadaptation to the ventilator after the period of time considered sufficient for elimination of the paralyzing effect, we should proceed to suspend midazolam as recommended for the global patient population.34,253,267–273
E15. The monitorization of sedation with BIS is recommended in patients subjected to MV with acute neurocritical disease or under the effects of neuromuscular relaxants, in order to avoid infra- or oversedation.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Olson et al.,101 in a clinical trial of 67 neurocritical patients subjected to MV and continuous sedation with propofol, evaluated the effect of two nursing-controlled protocols: Ramsay 4 versus Ramsay 4 plus BIS 60–70 during 12h. Addition of the BIS to the Ramsay scale was associated with a decrease in the dose of propofol (14.6 versus 27.9μg/kg/min) (p=0.003).
E16. It is advisable to consider a decrease in sedatives and analgesics in patients subjected to MV after performing tracheostomy.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: When MV is prolonged, the patient should not continue with the endotracheal tube.
It is not the purpose of this document to discuss the timing of tracheostomy or the best way of performing the technique. The absence of a tube through the glottis considerably lessens patient pain. Likewise, the level of sedation can be lowered under these circumstances, because the patient is more stable from the hemodynamic, neurological and respiratory perspectives. Nieszkowska et al., in a retrospective observational study of 72 patients subjected to MV, found the need for sedative and analgesics to decrease after tracheostomy.274
E17. It is suggested not to routinely increase the dose of sedatives, when accompanied by an infusion of morphine, in patients subjected to permissive hypercapnia.
Grade of recommendation: weak. Level of evidence: low (2C).
Justification: Permissive hypercapnia is a MV technique that can be used in two types of situations. The first corresponds to patients with severe airflow obstruction (asthma, COPD) requiring a low respiratory frequency and low tidal volumes in order to avoid high pressures in the airways and air trapping. The second refers to patients with severe ARDS who develop hypercapnia on being ventilated with a protective strategy.275 These situations often require the use of muscle relaxants.276 Vinayak et al.,277 in a retrospective study of 124 patients subjected to MV, found that in the first three days the patients with permissive hypercapnia treated with midazolam (n=13) did not require more doses than those who did not have permissive hypercapnia (n=51). In contrast, the patients with permissive hypercapnia treated with propofol (n=10) required greater doses than those who did not have hypercapnia (n=50). Other studies have obtained similar results.278,279
E18. In patients with ARDS, PaO2/FiO2<150, subjected to protective MV, consideration of the use of non-depolarizing neuromuscular blockers in continuous infusion is suggested during the first 48h.
Grade of recommendation: weak. Level of evidence: moderate (2B).
Justification: Needham et al. recently reported that patients with severe ARDS treated with a continuous infusion of non-depolarizing muscle relaxants (cisatracurium) during the first 48h of MV presented lesser mortality after 90 days, and a decrease in morbidity, with a shorter duration of MV, a briefer stay in the ICU, and a lesser incidence of organ failure and muscle weakness.276
What are the recommendations for patients with special conditions, such as individuals with COPD, ARDS, asthma, hemodynamic instability or multiorgan failure?
E19. Fentanyl is recommended as the analgesic of choice in patients with hemodynamic instability, bronchial asthma or COPD.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Fentanyl does not give rise to histamine release–a fact that can explain its lesser effect upon arterial pressure and bronchial smooth muscle.3
E20. An early physical and occupational therapy protocol is recommended in intubated patients subjected to MV, with the aim of reducing the duration of MV.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: In a series of 109 patients, Schweickert et al. evaluated the efficacy of physical and occupational therapy in the first three days of MV during the daily waking periods using the prescribed standard treatment that included daily awakening but without physiotherapy until prescription of the latter by the primary care team. Early therapy was associated with a greater percentage of functional independence at discharge (59% versus 35%) (OR 2.7; 95%CI: 1.2–6.1) (p=0.02) and a lesser duration of delirium (2 versus 4 days) (0.02).188 Needham et al., in a prospective observational study lasting 6 months in 57 patients subjected to MV for four days or more and enrolled in an early physical rehabilitation protocol with the reduction of deep sedation, reported a decrease in the incidence of delirium, with improved functional mobility, and a shortening of stay both in the ICU and in hospital.233
E21. Music therapy is recommended as a non-pharmacological adjuvant to sedation in patients subjected to MV, particularly during weaning.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Different studies have found music therapy to reduce anxiety, its physiological equivalents, and the administration of sedation during MV, particularly during weaning periods.280–282
F. Patients undergoing withdrawal of the endotracheal tube and mechanical ventilationWhat should be the pharmacological approach to patients undergoing withdrawal of MV and the oro/nasotracheal tube?
F1. A defined sedation and analgesia monitoring and dose adjustment protocol is recommended when withdrawal of the ventilator is planned, following resolution of the cause leading to the need for MV. This protocol should include daily evaluation of sedation, an awakening test and a spontaneous breathing test.30
Grade of recommendation: strong. Level of evidence: high (1A).
Justification: Patients undergoing withdrawal of MV and the tracheal tube must be in the waking state. All sedoanalgesia protocols must aim to prevent the accumulation of sedatives and analgesics, and the prolongation of their effect.
At this point the leading priority is to apply non-pharmacological measures for alleviating anxiety: the patient should receive a clear explanation of the situation, of the collaboration expected from him or her, and of the steps to be taken. Likewise, noise should be reduced to a minimum, along with the intensity of illumination, and flexible visiting hours should be allowed, since these are measures that help the patient in this phase. One of the basic principles for extubation is to assess sedation and determine whether its level is able to interfere with spontaneous breathing. Girard et al.30 in a study of 336 sedated patients subjected to MV, evaluated the combination of interrupting sedation and performing the spontaneous breathing test in the intervention group, versus use of the spontaneous breathing test in the control group. The patients assigned to the intervention group showed a clear reduction in the days without ventilation and in the duration of ICU stay (9.1 versus 12.9 days) (p=0.01). The mortality rate after 28 days showed no significant differences between the two groups (28% versus 35%) (p=0.21), though the difference in mortality after one year did reach statistical significance (44% versus 58%) (p<0.01). The interruption of sedation and the spontaneous breathing test can be regarded as standards of care.
F2. A defined sedation and analgesia monitoring and dose adjustment protocol is recommended, with daily suspension, when withdrawal of the ventilator is contemplated, once the cause leading to the need for MV has been resolved.
Grade of recommendation: strong. Level of evidence: high (1A).
Justification: During the withdrawal of MV and TI, the patient must be lucid and alert. If necessary, medication to control pain and psychomotor agitation should be provided.28,283,284
Which drugs may be contraindicated?
F3. It is advisable not to use midazolam or lorazepam in the withdrawal of MV.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Prolonged sedation with midazolam or lorazepam is associated to a prolongation of the time on MV, the time of withdrawal of MV and the orotracheal tube, and of patient stay in the ICU, since drug accumulation can occur, with the prolongation of sedation.253,285–288
Are there drugs that might be indicated?
F4. Low-dose remifentanil in continuous infusion is recommended for the sedation and analgesia of patients subjected to weaning from MV.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The choice of drugs depends on factors related to the patient (comorbidities, cause or indication of MV, time of MV), the pharmacological characteristics of the drugs, their adverse effects and costs.289 Pain can be one of the causes of withdrawal failure. In order to guarantee constant analgesia, continuous infusion is preferable to bolus dosing, since it allows more effective titration, a lesser total drug dose, and a shorter duration of MV. Bolus doses can be used via the intravenous route as rescue medication.253,285,286
Rozendaal et al. compared the effect of two analgesia and sedation protocols in 205 patients subjected to MV in the context of an open-label, cross-over clinical trial. One group received remifentanil, and propofol was only administered if sedation was not achieved at maximum doses (12μg/kg/h). The conventional group in turn received analgesia with morphine or fentanyl, and sedation with propofol, midazolam or lorazepam. The mean weaning time was 6h in the group treated with remifentanil and 25h in the group subjected to conventional treatment (p=0.0001), while the stay in the ICU was 6 versus 8 days (p>0.05), respectively.240 In the opinion of some authors, these results support the recommendation to use remifentanil in the weaning process.290
F5. Methadone via the enteral route is recommended in patients receiving opioids for more than 5 days and who are subjected to MV.
Grade of recommendation: weak. Level of evidence: moderate (2B).
Justification: In a group of 28 patients subjected to MV, 32% (95%CI: 13–51%) developed withdrawal syndrome after receiving opioids for 7 days or more.291 A recent double-blind, randomized controlled clinical study involving 68 patients showed that among the 54 survivors, the use of enteral methadone in subjects who had received opioids during more than 5 days reduced the weaning time from 7 days (95%CI: 2.5–11.5) to 4 days (95%CI: 1.99–6.01).292
F6. Dexmedetomidine is recommended in postsurgical patients.
Grade of recommendation: strong. Level of evidence: low (1C).
F7. Dexmedetomidine is recommended in patients with MV weaning difficulties and in patients with withdrawal syndrome.
Grade of recommendation: strong. Level of evidence: low (1C).
F8. Dexmedetomidine is recommended in patients with failed previous attempts of weaning from MV secondary to agitation and delirium.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Dexmedetomidine is an effective and safe sedative for most postsurgical patients in the ICU. The advantages of dexmedetomidine are its α agonist effect, conscious sedation, a lessened need for opioids, and respiratory stability. However, the drug is associated with hypotension and bradycardia, which generally resolve spontaneously or with only minimum intervention in the form of volume replacement and sometimes atropine and low-dose vasoactive drugs.287,293–295
When the patient develops agitation, delirium, suffers anxiety or develops withdrawal syndrome, it is useful to maintain a minimum level of sedation using drugs with a short half-life.1
Delirium, agitation and withdrawal have been associated to adverse outcomes in patients subjected to MV, particularly during the withdrawal of MV. In this process, dexmedetomidine has been used to guarantee the success of weaning in patients with previous failed weaning attempts due to agitation. The benefits of dexmedetomidine are possibly more significant when used with daily interruption of sedation, a weaning protocol, and early mobilization.125,296–299
F9. Propofol is recommended in postsurgical patients.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Propofol is the sedative of choice when quick patient awakening is required, due to the shorter waking time associated with this drug. The difference is more accentuated when the therapeutic objective is a Ramsay score of 4 or 5. In addition, the waking time is more predictable in patients administered propofol than in those receiving midazolam.253,285–287
G. Special populations: trauma patients, elderly subjects, pregnant patients and burn victimsWhat special considerations apply, and what are the pharmacological recommendations for the management of sedation and analgesia in these patients?
Trauma patientsG1. Propofol is recommended for the sedation of trauma patients requiring frequent neurological evaluations (Fig. 5).
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: The pharmacokinetic properties of propofol, resulting in fast recovery of patient consciousness within 10–15min after suspending infusion of the drug, have been commented in a number of publications.300–302 This characteristic is very useful in patients requiring frequent evaluations of central nervous system function.
G2. It is advisable to use propofol at doses of under 5mg/kg/h in order to avoid propofol infusion syndrome.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Propofol infusion syndrome is defined as the presence of heart failure, metabolic acidosis and rhabdomyolysis.303 In this study the symptoms were evidenced 24–48h after the start of infusion. The mortality rate associated to this syndrome in patients administered propofol for the control of intracranial hypertension was 10%. The probability of appearance of the phenomenon is 1.93 for every mg/kg of increase over the mean dose of 5mg/kg/h (equivalent to 83μg/kg/min). None of the patients administered less than this dose developed the syndrome.303 The recommendation underscores the avoidance of propofol doses above 5mg/kg/h until the safety of other doses has been assessed.
G3. Use is recommended of protocols for sedoanalgesia and the detection of delirium in critical trauma patients.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: A controlled, prospective study involving patients that cannot receive daily interruptions of the infusion of sedatives found that objective evaluation using protocols for analgesia, conscious sedation and the detection of delirium reduces the days of MV and hospital stay in critical trauma patients.62
G4. Use of the CAM-ICU is recommended in the detection of delirium in trauma patients.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: A retrospective study compared 59 trauma patients treated with a protocol for sedation (RASS), analgesia (visual analog scale) and the estimation of delirium (Confusion Assessment Method for the Intensive Care Unit, CAM-ICU) versus 61 patients treated without this protocol two years earlier. During the period in which the protocol was used, the duration of MV was reduced from a median of 3.2–1.2 days (p=0.03), and the median duration of stay in the ICU was 5.9 versus 4.1 days (p=0.21), respectively.304
Elderly subjectsG5. Caution is recommended when using propofol in elderly patients.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: The initial recovery times in patients sedated with propofol are similar in young individuals (20–50 years of age) and in elderly patients (65–85 years of age). However, total psychomotor recovery is slower in the latter age group, and can take up to 120min after maintaining the patients sedated with BIS 60–70.305 The postural stability recovery times in elderly patients are likewise longer than in younger individuals, taking up to 120min.306
G6. Caution is recommended when using benzodiazepines in elderly patients.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Among patients over 65 years of age there is a correlation between the increased appearance of delirium and consequent increases in morbidity–mortality and management costs. The probability of developing delirium in this group increases 2% in relative terms (OR 1.02; 95%CI: 1.01–1.03) per year beyond 65 years of age. Other factors independently associated to the appearance of delirium are the APACHE II scale and the use of lorazepam.307
G7. The prophylactic use of low-dose haloperidol is suggested in the postoperative period among elderly patients.
Grade of recommendation: weak. Level of evidence: moderate (2B).
Justification: A randomized and controlled clinical trial involving 457 patients found that prophylactic treatment with low-dose haloperidol (initial 0.5mg intravenous bolus followed by intravenous infusion at a rate of 0.1mg/h) for a short period of time (12h) can reduce the incidence of delirium in elderly patients in the 7 days after non-cardiac surgery from 23% to 15% (p=0.03), and the stay in the ICU from 23 to 21h. Another observed effect was improvement in the number of days without delirium.308
Pregnant patientsG8. In evaluating the administration of analgesics and sedatives during pregnancy, it is advisable to follow the classification of the United States Food and Drug Administration (FDA).
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: The management of analgesia and sedation in pregnant patients must take two factors into account: (a) the capacity of the drug to produce damage in the embryo and fetus and (b) the possible reversible physiological effects of the drug in the newborn infant (sedation, respiratory depression, withdrawal syndrome) when used during the peripartum.
The classification of the FDA is shown in Table 11.309
Classification of drugs in pregnancy of the United States Food and Drug Administration (FDA).
| A | Controlled human studies show that there is no risk |
| B | No evidence of risk in studiesStudies in animals in which no risk is evidenced, without controls in pregnant subjects. Studies in animals show risk, but without risk in controlled studies with pregnant subjects |
| C | Risk cannot be ruled outNo controlled studies in pregnant subjects, and there is risk in animal studiesShould only be used if the potential benefit outweighs the potential risk for the fetus |
| D | Evidence of risk for the fetusShould only be used when the benefit for the seriously ill pregnant patient can be acceptable despite the risk for the fetus |
| X | Contraindicated in both pregnant women and in women who may become pregnant |
The potential risks of sedoanalgesia in pregnant women are described below:
Propofol. Category B.
Propofol produces reversible fetal effects. It can induce neonatal depression particularly in the peripartum.309 Propofol can be used at sub-hypnotic doses for the control of emesis associated to cesarean section.310 There are no differences in metabolism or response in pregnant women.311,312
Fentanyl and remifentanil. Category C.
These drugs can produce neonatal depression and must be used with caution. Chronic administration in pregnancy has been associated to withdrawal syndrome in the newborn infant.309
Benzodiazepines. Category D.
Benzodiazepines produce reversible fetal effects, neonatal depression and hypotonus.309 There is statistical evidence of a possible association to malformations of the digestive tube. In particular, the use of lorazepam during embryogenesis has been associated to anal atresia.313,314
Dexmedetomidine. Category C.
Adverse fetal effects have been observed in studies in animals, including low body weight and fetal death, though there are no controlled studies or reports of teratogenicity in humans.309
Haloperidol. Category C.
Adverse fetal effects have been observed in studies in animals, including fetal loss and cleft palate, though no controlled studies have been made in humans.309
Lurasidone. Category B.
Studies in animals have not demonstrated fetal effects, and studies in pregnant women have not revealed risk for the fetus.309
Ketamine. Category B.
Studies in animals have not demonstrated fetal effects. There are no human studies or reports of possible teratogenicity.309
G9. The concomitant use of intravenous paracetamol is recommended in situations requiring immediate analgesia, in order to lessen the need for opioids and their potential adverse effects.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The intravenous administration of paracetamol (acetaminophen) increases its bioavailability and efficacy, compared with enteral administration, by avoiding first-pass metabolization in the liver. This drug exerts synergic effects with other more potent parenteral analgesics; consequently, the necessary dose of the latter can be reduced. On the other hand, paracetamol at the usual doses is a very safe drug.315
Sedation and analgesia: special procedures (burn victims)What special considerations apply in the management of sedation and analgesia in burn victims?
G10. Ketamine is recommended as first line medication for sedoanalgesia in routine painful procedures in burn victims, with the use of opioids as second line treatment.
Grade of recommendation: strong. Level of evidence: moderate (1B).
G11. It is advisable not to use ketamine alone. The drug should be accompanied by midazolam, propofol or dexmedetomidine.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The management of painful procedures in burn victims, including the changing of bandages, cures, etc., requires the use of analgesics with potent hypnotic effects. In this context, ketamine is the most widely accepted option, due to its rapid action and absence of effects upon patient respiratory or hemodynamic function. Current studies aim to evaluate the ideal drug to be administered with ketamine, in order to lessen the side effects.316–318
H. Sedoanalgesia in the immediate postoperative period of cardiovascular surgeryAre there benefits in using sedation and analgesia in patients during the postoperative period of heart surgery?
H1. It is advisable for all patients in the immediate postoperative period of heart surgery to receive adequate analgesia and sedation, in order to lessen the appearance of possible complications.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The absence of pain and anxiety is a fundamental right of the patient, and moreover reduces the incidence of complications.67,319 Postoperative sedoanalgesia reduces morbidity in heart surgery. Tachycardia and catecholamine release contribute to the development of arterial hypertension, myocardial ischemia and atheroma plaque rupture. At the time of admission to the ICU, the patients are under the residual effects of anesthetics, and in this context the anesthetic technique used in the operating room is an important factor for defining the required grade of sedoanalgesia.
During the immediate postoperative period there is an interval in which MV is needed, and sedoanalgesia is therefore required while thermal homeostasis and hemodynamic stability are restored. Optimum sedation in this period minimizes cardiovascular response to stimulation, reduces the time required for awakening, and allows withdrawal of the endotracheal tube without increasing the incidence of cardiovascular complications.320 At present, with the use of fast-track anesthetic systems, the administration of short acting drugs allows rapid postanesthetic recovery.321
H2. Postoperative conscious sedation adequately controlling stress response is recommended. A sedation measurement scale should be used to control this effect (RASS score −1 and −2).
Grade of recommendation: weak. Level of evidence: moderate (2B).
Justification: Traditionally it was assumed that patients in the postoperative period of heart surgery require deep sedation in order to reduce the endocrine response to stress and prevent myocardial ischemia.320 However, there are no statistically significant differences in the stress response indicators (e.g., catecholamine and cortisol levels) between superficial and deep sedation. In contrast, those patients subjected to deep sedation experience greater myocardial ischemia, higher levels of pain, and prolongation of the duration of ventilatory support compared with those receiving mild sedation.322
H3. The use of remifentanil or other opioids and PCA is recommended as a first measure in the management of cardiovascular postoperative analgesia.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The use of remifentanil/propofol in comparison with fentanyl/midazolam in patients during the postoperative period of cardiovascular surgery while subjected to MV was shown to maintain better levels of analgesia, reducing the duration of MV (20.7 versus 24.2h [p<0.05]) and the duration of stay in the ICU (46.4 versus 64.7h [p<0.05]).323 Following the withdrawal of MV, analgesia with morphine or its analogs remains the treatment of choice for the management of pain, thanks to its potency, with variations in the method of administration (i.e., in bolus form, as a continuous infusion, or as patient-controlled analgesia [PCA]).
No significant differences have been found on comparing the use of PCA with analgesia provided by the nursing personnel, since health professionals tend to closely monitor the presence of pain in these patients and offer analgesia in a timely manner.324 Likewise, no differences in the level of sedoanalgesia obtained have been found on comparing the use of PCA with peridural analgesia.325,326
H4. Consideration of the use of subarachnoid and/or peridural analgesia for the management of postoperative pain is suggested.
Grade of recommendation: weak. Level of evidence: moderate (2B).
Justification: The combination of general anesthesia with regional anesthesia has resulted in reduction of the time to withdrawal of the endotracheal tube and better pain control in heart surgery patients. The administration of intrathecal sufentanil, clonidine or morphine reduces the need for postoperative analgesia and affords better pain control. The use of intrathecal morphine is safe in comparison with the conventional administration methods.327–332 However, the administration of intrathecal morphine does not shorten the time to withdrawal of TI in comparison with placebo.333 On the other hand, the addition of epidural or subdural opioids to those administered via the intravenous route during surgery can produce respiratory depression and delay withdrawal of the endotracheal tube.334
In fast-track heart surgery protocols, the administration of low-dose intrathecal morphine is associated with a 40% decrease in the use of intravenous morphine during the first 24h after withdrawal of the endotracheal tube.335
Peridural analgesia using low doses of 0.125% bupivacaine improves the myocardial oxygen supply/demand ratio, increases subendocardial blood flow, and reduces postoperative hypercoagulability. Consequently, its use in combination with inhalatory general anesthesia has been associated to a lessened stress response among patients subjected to coronary surgery.336
There have been no reports of hematomas secondary to neuroaxial block. Nevertheless, while low, the risk exists and must be taken into account (1:150,000 for block peridural and 1:220,000 for subdural block).337
Globally, therefore, the risks and benefits of regional anesthetic techniques should be evaluated in each individual patient, considering that there is no definitive evidence supporting its use in securing faster withdrawal of the endotracheal tube, when compared with the traditional administration of intravenous opioids and nonsteroidal antiinflammatory drugs (NSAIDs).
It has been suggested that other techniques such as parasternal block, infiltration of the surgical wound, the use of paravertebral blocks and continuous infusions of local anesthetics can be useful as analgesic treatment in patients subjected to heart surgery.338–341
H5. The use of NSAIDs is suggested for the management of postoperative pain.
Grade of recommendation: weak. Level of evidence: moderate (2B).
Justification: NSAIDs allow us to reduce the dose of opioids, maintain or improve analgesia, and reduce the undesirable side effects of opioid drugs.342,343
Diclofenac, indomethacin and ketoprofen have been shown to be effective in the management of postoperative pain.343 However, these drugs are contraindicated in patients with coronary disease, renal failure, gastrointestinal bleeding and bronchospasm.
H6. The use of dexmedetomidine, remifentanil or their combination, the combination of low-dose propofol and midazolam, or the combination of propofol and fentanyl are recommended for postoperative sedation and analgesia.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The use of dexmedetomidine significantly reduces the incidence and duration of delirium in patients in the postoperative period of cardiovascular surgery requiring sedation, when compared with midazolam and propofol, and when compared with patients treated with morphine only. At the same time, it shortens the stay in the ICU and in hospital.344,345 Remifentanil in turn has been shown to reduce the levels of biochemical markers of myocardial damage after coronary surgery, the duration of MV, and the length of hospital stay.346,347
It is now known that the adequate combination of short-acting sedating agents can reduce the analgesic needs and the adverse effects of long-acting opioids.322
Different protocols have evaluated the efficacy of the combination of propofol with midazolam, and of propofol with fentanyl.
The combination of low doses of propofol and midazolam has received widespread acceptance, since these drug substances exert a synergic effect by acting upon the same γ-aminobutyric acid (GABA) receptor–thus affording greater hemodynamic stability than propofol used as sole agent.321 Moreover, the combination of these drugs has been found to be an effective and safe alternative among patients in the postoperative period of heart surgery, since it exerts only minimum effects upon arterial pressure.348
Propofol as single agent for sedation has not been found to be superior to midazolam, since it is not associated with significant shortening of the time to withdrawal of the endotracheal tube, a reduction in the incidence of reintubation, postoperative hypertension and hypotension, or in the patient requirements.349 Nevertheless, during the withdrawal of TI, when the patient is still sedated, the administration of propofol is safe and reduces the incidence of hemodynamic alterations and myocardial ischemia.
No differences have been found in the duration of the awakening period or in sedation time on comparing propofol as single agent versus the combination propofol-fentanyl. However, the concentration of propofol, the infusion rate, and the total dose were all higher in the group in which propofol was used as only drug. Among the hemodynamic parameters, the drop in mean arterial pressure (over 20%) was greater in the group in which propofol was used as only drug than in the propofol-fentanyl group. Therefore, the combination of propofol-fentanyl is superior in terms of sedation, especially in preventing periods of hypotension in patients during the postoperative period.210,343
H7. Dexmedetomidine is recommended among patients in the postoperative period of cardiovascular surgery, either as single drug or combined with opioid analgesics.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The characteristics of dexmedetomidine, which have already been commented above, define it as an appropriate option for short-action sedoanalgesia. In patients recovering from heart surgery, dexmedetomidine affords effective sedoanalgesia. In a clinical trial involving 89 patients subjected to prolonged coronary surgery, dexmedetomidine at a loading dose of 1μg/kg, followed by 0.4μg/kg/h and propofol 5μg/kg/h showed similar efficacy in sedoanalgesia.41,344,345,350–353 The prevention of postoperative trembling is an additional benefit of dexmedetomidine.354,355
H8. The use of pregabalin is recommended in elderly patients, since it reduces the need for opioids.
Grade of recommendation: weak. Level of evidence: moderate (2B).
Justification: A randomized, double-blind, controlled clinical trial on the effect of pregabalin upon the consumption of oxycodone, postoperative confusion and pain in 60 patients ≥75 years of age subjected to heart surgery found the use of pregabalin to reduce the postoperative consumption of oxycodone before extubation and in the first 24h after extubation from 16 to 9mg (p<0.001). Likewise, it reduced the incidence of confusion only on the first postoperative day (CAM-ICU in the pregabalin group 21 versus 24 in the placebo group [p=0.04]), though it increased the extubation time (10.6h) compared with the patients given placebo (8.3h).356
Fig. 6 details the management of patients in the postoperative period of cardiovascular surgery.
I. Neurological and neurocritical patientsWhat medical considerations should be made before starting any type of sedation and analgesia?
I1. An initial neurological evaluation based on the clinical manifestations is recommended, with exclusion of the presence of space-occupying lesions or hematomas that can be surgically resolved, and adopting a local protocol or guide for the management of these patients.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: As in other patients admitted to the ICU, sedoanalgesia in neurocritical patients must seek to control or reduce the metabolic response to stress (tachycardia, hypertension, increased protein catabolism, etc.), maintain patient synchronization with the ventilator, and lessen the pain, anxiety, and agitation.357–359
However, some specific considerations apply in the neurocritical patient with central nervous system alterations due to brain injury or other CNS lesions, including the prevention of situations capable of increasing intracranial pressure (ICP); the reduction and maintenance of brain metabolic needs; elevation of oxygen uptake; optimization of systemic hemodynamics; and reduction of the brain metabolic oxygen demands.358–361 To this effect the following is required: (a) patient monitorization of the systemic and cerebral hemodynamic effects such as systemic arterial pressure, central venous pressure (CVP) and ICP; (b) follow-up of sedation using adequate scales that prove easy to use in clinical practice and are reproducible and widely accepted; and (c) protocolization and standardization of medical care.
When performing sedoanalgesia in neurocritical patients we must take into account that: (a) it must not interfere with continuous neurological assessment in the first hours, in order to be able to detect treatable intracranial complications requiring prompt intervention, and which can manifest as agitation or discomfort and (b) we must prevent secondary neurological damage, associated to hypoxemia and hypotension, which should be corrected as quickly as possible.358,360,362
Some deleterious systemic factors must be corrected or prevented before starting sedation, such as systemic arterial hypotension (maintaining a systolic blood pressure of over 90mmHg, with an ICP of 70mmHg), hypoxemia, fever or hypothermia and hyperglycemia.358–360
Which drugs are indicated when frequent evaluations of the patient neurological condition are required?
I2. It is advisable to use drugs with a short half-life and scant accumulation (propofol, dexmedetomidine and remifentanil), allowing frequent neurological evaluations. A sedation regimen based on analgesia with short-acting opioids such as remifentanil is indicated, since it allows adequate evaluation of the neurological condition and rapid awakening once the medication is suspended.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The ideal sedative in the neurocritical patient is a drug that can prevent ICP elevations, keep the patient hemodynamically stable, and avoid deep sedation (rapid effect and short action). No single ideal agent is able to offer these properties, however, and a combination of different drugs is therefore needed in order to achieve the desired effects. Benzodiazepines are still used associated to analgesics, including particularly the morphine derivates.357 The use of hypnotics such as propofol in combination with an opioid like remifentanil is considered adequate for these patients, in which daily or more frequent interruption of sedation is essential.284,363
Propofol, in the same way as other barbiturates, reduces cerebral blood flow and oxygen consumption by up to 40–60%, in proportion to the administered dose. Furthermore, the drug exerts a vasoconstrictor effect upon the cerebral vascular system. When administered at sufficient doses to produce coma, its antiepileptic activity is comparable to that of the barbiturates, reducing ICP and, as an adverse effect, reducing cerebral perfusion pressure secondary to systemic hypotension, particularly in hypovolemic patients. The pharmacokinetic properties of propofol therefore allow it to be recommended as an ideal hypnotic agent in both the induction and maintenance of sedation in neurocritical patients, even in the management of intracranial hypertension, due to its easy titration and the rapid reversibility of the effect once infusion is suspended. These properties allow propofol to produce predictable sedation. Other potentially beneficial properties in patients with brain injuries are inhibition of the GABA system and the N-methyl-d-aspartate (NMDA) receptors, and the antioxidant effect of the drug.303,364
Opioids are the first choice for analgesia. These drugs should be used to reduce anxiety, pain and distress. The opioids in general do not exert important effects upon brain oxygen consumption. At analgesic doses, the hemodynamic, respiratory and neurological actions of the four derivatives (fentanyl, alfentanil, sufentanil and remifentanil) are comparable. The choice among them should be based on their respective pharmacodynamic characteristics. In patients with brain injuries it has been seen that the administration of opioids can reduce the cerebral perfusion pressure secondary to a reduction in mean arterial pressure and an increase in ICP. This effect has been described when the drugs are administered in bolus form, not as a continuous infusion.46
Sedation based on analgesia (remifentanil with the addition of boluses of propofol and midazolam) allows daily evaluation and faster awakening compared with the classical scheme (fentanyl or morphine, with the combination of propofol or midazolam).242,284 Remifentanil offers a concrete advantage in the neurocritical patient, since its pharmacokinetic profile ensures fast awakening once the medication is suspended–this in turn facilitating evaluation of the neurological condition of the patient.242 Morphine was one of the most widely used analgesics. However, its use is limited by slow onset of action (20min on average), the risk of accumulation in the presence of renal failure, and histamine release. As a result, the use of short-acting opioids such as remifentanil is presently preferred.284,365
No studies are available demonstrating the superiority of remifentanil versus other opioids in terms of a decrease in mortality rate, though remifentanil does offer advantages in terms of a shorter duration of hospital stay, a shorter duration of MV, and a shorter time to extubation. An inconvenience of remifentanil is the hyperalgesia produced when the drug is suspended, making it necessary to prescribe transition analgesics.
Table 12 details the properties of the above described drug substances.
Characteristics of the drugs used in neurological and neurosurgical patients.
| Characteristic | Propofol | Midazolam | Lorazepam | Fentanyl | Remifentanil |
| Rapid onset | +++ | +++ | + | +++ | +++ |
| Easy recovery | +++ | ++ | + | ++ | +++ |
| Easy titration | +++ | ++ | + | ++ | +++ |
| Intracranial pressure | ↓↓ | ↓↓ | ↓ | = | = |
| Cerebral blood flow | ↓↓ | ↓↓ | ↓ | = | = |
| Cerebral O2 consumption | ↓↓ | ↓↓ | ↓ | ↓ | ↓ |
| Mean arterial pressure | ↓↓ | ↓ | ↓ | ↓ | ↓ |
Which sedative is advised for patients with intracranial hypertension and subjected to MV?
I3. The use of propofol in infusion is recommended, without exceeding 5mg/kg/h and a duration of 5 days. Midazolam should be considered in patients in which propofol is contraindicated.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: As commented above, propofol and midazolam are equally effective in sedation of the neurocritical patient, in accordance with their systemic and cerebral hemodynamic effects.284,364,366
I4. Thiopental sodium is recommended as a treatment measure reserved for patients with serious brain injuries presenting intracranial hypertension refractory to medical treatment and without coexisting major hemodynamic limitations.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Barbiturates reduce blood flow to the brain and cerebral metabolism in direct proportion to the administered dose, until brain activity is ultimately abolished. The decrease in ICP is secondary to the effect of the drug upon cerebral perfusion, and in turn results from lowered brain metabolism, provided cerebral hemodynamics and the coupling of cerebral blood flow/oxygen consumption are maintained. If oxygen consumption decreases as a consequence of ischemic alterations or secondary neurological damage, the barbiturates increase blood flow through a compensatory mechanism, provided the systemic arterial pressure is maintained above the values determined by the autoregulatory mechanisms (mean arterial pressure over 90mmHg). However, it is important to remember that in addition to affecting the hemodynamic situation, barbiturates also cause immunosuppression and a prolongation of coma once their administration is suspended, due to drug redistribution in the adipose compartments.367–371
I5. It is not advisable to systematically use ketamine in patients with brain injuries or other neurocritical conditions.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Ketamine is a non-competitive NMDA receptor antagonist with a primary action site located in the cortical neothalamus. Ketamine has been related to an increase in partial CO2 pressure in arterial blood and in ICP in patients without MV. However, it recently has been described that in patients with intracranial hypertension and controlled MV, ketamine in continuous infusion and midazolam maintain cerebral hemodynamics and control of brain perfusion to a degree comparable to that of the opioids.372 Certain adverse effects such as an increase in heart rate and longer neurological recovery after suspension (related to the presence of an active metabolite: norketamine) advise against the use of ketamine in neurocritical patients when frequent neurological evaluations are needed.
J. Patients with kidney or liver failureWhich medical conditions should be evaluated before starting any type of sedation and analgesia?
J1. Evaluation of liver and kidney function is recommended in all patients requiring sedoanalgesia in the ICU.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: The clinical usefulness of most hypnotics, sedatives and analgesics is negatively affected by liver or renal failure, or (in the worst of cases) both.3 Failure of such organ function poses a genuine challenge for clinicians, and requires constant weighing of the risk–benefit ratio of the treatment provided, often without the support of adequate scientific evidence.373 Liver or renal failure produces changes not only in reference to clearance, distribution volume, free fraction or elimination of the original drug substance, but can also result in the accumulation of active metabolites, whether toxic or otherwise.
In the presence of liver dysfunction, most drug substances experience diminished clearance, a prolongation of the half-life, and the accumulation of metabolites that are potentially toxic or possess metabolic activity–with poorly known clinical repercussions. Experimental studies have shown that opioidergic transmission can be altered in cirrhotic patients, with an increase in receptor affinity for opioids. Furthermore, these patients show increased sensitivity to GABA, with reinforcement of the effects of benzodiazepines. Some forms of subclinical encephalopathy can be induced by the administration of sedatives and opioids. In patients with liver failure, the Child–Pugh classification374,375 is used as a guide in choosing the drug and in defining the dosage3,373,376–379 (Table 13).
Child–Pugh classification modified for hepatic patients.
| Item | Score | ||
| 1 | 2 | 3 | |
| Ascites | Absent | Mild | Moderate |
| Encephalopathy | No | Grade 1–2 | Grade 3–4 |
| Albumin (g/l) | >3.5 | 2.8–3.5 | <2.8 |
| Bilirubin (mg/dl) | <2 | 2–3 | >3 |
| (for cholestatic diseases) | (<4) | (4–10) | (>10) |
| Prothrombin time | >50 (<1.7) | 30–50 (1.8–2.3) | <30 (>2.3) |
| Class A: 5–6 points | |||
| Class B: 7–9 points | |||
| Class C: 10–15 points | |||
J2. Evaluation of the risk–benefit ratio of sedoanalgesia in patients with chronic renal failure is recommended.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: In patients with chronic renal failure, whether on dialysis or not, the prediction of the pharmacokinetic profiles using formulas to calculate the glomerular filtration rate (GFR) has not been established for most drugs. In this context, most drug substances have not been extensively studied prior to their use in clinical practice in critical patients.373,380–382 Due to the changes in drug clearance and the potential accumulation of active metabolites, the clinician must evaluate the risk–benefit ratio taking into consideration other pharmacokinetic and pharmacodynamic interaction factors, as well as the potential adverse effects.
Which analgesics and sedatives are contraindicated, and why, in patients of this kind?
Renal failureJ3. It is advisable not to use morphine in critical patients with renal failure and on dialysis.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Morphine undergoes biotransformation in the form of glucuronidation in the liver, yielding dialyzable active metabolites (morphine-6-glucuronide and morphine-3-glucuronide) that are excreted through the kidneys. The metabolite morphine-6-glucuronide, which accumulates in situations of renal failure, is the cause of the respiratory depression observed in patients with renal failure. Despite dialysis, the effect is prolonged, probably because of decreased diffusion from the central nervous system. In chronic patients, morphine has been shown to be less well tolerated than hydromorphone.373,376,378,379
Depending on the degree of renal failure, we can adjust the dosage according to the GFR as follows: between 20 and 50ml/min, 75% of the dose; between 10 and 20ml/min, 50% of the dose.
Liver failureJ4. It is advisable not to use midazolam in cirrhotic patients.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: In patients with liver failure, the elimination half-life of midazolam increases two- to threefold, while its clearance decreases 50%. Encephalopathy has been demonstrated up to 6h after administration of the drug in cirrhotic patients (Child–Pugh class A or B) as an infusion for sedation in digestive tract endoscopy. However, midazolam can be used as a sedative in cirrhotic patients, for performing upper digestive endoscopy, at doses of 30–50μg/kg in subjects corresponding to Child–Pugh class A or B.3,380,381,383–385
Which drugs can be used?
Renal failureJ5. The use of remifentanil is recommended during MV for more than 48h with daily interruption in patients with renal failure who are not on dialysis.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Remifentanil is quickly metabolized by nonspecific plasma esterases. It does not undergo accumulation. The drug produces an active metabolite, remifentanil acid, that is largely (90%) eliminated in urine but which lacks any practical effect, due to its low potency. In comparison with patients without renal failure, the plasma concentration of remifentanil is 30% greater after a loading dose, though the recovery time is similar after suspending the infusion when the latter has been administered during 72h.373,386,387
In a randomized, double-blind and controlled study of 19 patients comparing fentanyl-midazolam versus remifentanil-midazolam in patients subjected to MV, the use of remifentanil was associated with a decrease in the time to extubation in patients with renal failure (24.7 versus 48h [p=0.04]) and in the duration of the weaning process. The group treated with remifentanil required larger doses of morphine after extubation. The incidence of agitation was similar in the two groups. No evaluation was made of the impact of these strategies upon the incidence of pulmonary complications.272
J6. Hydromorphone is recommended, reducing its dose and monitoring signs of central nervous system excitation and confusion states.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Hydromorphone undergoes glucuronidation in the liver, yielding an active metabolite that is eliminated through the kidneys, and which undergoes a fourfold increase in plasma concentration during renal failure. This metabolite very probably is responsible for the reported excitatory adverse effects upon the central nervous system (myoclonus, agitation and confusion). During dialysis, the plasma concentration decreases by up to 40%. Hydromorphone shows no substantial accumulation, because it is quickly converted to 3G-hydromorphone, which appears to be effectively removed during hemodialysis. 3G-hydromorphone accumulates between dialysis sessions, and such accumulation is associated to increased pain perception and a reduction in the duration of analgesia.3,373,388,389
J7. The use of fentanyl is recommended, starting with a lower loading dose in patients with a glomerular filtration rate of less than 50ml/min.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Fentanyl undergoes biotransformation in the form of oxidation in the liver, with the production of inactive metabolites. It is not clear whether the drug is dialyzable. In renal failure the clearance of fentanyl decreases in relation to the GFR, exhibiting a safer pharmacological profile than morphine or hydromorphone. There have been reports of prolonged sedation and respiratory depression in patients subjected to continuous infusion of fentanyl.3,373,388
J8. The use of dexmedetomidine is recommended, reducing the loading dose and adjusting the infusion according to the clinical response obtained.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Dexmedetomidine is metabolized in the liver through glucuronidation and the cytochrome P450 system. Its inactive metabolites are largely eliminated in urine (90%) and, to a lesser extent, in stools (10%). There is only one report of a patient subjected to hemofiltration in which dexmedetomidine was described as not amenable to filtration.390 Its elimination half-life decreases in chronic renal failure. Nevertheless, the recovery time is shorter–possibly because of the plasma concentration peak that occurs with the loading dose, or because of decreased diffusion from the central nervous system toward the central compartment. The clearance and distribution volume are similar in healthy individuals and in patients with chronic renal failure. Studies are needed to determine whether dexmedetomidine is dialyzable or not.373,391,392
J9. The use of midazolam is suggested only for periods of under 48–72h, with reduction of the dose by 50%.
Grade of recommendation: weak. Level of evidence: low (2C).
Justification: Midazolam is oxidized in the liver to hydroxymidazolam (an equipotent metabolite), which is eliminated through the kidneys. It accumulates in patients with chronic renal failure and induces prolonged sedation. Midazolam is not dialyzable, in contrast to its metabolites. Continuous extrarenal filtration prevents the accumulation of its active metabolite. The drug can be used in patients with chronic renal failure, provided the dose is lowered.3,379
J10. It is advisable not to use lorazepam in this group of patients. If use of the drug proves necessary, monitorization is required of the concentration of propyleneglycol, lactic acid, serum osmolarity and the anion gap, particularly in the case of infusions lasting more than 72h.
Grade of recommendation: weak. Level of evidence: low (2C).
Justification: Lorazepam is conjugated in the liver to an inactive metabolite that is eliminated through the kidneys. In chronic renal failure the elimination half-life is prolonged, but clearance is similar to that found in healthy individuals. The drug is not amenable to either dialysis or ultrafiltration. Propyleneglycol (dialyzable), an excipient in the vial formulation of lorazepam, is responsible for the metabolic acidosis and worsened renal function that have been observed in patients with GFR<50ml/min.3,150,379,393
J11. The use of propofol is recommended, increasing its dose at the start of dialysis and monitoring the serum triglyceride levels after 12h of continuous infusion.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Propofol undergoes hepatic and extrahepatic metabolization through glucuronidation and sulfurization, yielding inactive metabolites. Metabolic transformation through cytochrome P450 phase 1 metabolism has also been demonstrated. The drug is not dialyzable, and its pharmacokinetics and pharmacodynamics are not significantly altered in patients with chronic renal failure. The dose should be increased during dialysis, due to the decrease in plasma drug concentration secondary to hemodilution and albumin binding to the filters. Likewise, lesser doses are needed in continuous infusion for sedation, since when infusion is prolonged for more than 12h it gives rise to a significant increase in triglyceride levels. Close monitoring is therefore recommended.3,394–396
J12. The use of haloperidol is recommended in patients with delirium, reducing the dose by 30%.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Haloperidol is metabolized in the liver through oxidation. Its active metabolite is responsible for extrapyramidal manifestations. In healthy individuals the drug has a half-life of 18–54h, though its half-life in patients with chronic renal failure is not known, since no pharmacokinetic or pharmacodynamic studies have been made in such individuals. The hypomagnesemia and hypopotassemia resulting from dialysis theoretically can increase the risk of polymorphic ventricular tachycardia.3,379,397,398
Liver failureJ13. The use of remifentanil is recommended, reducing its dose and monitoring patient ventilation.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Because of its metabolism and elimination, remifentanil is the opioid of choice in patients with liver dysfunction. However, the therapeutic window or margin between analgesia and respiratory depression is narrow, and strict monitoring of breathing is thus required in patients who are not subjected to MV.3,386,399,400
J14. The use of fentanyl is recommended, reducing its maintenance dose.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Fentanyl has prolonged clearance and a long elimination half-life, and undergoes accumulation. Its dose therefore should be lowered. Fentanyl does not have active metabolites. It is indicated in patients with liver failure, with a lowering of the administered dose, though there are no concrete guidelines regarding dosification of the drug in liver failure. It has been used in patients with bleeding esophageal varicose veins, reducing the hemodynamic response to TI.3,401
J15. The use of morphine and hydromorphone is suggested, administering low and fractionated doses, with close clinical monitoring of the patient.
Grade of recommendation: weak. Level of evidence: low (2C).
Justification: Pharmacological studies have shown the clearance of morphine to be reduced in cirrhotic patients. The elimination half-life and distribution volume are increased. Morphine can induce hepatic encephalopathy. There have been some reports of its use in single epidural administration in Child–Pugh class A cirrhotic patients without coagulation disorders (3–5mg), for the control of postoperative pain. There have also been some descriptions of the continuous infusion of 2mg/h for the treatment of postoperative pain.3,377,382,385,402,403 No clear recommendations are available on its use, however, since the drug does not have a safe pharmacological profile, and close monitoring is required during administration.378
Because of its extensive hepatic metabolization, hydromorphone can exhibit increased bioavailability in the presence of liver dysfunction, triggering the appearance of hepatic encephalopathy and respiratory depression. Its safety profile has not been assessed in patients with liver dysfunction.3,388
J16. Dexmedetomidine is suggested as coadjuvant treatment in cirrhotic patients with alcohol withdrawal syndrome, when conventional management fails. The dose should be lowered.
Grade of recommendation: weak. Level of evidence: moderate (2B).
Justification: A study in cirrhotic patients revealed an increase in the half-life and distribution volume of dexmedetomidine, with prolongation of its clearance, in comparison with subjects without liver failure. It has been reported to be useful in alcoholics with withdrawal syndrome who have not responded adequately to conventional treatment.3,392,404,405
J17. The use of lorazepam is suggested in patients with alcohol withdrawal syndrome.
Grade of recommendation: weak. Level of evidence: low (2C).
Justification: Lorazepam, like all benzodiazepines, can induce hepatic encephalopathy. Its clearance is reduced in cirrhotic patients. Lorazepam can be used in patients with alcohol withdrawal syndrome as first line benzodiazepine for the prevention of seizures.406–408
J18. Propofol is recommended as the hypnotic agent of choice in the management of patients with fulminant liver failure requiring TI and the control of intracranial hypertension up to a dose of 50μg/kg/min (3mg/kg/h).
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: The elimination half-life of propofol and its clearance are scantly affected in liver failure, though its distribution volume doubles. Compared with healthy individuals, the recuperation is longer. Propofol offers better quality of sedation than midazolam and faster awakening, with less psychomotor dysfunction when used for sedation in upper digestive tract endoscopy.3,383,385 The drug has also been shown to reduce ICP in patients with fulminant liver failure.409–411
J19. The use of haloperidol is recommended in cirrhotic patients with delirium. The starting dosage should be lower than that recommended in patients without liver failure, and monitoring of the electrolytic and electrocardiographic alterations is required (particularly the QT-interval).
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: No pharmacokinetic studies of haloperidol in liver failure are known. The drug has been used in alcoholics with delirium, administering lesser doses than in patients without liver damage. There have been reports of polymorphic tachycardia in cirrhotic patients, associated to hypopotassemia or hypomagnesemia.398,412–414
Table 14 describes the adjusted drug dosages in the treatment of patients with kidney or liver failure.415
Sedative and opioid drug dose adjustment in renal and liver failure.
| Drug | Active metabolites | Metabolic pathway | Dose renal failure (observations) | Dose liver failure (observations) |
| Dexmedetomidine | No | Glucuronidation | Impregnation dose 0.5μg/kg in 10minInfusion 0.2–0.7μg/kg/h | In alcohol withdrawal: 1μg/kg in 10min. Continue 0.2–0.7μg/kg/h |
| Fentanyl | No | Oxidation | 0.7–10μg/kg/h. Start with 50% of the dose if GFR is <50ml/min(Can produce prolonged sedation) | 1–2μg/kg iv for brief proceduresChild A 0.7–10μg/kg/hChild B–C reduce dose according to response(Can trigger hepatic encephalopathy) |
| Hydromorphone | Yes (H3G) | Glucuronidation | 10–30μg/kg iv every 2–4h(Can produce myoclonus, hallucinations and/or confusion) | Child A: 10–30μg/kg iv every 2–4hChild B–C: use not recommended in continuous infusion(Can trigger hepatic encephalopathy) |
| Morphine | Yes (M3G and M6G) | Glucuronidation | GFR>50ml/min: 0.02–0.15mg/kg iv every 4hGFR 20–50ml/min: 75% of the doseGFR 10–20ml/min: 50% of the dose(Use not recommended in patients on dialysis) | Child A 0.02–0.1mg/kg. iv every 4hChild B–C 0.02–0.04mg/kg every 4–6h(Use not recommended in continuous infusion. Can trigger hepatic encephalopathy) |
| Remifentanil | Yes (remifentanil acid) | Hydrolysis (esterases) | 0.05–0.3μg/kg/min(Opioid of choice in renal failure) | 0.05–0.3μg/kg/min. Adjust dose according to response(Probably the opioid of choice) |
| Midazolam | Yes (OH) | Oxidation | Reduce loading dose by 50%Infusion 0.02–0.1mg/kg/h no more than 48h(OH-midazolam is dialyzable) | Child A: 20–50μg/kg for brief proceduresChild B–C: use not recommended in continuous infusion(Can trigger hepatic encephalopathy) |
| Lorazepam | Yes (OH-midazolam) | Glucuronidation | 0.01–0.1mg/kg/h(Possible toxicity due to propyleneglycol solvent) | Doses 0.01–0.1mg/kg/h(In alcohol withdrawal it is the first choice for prevention of seizures. Can trigger hepatic encephalopathy) |
| Propofol | No | Oxidation | Increase loading dose at start of dialysis 2–3mg/kg. Continue infusion dose 5–40μg/kg/min(Triglyceride measurement in infusions >12h) | 5–40μg/kg/min(Useful in fulminant liver failure for intracranial pressure control) |
| Haloperidol | No | Oxidation | 2mg iv every 20min until control of symptoms(Not recommended in patients with hypopotassemia or hypomagnesemia, or with prolonged QT-interval) | 2mg iv every 20min until control of symptoms2–4mg iv every 6h(Not recommended in patients with hypopotassemia or hypomagnesemia, or with prolonged QT-interval, or simultaneous to vasopressin) |
GFR: glomerular filtration rate; iv: intravenous; M3G: morphine-3-glucuronide; M6G: morphine-6-glucuronide.
J20. The use of propofol is suggested in Child–Pugh class A or B patients scheduled for upper digestive tract endoscopy in the absence of active bleeding.
Grade of recommendation: weak. Level of evidence: low (2C).
Justification: Cirrhotic patients are more susceptible to sedation-related complications than non-cirrhotic individuals. In this context, propofol compared with midazolam results in faster recovery and greater effectiveness, and does not cause acute worsening in patients with minimal encephalopathy assessed by psychomotor tests. Although the studies of this treatment have been made in gastroenterology units, with sedation exclusively administered by gastroenterologists or intensivists, moderate sedation with propofol without the need for TI is effective and safe. The suggested fentanyl dosing is 50μg followed by propofol 0.25mg/kg with fractionated doses between 20 and 30mg/min when necessary, without exceeding a total dose of 400mg.416,417
K. Patients requiring special procedures (tracheostomy, thoracic catheters or tubes, peritoneal lavage, wound or burn lavage and debridement)Which drugs are indicated for these procedures?
K1. An opioid analgesic, fentanyl or remifentanil, associated to a sedative (propofol, midazolam) is advised for these special procedures. The doses are to be modified taking into account the previous use of other sedatives and analgesics.
Grade of recommendation: weak. Level of evidence: low (2C).
K2. Protocolized opioid use is recommended in case of painful or unpleasant routine procedures (endotracheal suction and changes in body position).
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: No drug or drug combination has been adequately evaluated for anesthesia or analgesia in special procedures in the ICU. In the case of minor procedures carried out in the ICU, the sedatives, hypnotics or analgesics administered to the patient before performing the procedure must be taken into account. Among the alternatives, we can consider nerve blocks or infiltrations of local anesthetics, or may administer boluses equivalent to 25–50% of the basal opioid dose which the patient is receiving. When brief sedation is expected (<24h), propofol in bolus form is indicated. If sedation after the procedure is expected to last 48h or more, we should use midazolam or lorazepam as first choice.418–421
K3. The routine use of neuromuscular relaxants for performing special procedures in the ICU is not advised.
Grade of recommendation: weak. Level of evidence: low (2C).
Justification: Neuromuscular relaxants should only be used in special cases in which correct and safe performance of the procedure requires muscle relaxation (abdominal interventions, certain tracheostomies, fiberoptic bronchoscopy), or when the patient requires brain protective measures, as in intracranial hypertension.
When are regional analgesia techniques indicated for the management of pain in the ICU?
K4. Regional analgesia is recommended in the postoperative period of thoracotomy and major thoracoabdominal surgery, and in chest traumatisms.
Grade of recommendation: strong. Level of evidence: high (1A).
Justification: Regional analgesia can be useful in selected patients in the ICU for the management of chest injuries (multiple rib fractures, sternal fracture and lung contusion), or postoperative pain in the context of chest surgery and major thoracoabdominal interventions.422,423
Which regional analgesia technique is recommended for the management of pain in the ICU?
K5. The continuous peridural approach is recommended as the regional analgesia technique of choice in the postoperative period of thoracotomy, thoracoabdominal surgery and chest injuries. The paravertebral approach with continuous infusion is an alternative to the peridural route in the postoperative period of thoracotomy.
Grade of recommendation: strong. Level of evidence: high (1A).
Justification: The oldest and most widespread technique is peridural analgesia, which in comparison with intravenous analgesia using opioids in bolus doses or PCA, affords adequate analgesic control, facilitates respiratory physiotherapy, and reduces the duration of MV and the incidence of nosocomial pneumonia.422–424
The results of the trials that have compared the paravertebral technique versus the peridural approach indicate that both procedures offer similar analgesic potency, though with a lesser incidence of adverse effects such as hypotension, nausea, vomiting and urinary retention, when the paravertebral approach is used.425–428
K6. Intercostal block is suggested in the postoperative period of thoracotomy.
Grade of recommendation: weak. Level of evidence: moderate (2B).
Justification: Comparison of intercostal block versus peridural analgesia shows a decrease in opioid consumption (PCA) and in the incidence of urinary retention when the intercostal technique is used in programmed thoracotomy procedures.429
L. Non-pharmacological strategies or complementary treatmentsWhat should be done to modulate the external conditions that can affect patient tranquility, such as noise, waking-sleep, visits, inappropriate conversations?
L1. Sleep in the ICU is to be facilitated, adopting all the necessary measures, particularly non-pharmacological measures.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Sleep is important for patient recovery. Objective and subjective measures of sleep quality in the ICU reveal the existence of important sleep disturbances. This in turn represents an additional source of stress that can have a negative impact upon the humoral and cellular immune system, with an increase in oxygen consumption and CO2 production, and alterations in thermoregulation.430,431
The causes of sleep disturbances among patients in the ICU include medical-nursing evaluations, diagnostic tests, noise, lights during the night, pain, discomfort and invasive procedures.432–434
Complementary measures can be used to facilitate sleep,435 such as the control of environmental illumination, massages, music therapy,436 the synchronization of activities with the circadian rhythm, and the lessening of noise.437 Nursing assessment or the use of a scale for sleep control is also important.438
L2. It is advisable to adopt all necessary measures to reduce noise in the ICU. As a complementary measure we can use earplugs to lessen noise perception.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Noise in the ICU creates a hostile environment for the patient, producing sleep disturbances and anxiety.431–434,437,439–441 Noise in the ICU is produced by alarms, mechanical ventilators, telephones and conversations among the personnel members. Levels above 80dB are to be avoided, while levels below 35dB favor sleep.442 As a complementary measure we can use earplugs to lessen noise perception on the part of the patient.3,191,431,443,444
L3. As far as possible, it is advisable to observe the waking-sleep rhythm, lowering the lights at night, and reducing the nursing interventions or procedures.
Grade of recommendation: strong. Level of evidence: strong (1B).
Justification: The waking-sleep rhythm should be observed as far as possible, attempting to minimize sleep disturbances at night attributable to procedures, and affording an environment with the least illumination possible. A guide promoting the control of noise and lights at night in the ICU should be used.445,446
When and what relaxation and massage techniques can be used?
L4. Massages can be used as an alternative or adjuvant to drug treatment.
Grade of recommendation: strong. Level of evidence: weak (1C).
Justification: Back massaging for an average of 5–10min promotes relaxation and improves sleep,447 in the same way as foot massage applied for 5min.448 The combination of massages with acupressure has shown the same benefits.449
When should music therapy be used?
L5. Music therapy is recommended in patients admitted to the ICU, especially in those subjected to MV.
Grade of recommendation: strong. Level of evidence: moderate (1B).
Justification: Music therapy can help relax and lessen the pain in patients in the ICU. Music can also mask noise. In the postoperative period of heart surgery, listening to music during the first day has been associated with a decrease in discomfort produced by noise, as well as a drop in heart rate and systolic blood pressure.450 A similar effect has been documented in cancer patients admitted to the ICU.451 In patients subjected to MV, music therapy is associated with a decrease in anxiety, systolic and diastolic blood pressure, and heart rate.281,452–457 Nevertheless, larger studies are needed in order to confirm that music therapy is effective in all groups of patients. Since music therapy is an intervention without adverse effects and has a low cost, it should be considered among the measures for controlling anxiety and noise in the ICU.280–282,458–461
L6. It is advisable to inform the patient about his or her illness and the procedures to be carried out.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: The lack of information, or the inadequate management of the information which the patient receives, can contribute to increase anxiety.462 No adequate studies have been carried out on the appropriate way to inform patients in the ICU. Having a better understanding of their illness and of the interventions carried out can improve patient collaboration and lessen anxiety. Likewise, it seems reasonable to avoid inappropriate conversations among the medical or nursing personnel that may be heard by the patients.463,464
L7. It is advisable to establish an effective means of communication with patients subjected to MV.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: Patients who are subjected to MV and are not sedated or are under cooperative sedation, are better able to communicate with the attending healthcare personnel and with their relatives; as a result, they are better able to assess their condition and experience greater autonomy and wellbeing. Some recommended communication methods are gestures, movements of the head, writing, letter cards, words, phrases and images, and even electronic devices. In patients subjected to tracheostomy, we should consider the possibility of deflating the cuff to allow speech.465,466
L8. It is advisable to encourage the use of devices allowing patient orientation in the intensive care environment, such as wall clocks, calendars, windows with natural light, photos of relatives in visible places within the ICU, and patient personal items or objects of daily use.
Grade of recommendation: strong. Level of evidence: low (1C).
Justification: The presence of a clock, calendar, wall boards and similar objects allowing patients and their relatives to personalize the room, together with good wish cards, photos and other personal items, all create a comfortable environment for patient recovery. The room may even be equipped with television and an education/training system that can be used in support of the patient educational objectives and for providing entertainment.467
As a summary, Appendix C suggests a bundle of measures for the management of analgesia, sedation and delirium in the critical adult patient.
Conflicts of interestThe members of the consensus group declare the following conflicts of interest: G. Castorena is a speech representative of MSD for Sugamadex; J.C. Díaz is a speech representative of Hospira; A. Hernández organizes academic meetings for Hospira and Boehringer Ingelheim; T. Muñoz is a consultant (Advisory Board) for Orion Pharma S.L.; F. Pálizas is a speech representative of Bayer for rivaroxaban and receives support for attending congresses on sepsis; J.M. Pardo receives support for attending congresses from MSD and Sanofi-Aventis; C. Righy is a speech representative of Hospira; M. Suárez is a speech representative of Hospira and receives support for attending congresses from Victus Laboratories and Abbot Laboratories. The rest declare that they have no conflicts of interest.
Thanks are due to the Asociación Colombiana de Medicina Crítica y Cuidado Intensivo (AMCI), for obtaining the support of Hospira Laboratories in receiving the grant necessary to develop the guide and for administration of the resources. We also thank Dr. Sandra Rubiano, MD, for her support in drafting the guide and methodological help;
Dr. Olga Lucia Quintero, MD, for her help in organizing the literature references and in translating the guides into English;
Dr. Carlos Eduardo Pinzón, MD, of the Cochrane Collaboration Colombia, for the literature search and methodological counseling; and Ms. Alexandra Suárez for her help in organizing the logistics and for secretarial work in drafting the guide.
Coordinator (Chairman):
Edgar Celis-Rodríguez, MD (Colombia)
Professor of Anesthesia and Critical Care Medicine
Head of the Department of Critical Care Medicine
Hospital Universitario Fundación Santa Fe de Bogotá
Universidad de Los Andes
Bogotá, Colombia
Treasurer WFSICCM
edgarcelis.md@gmail.com
Members:
Sociedad Argentina de Terapia Intensiva (SATI)
Fernando Pálizas, MD
Director of the Department of Intensive Care Medicine of Clínicas Bazterrica and Santa Isabel
Director of Specialization in Intensive Care Medicine, Bazterrica center, University of Buenos Aires
Buenos Aires, Argentina
palizasfernando@gmail.com
Daniel Ceraso MD, FCCM
Specialist in Intensive Care Medicine SATI-FCCM
Head of the Unit of Intensive Care Medicine, Hospital Juan A. Fernández, Buenos Aires
Head of the Unit of Intensive Care Medicine, Sanatorio San Lucas, San Isidro, Argentina
Director of Specialization in Intensive Care Medicine, University of Buenos Aires
dceraso@gmail.com
Néstor Raimondi, MD, FCCM
Clinical coordinator, Intensive Care Medicine A. Hospital Juan A. Fernández
President, Sociedad Argentina de Terapia Intensiva
Vice-president of the FEPIMCTI
Buenos Aires, Argentina
nestor.raimondi@gmail.com
Associação de Medicina Intensiva Brasileira-AMIB
Cássia Righy Shinotsuka
Coordinator of the Neurointensive Care Unit, Hospital Copa D’Or, Rio de Janeiro, Brazil
Pesquisadora Associada do Instituto D’Or de Pesquisa e Ensino
Sociedad Chilena de Medicina Intensiva
Sebastián Ugarte, MD
Professor of Andrés Bello University
Director of the Department of Critical Care Medicine, Clínica INDISA
Director of the Intensive Care Network, Santiago de Chile, Chile
President of the Federación Panamericana e Ibérica de Medicina Crítica y Terapia Intensiva
Council member of the World Federation of Societies of Intensive and Critical Care Medicine
sugarteu@gmail.com
Antonio Hernández
Specialist in Intensive Care Medicine and Respiratory Diseases
Head of the Department of Critical Care Medicine, Hospital Militar de Santiago
Professor of Universidad de Los Andes
Professor of Universidad de Valparaíso
antoniohzd@gmail.com
Asociación Colombiana de Medicina Crítica y Cuidado Intensivo-AMCI
Fernando Raffán-Sanabria, MD
Anesthetist-intensivist, specialist in transplant anesthesia
Department of anesthesia and Department of Critical Care Medicine
Hospital Universitario Fundación Santa Fe de Bogotá
Clinical Professor, Faculty of Medicine, Universidad de Los Andes, Bogotá
Professor of anesthesiology, Universidad El Bosque. Bogotá
raffanmago@gmail.com
Carmelo Dueñas-Castell
Professor of the University of Cartagena
UCI Gestión Salud, UCI Santa Cruz de Bocagrande
Secretary of the Federación Panamericana e Ibérica de Medicina crítica y Terapia Intensiva
Scientific consultant for Linde Healthcare
crdc2001@gmail.com
Dario I. Pinilla
Manager of Critical Care Medicine, Corporación Mederi
Professor of Universidad del Rosario in the area of Critical Care Medicine
darioipinilla@gmail.com
Claudia Birchenall, MD
Internist-intensivist
Statistician-Clinical epidemiologist
Clínica Universitaria de Colombia
Hospital Universitario Mayor-Mederi
cibirchenall@yahoo.com
Juan Carlos Díaz-Cortés
Anesthetist, intensivist, epidemiologist
UCI, Hospital Universitario Fundación Santa Fe de Bogotá
Instructor in Anesthesia and Intensive Care Medicine, Universidad El Bosque, Universidad del Rosario and Universidad de Los Andes
judiazco@gmail.com
Juan Mauricio Pardo-Oviedo
Specialist in Internal Medicine and Intensive Care Medicine, Universidad del Rosario
Specialist in Philosophy of Science, Universidad El Bosque
Head of Medical Training, Hospital Universitario Mayor–Mederi
Physician of the Intensive Care Unit, Fundación Cardio-infantil
Professor of Medicine, Universidad del Rosario
Juan.pardo@urosario.edu.co
Society of Critical Care Medicine-SCCM
Edgar J. Jimenez, MD, FCCM
President of the World Federation of Societies of Intensive and Critical Care Medicine
Chairman, Section of Critical Care Medicine, Orlando Health Corporation and Orlando Health Physicians Group Director, Intensive Care Units, Orlando Regional Medical Center
Professor of Medicine, University of Central Florida
Associate Professor of Medicine, University of Florida and Florida State University
edgar.Jimenez@orlandohealth.comedgar.Jimenez@orhs.org
Asociación Mexicana de Medicina Crítica y Terapia Intensiva-AMMCTI
Guillermo Castorena-Arellano
Subdirector of Anesthesia and Critical Care, Hospital General Manuel Gea González, Ciudad de México
Assistant Professor of Anesthesia, UNAM, Fundación Clínica Médica Sur.
Ciudad de México, Mexico
drmicky@prodigy.net.mx
Jesús Ojino Sosa-García
Specialist in Internal Medicine and Critical Care Medicine
Physician ascribed to the Intensive Care Unit, Hospital Médica Sur
Clinical practice guide methodological consultant. Centro Nacional de Excelencia Tecnológica en Salud. Secretaría de Salud (CENETEC-SALUD)
drintervista@gmail.com
Sociedad Peruana de Medicina Intensiva-SOPEMI
Juan Carlos Meza, MD
Internist and intensivist
Head of the Department of Critical Care Medicine and of the Continued Medical Training Office of the Naval Medical Center, CMST
Lecturer of Universidad Nacional Mayor de San Marcos and of Universidad San Martín de Porres
jcmezagarcia@gmail.com
Mario Suárez, MD
Internist and intensivist
Director of the Hospital Nacional Hipólito Unanue, Lima, Peru.
Intensive Care Medicine resident tutor, Hospital Nacional Hipólito Unanue
mariosuarez125@yahoo.com
Sociedad Venezolana de Medicina Crítica-SVMC
Clara Pacheco-Tovar
Specialist in Internal Medicine and Intensive Care Medicine
Head of the Department of Intensive Care Medicine, Hospital Universitario de Caracas. Universidad Central de Venezuela
Intensivist of La Trinidad teaching medical center
President of the Sociedad Venezolana de Medicina Crítica
pacheco.clara@gmail.com
Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias (SEMICYUC)
Tomás Muñoz-Martínez, MD, PhD
Doctor of Medicine and Surgery, Universidad del País Vasco
Specialist in Intensive Care Medicine. Advanced training in Epidemiology and Public Health (Universidad del País Vasco)
Area specialist of the Department of Intensive Care Medicine, Hospital de Cruces (Vizcaya)
Coordinator of the Analgesia and Sedation Work Group of the SEMICYUC
tomas.munozmartinez@osakidetza.nettomas@arconte.jazztel.es
Miguel Ángel de la Cal, MD
Section Chief of Intensive Care Medicine
Hospital Universitario de Getafe
Centro de Investigación Biomédica en Red (CIBER) de Enfermedades Respiratorias. Instituto de Salud Carlos III
Madrid, Spain
mcal@ucigetafe.com
Methodology and logistic support:
Miguel Ángel de la Cal, MD (Spain)
Claudia Birchenall, MD (Colombia)Juan Carlos Díaz, MD (Colombia)
What are the recommendations for patients with special conditions such as COPD, asthma, hemodynamic instability or multiorgan failure?
(“Conscious Sedation” [MeSH] OR “Deep Sedation” [MeSH] OR “Anesthetics, Dissociative” [MeSH] OR SEDATION [All Fields] OR “Conscious Sedation” [tw] OR “Deep Sedation” [tw] OR “Anesthetics, Dissociative” [tw] OR “sedation” [All Fields] OR “sedation, conscious” [All Fields] OR “sedation, deep” [All Fields] OR “sedation, moderate” [All Fields] OR “sedations” [All Fields] OR “sedations, deep” [All Fields] OR “sedative” [All Fields] OR “sedative action” [All Fields] OR “sedative and hypnotic” [All Fields] OR “sedative effect” [All Fields] OR “sedative effects” [All Fields]) AND (comparative study [mh] OR placebos [mh] OR clinical trial [pt] OR random* [tiab] OR controlled clinical trial [pt] OR randomized controlled trial [pt] OR double blind method) AND (“Intensive Care” [MeSH] OR “Intensive Care Units” [MeSH] OR “Critical Care” [MeSH] OR Intensive Care [tw] OR Intensive Unit* [tw] OR Critical Care [tw] OR ICU [tw] OR Coronary Care Units [MeSH] OR Burn Units [MeSH] OR Respiratory Care Units [MeSH] OR UTI [TW] OR Neurointensive care) AND (“sepsis” [MeSH Terms] OR sepsis [Text Word])
What are the factors that contribute to the appearance of agitation?
Search (Cohort studies [mh] OR Risk [mh] OR (Odds [tw] AND ratio* [tw]) OR (Relative [tw] AND risk [tw]) OR Case control* [tw] OR Case–control studies [mh] OR “clinical indicators” [tw] OR forecasting [tw]) AND (Psychomotor Agitation [mh] OR Excitement [tw] OR Psychomotor Hyperactivity [tw] OR Agitation [tw] OR Psychomotor Agitation [tw] OR Akathisia [tw] OR Dyskinesias [tw] OR Delirium [MeSH] OR “Alcohol Withdrawal Delirium” [MeSH] OR “Delirium, Dementia, Amnesic, Cognitive Disorders” [MeSH] OR Delirium of Mixed Origin OR Delirium* [tw] OR Confusion [mh] OR bewilderment [tw] OR emotional disturbance [tw] OR perceptual disorientation [tw] OR Confusional State* [tw] OR Bewilderment [tw] OR Confusion [tw] OR Disorientation [tw] OR Neurobehavioral Manifestations [mh] OR Cognitive Symptoms [tw] OR Cognitive Manifestation* [tw] OR Alcohol Withdrawal Delirium [mh] OR DELUSIONS [tw] OR hallucination* [tw] OR tremor [tw] OR agitation [tw] OR insomnia [tw] OR autonomic hyperactivity [tw] OR Hallucinosis [tw]) AND (“Intensive Care” [MeSH] OR “Intensive Care Units” [MeSH] OR “Critical Care” [MeSH] OR Intensive Care [tw] OR Intensive Unit* [tw] OR Critical Care [tw] OR ICU [tw] OR Coronary Care Units [MeSH] OR Burn Units [MeSH] OR Respiratory Care Units [MeSH] OR UTI [TW] OR Neurointensive care [tw])Limits: Publication Date from 2006/01/01 to 2011/12/31.
Which are the most widely used measurement instruments (scales, checklists, measurement systems) for monitoring and diagnosis?
((“Intensive Care” [MeSH] OR “Intensive Care Units” [MeSH] OR “Critical Care” [MeSH] OR (Intensive Care [tw]) OR Intensive Unit* [tw] OR Critical Care [TW] OR ICU [tw] OR Coronary Care Units [MeSH] OR Burn Units [MeSH] OR Respiratory Care Units [MeSH] OR UTI [TW] OR Neurointensive care [tw]) AND (“Delirium” [MeSH] OR “Delirium, Dementia, Amnesic, Cognitive Disorders” [MeSH] OR “delirium” [All Fields]) AND (sensitiv* [Title/Abstract] OR sensitivity and specificity [MeSH Terms] OR diagnos* [Title/Abstract] OR diagnosis [MeSH:noexp] OR diagnostic* [MeSH:noexp] OR diagnosis, differential [MeSH:noexp] OR diagnosis [Subheading:noexp] OR Scales [tw] OR “weights and measures” [MeSH Terms] OR SCALE [Text Word]) OR measurement [All Fields] OR “scale” [All Fields].
Which are the most widely used scales and elements for the monitorization and diagnosis of agitation?
Search (Scales [tw] OR Pain Measurement [tw] OR Analgesia test* [tw] OR pain score [tw] OR pain scale [tw] OR sedation score [tw] OR Apache [tw] OR Glasgow coma scale [tw] OR point scoring systems [tw] OR Ramsey sedation score [tw] OR addenbrooke* sedation score* [tw] OR sedation item* [tw] OR “Psychiatric Status Rating Scales” [MeSH] OR “Pain Measurement” [MeSH] OR “Manifest Anxiety Scale” [MeSH] OR “Test Anxiety Scale” [MeSH] OR pain questionnaire [tw]) AND (“Intensive Care” [MeSH] OR “Intensive Care Units” [MeSH] OR “Critical Care” [MeSH] OR (Intensive Care [tw]) OR Intensive Unit* [tw] OR Critical Care [TW] OR ICU [tw] OR Coronary Care Units [MeSH] OR Burn Units [MeSH] OR Respiratory Care Units [MeSH] OR UTI [TW] OR Neurointensive care [tw]) Limits: Publication Date from 2006/01/01 to 2011/12/31.
What are the risk factors that contribute to its appearance?
(“Intensive Care” [MeSH] OR “Intensive Care Units” [MeSH] OR “Critical Care” [MeSH] OR (Intensive Care [tw]) OR Intensive Unit* [tw] OR Critical Care [TW] OR ICU [tw] OR Coronary Care Units [MeSH] OR Burn Units [MeSH] OR Respiratory Care Units [MeSH] OR UTI [TW] OR Neurointensive care [tw]) AND (“Delirium” [MeSH] OR “Delirium, Dementia, Amnestic, Cognitive Disorders” [MeSH] OR “delirium” [All Fields]) AND (random*[tiab] OR cohort* [tiab] OR risk* [tiab] OR cause* [tiab] OR predispos* [tiab] OR odds ratio [mh] OR case control* OR odds ratio* OR controlled clinical trial [pt] OR randomized controlled trial [pt] OR risk [mh] OR practice guideline [pt] OR epidemiologic studies [mh] OR case control studies [mh] OR cohort studies [mh] OR age factors [mh] OR comorbidity [mh] OR epidemiologic factors [mh])
What are the specific recommendations in pregnant patients?
“pregnancy” [MeSH Terms] OR pregnancy [Text Word] OR “pregnancy” [All Fields] AND (“Conscious Sedation” [MeSH] OR “Deep Sedation” [MeSH] OR “Anesthetics, Dissociative” [MeSH] OR SEDATION [All Fields] OR “Conscious Sedation” [tw] OR “Deep Sedation” [tw] OR “Anesthetics, Dissociative” [tw] OR “sedation” [All Fields] OR “sedation, conscious” [All Fields] OR “sedation, deep” [All Fields] OR “sedation, moderate” [All Fields] OR “sedations” [All Fields] OR “sedations, deep” [All Fields] OR “sedative” [All Fields] OR “sedative action” [All Fields] OR “sedative and hypnotic” [All Fields] OR “sedative effect” [All Fields] OR “sedative effects” [All Fields]) AND (“Intensive Care” [MeSH] OR “Intensive Care Units” [MeSH] OR “Critical Care” [MeSH] OR Intensive Care [tw] OR Intensive Unit* [tw] OR Critical Care [tw] OR ICU [tw] OR Coronary Care Units [MeSH] OR Burn Units [MeSH] OR Respiratory Care Units [MeSH] OR UTI [TW] OR Neurointensive care).
What are the specific recommendations in patients with intracranial hypertension?
“intracranial hypertension” [MeSH Terms] OR intracranial hypertension [Text Word] OR “pseudotumor cerebri” [MeSH Terms] OR idiopathic intracranial hypertension [Text Word] AND (“Conscious Sedation” [MeSH] OR “Deep Sedation” [MeSH] OR “Anesthetics, Dissociative” [MeSH] OR SEDATION [All Fields] OR “Conscious Sedation” [tw] OR “Deep Sedation” [tw] OR “Anesthetics, Dissociative” [tw] OR “sedation” [All Fields] OR “sedation, conscious” [All Fields] OR “sedation, deep” [All Fields] OR “sedation, moderate” [All Fields] OR “sedations” [All Fields] OR “sedations, deep” [All Fields] OR “sedative” [All Fields] OR “sedative action” [All Fields] OR “sedative and hypnotic” [All Fields] OR “sedative effect” [All Fields] OR “sedative effects” [All Fields]) AND (“Intensive Care” [MeSH] OR “Intensive Care Units” [MeSH] OR “Critical Care” [MeSH] OR Intensive Care [tw] OR Intensive Unit* [tw] OR Critical Care [tw] OR ICU [tw] OR Coronary Care Units [MeSH] OR Burn Units [MeSH] OR Respiratory Care Units [MeSH] OR UTI [TW] OR Neurointensive care).
What are the specific recommendations in patients subjected to tracheostomy?
(“Conscious Sedation” [MeSH] OR “Deep Sedation” [MeSH] OR “Anesthetics, Dissociative” [MeSH] OR SEDATION [All Fields] OR “Conscious Sedation” [tw] OR “Deep Sedation” [tw] OR “Anesthetics, Dissociative” [tw] OR “sedation” [All Fields] OR “sedation, conscious” [All Fields] OR “sedation, deep” [All Fields] OR “sedation, moderate” [All Fields] OR “sedations” [All Fields] OR “sedations, deep” [All Fields] OR “sedative” [All Fields] OR “sedative action” [All Fields] OR “sedative and hypnotic” [All Fields] OR “sedative effect” [All Fields] OR “sedative effects” [All Fields]) AND (“tracheostomy” [MeSH Terms] OR tracheostomy [Text Word] OR “tracheostomy” [All Fields]) OR (“tracheotomies” [All Fields] OR “tracheotomy” [All Fields]) AND (“Intensive Care” [MeSH] OR “Intensive Care Units” [MeSH] OR “Critical Care” [MeSH] OR Intensive Care [tw] OR Intensive Unit* [tw] OR Critical Care [tw] OR ICU [tw] OR Coronary Care Units [MeSH] OR Burn Units [MeSH] OR Respiratory Care Units [MeSH] OR UTI [TW] OR Neurointensive care)
What are the specific recommendations in burn victims?
(“Conscious Sedation” [MeSH] OR “Deep Sedation” [MeSH] OR “Anesthetics, Dissociative”[MeSH] OR SEDATION [All Fields] OR “Conscious Sedation” [tw] OR “Deep Sedation” [tw] OR “Anesthetics, Dissociative” [tw] OR “sedation” [All Fields] OR “sedation, conscious” [All Fields] OR “sedation, deep” [All Fields] OR “sedation, moderate” [All Fields] OR “sedations” [All Fields] OR “sedations, deep” [All Fields] OR “sedative” [All Fields] OR “sedative action” [All Fields] OR “sedative and hypnotic” [All Fields] OR “sedative effect” [All Fields] OR “sedative effects” [All Fields]) AND (“Intensive Care” [MeSH] OR “Intensive Care Units” [MeSH] OR “Critical Care” [MeSH] OR Intensive Care [tw] OR Intensive Unit* [tw] OR Critical Care [tw] OR ICU [tw] OR Coronary Care Units [MeSH] OR Burn Units [MeSH] OR Respiratory Care Units [MeSH] OR UTI [TW] OR Neurointensive care) AND (“burn injury” [All Fields] OR “burned patients” [All Fields] OR “burn, eye” [All Fields] OR “burn, electric” [All Fields] OR “burn, chemical” [All Fields] OR “burn wounds” [All Fields] OR “burn wound” [All Fields] OR “Burns” [MeSH] OR “Burns, Inhalation” [MeSH] OR “Eye Burns” [MeSH] OR “Burns, Electric” [MeSH] OR “Burns, Chemical” [MeSH] OR “Burns” [tw] OR “Burns, Inhalation” [tw] OR “Eye Burns” [tw] OR “Burns, Electric” [tw] OR “Burns, Chemical” [tw])
Which are the best therapeutic options?
(“Intensive Care” [MeSH] OR “Intensive Care Units” [MeSH] OR “Critical Care” [MeSH] OR (Intensive Care [tw]) OR Intensive Unit* [tw] OR Critical Care [TW] OR ICU [tw] OR Coronary Care Units [MeSH] OR Burn Units [MeSH] OR Respiratory Care Units [MeSH] OR UTI [TW] OR Neurointensive care [tw]) AND (“Delirium” [MeSH] OR “Delirium, Dementia, Amnestic, Cognitive Disorders” [MeSH] OR “delirium” [All Fields]) AND “antipsychotic agents” [MeSH Terms] OR “antipsychotic agents” [All Fields] OR antipsychotic drugs [Text Word] OR “haloperidol” [MeSH Terms] OR haloperidol [Text Word] OR “olanzapine” [Supplementary Concept] OR olanzapine [Text Word] OR “quetiapine” [Supplementary Concept] OR quetiapine [Text Word] OR “risperidone” [MeSH Terms] OR RISPERIDONE [Text Word].
As a byproduct of this guide, we wish to underscore a series of measures addressed to adult critical patients with or without MV, and intended for application (where possible) from the moment of admission to the ICU.
All these interventions share the following characteristics: (a) most of the recommendations are based on high or moderate levels of evidence (A or B); (b) they have a strong impact upon patient outcome; (c) they are consistent among the different studies; and (d) they have been approved by all the members of the group of experts.
The outcomes referred to by this bundle of measures are the following: mortality, delirium, cognitive deficit, withdrawal syndrome, lack of adaptation, duration of MV, stay in the ICU, hospital stay, side effects of drugs, cost and patient comfort.
Each of these interventions have yielded good results when used separately. The aim of applying them jointly is to exert a maximum impact upon the aforementioned outcomes, in a way similar to other bundles (prevention of the infections associated to intravascular catheter use, ventilator associated pneumonia and urinary tract infections associated to bladder catheterization). Achievement of the objectives requires full implementation of these measures, except when contraindicated or not applicable.
- 1.
Analgesia (Fig. 7).
- 1.1.
Evaluate the level of pain (visual analog scale, BPS or Campbell). Maintain level of pain ≤4/10.
- 1.2.
Start the treatment of pain: titer the opioid or non-opioid drug and use multimodal analgesia techniques.
- 1.1.
- 2.
Sedation and agitation
- 2.1.
Evaluate the need for sedation and define the level of sedation required (RASS scale or BIS).
- i.
No sedation
- ii.
Cooperative sedation (RASS about −1)
- iii.
Deep sedation (RASS about −4)
- i.
- 2.2.
Chose the appropriate medication and titer the dose to secure the required level of sedation (the use of non-benzodiazepine drugs such as propofol or dexmedetomidine is advised).
- 2.3.
Prefer superficial levels of sedation and promote early mobilization.
- 2.4.
Use containment measures in episodes of severe agitation: first verbal, then pharmacological, and finally physical.
- 2.1.
- 3.
Delirium
- 3.1
Identify modifiable risk factors of delirium (infection, hypoxemia, metabolic acidosis, uremia, disease severity, patient environment, GABA type sedatives, high dose opioids, alteration of the natural sleep pattern and memory).
- 3.2
Detect the appearance of delirium (use CAM-ICU).
- 3.3
Select the most appropriate drug and titer its dose (haloperidol, atypical antipsychotics or dexmedetomidine; avoid the use of benzodiazepines).
- 3.1
Please cite this article as: Celis-Rodríguez E, Birchenall C, de la Cal MÁ, Castorena Arellano G, Hernández A, Ceraso D, et al. Guía de práctica clínica basada en la evidencia para el manejo de la sedoanalgesia en el paciente adulto críticamente enfermo. Med Intensiva. 2013;37:519–574.