To identify clinical and radiological factors associated to early evolution to brain death (BD), defined as occurring within the first 24 h.
DesignA retrospective cohort study was made covering the period 2015−2017.
SettingAn adult Intensive Care Unit (ICU).
Patients/methodsEpidemiological, clinical and imaging (CT scan) parameters upon admission to the ICU in patients evolving to BD.
ResultsA total of 166 patients with BD (86 males, mean age 62.7 years) were analyzed. Primary cause: intracerebral hemorrhage 42.8%, subarachnoid hemorrhage 18.7%, traumatic brain injury 17.5%, anoxia 9%, stroke 7.8%, other causes 4.2%. Epidemiological data: arterial hypertension 50%, dyslipidemia 34%, smoking 33%, antiplatelet medication 21%, alcoholism 19%, anticoagulant therapy 15%, diabetes 15%. The Glasgow Coma Score (GCS) upon admission was 3 in 68.8% of the cases in early BD versus in 38.2% of the cases in BD occurring after 24 h (p = 0.0001). Eighty-five patients presented supratentorial hematomas with a volume of 90.9 ml in early BD versus 82.7 ml in BD > 24 h (p = 0.54). The mean midline shift was 10.7 mm in early BD versus 7.8 mm in BD > 24 h (p = 0.045). Ninety-one patients presented ventriculomegaly and 38 additionally ependymal transudation (p = 0.021). Thirty-six patients with early BD versus 24 with BD > 24 h presented complete effacement of basal cisterns (p = 0.005), sulcular effacement (p = 0.013), loss of cortico-subcortical differentiation (p = 0.0001) and effacement of the suprasellar cistern (p = 0.005). The optic nerve sheath measurements showed no significant differences between groups.
ConclusionsEarly BD (>24 h) was associated to GCS < 5, midline shift, effacement of the basal cisterns, cerebral sulci and suprasellar cistern, and ependymal transudation.
Identificar los factores clínico-radiológicos que se asocian a evolución precoz a muerte encefálica (ME), definida esta como la ocurrida en ≤24 horas.
DiseñoEstudio de cohortes retrospectivo desde 2015 hasta 2017, ambos incluidos.
ÁmbitoServicio de Medicina Intensiva (SMI) de adultos.
Pacientes y métodoAnálisis de variables clínico-epidemiológicas y de la TC craneal de ingreso en pacientes con evolución a ME.
ResultadosSe analizaron 166 ME, 86 varones, edad media 62,7 años, 42,8% hemorragia intracerebral, 18,7% HSA, 17,5% TCE, 7,8% ictus isquémico, 9% anoxia y 4,2% otras causas; 50% HTA, 34% dislipemia, 33% tabaquismo, 21% antiagregación, 19% enolismo. El 15% anticoagulación, 15% diabetes. El GCS fue tres en el 68,8% en ME precoz frente 38,2% en ME >24 h (p 0,0001); 85 hematoma supratentorial (90,9 mL en ME precoz vs. 82,7 mL ME tardía, p 0,54); 12 hematoma infratentorial. Desplazamiento medio de línea media 10,7 mm en ME precoz vs. 7,8 mm en ME tardía (p 0,045); 91 pacientes ventriculomegalia y 38 trasudado periependimario (p 0,021). Borramiento completo de cisternas basales 36 en ME precoz frente a 24 en ME tardía (p 0,005), borramiento de surcos (p 0,013), pérdida de diferenciación córtico-subcortical (p 0,0001) y ausencia de cisterna supraselar (p 0,005). La medición de la vaina del nervio óptico no mostró diferencias significativas entre los dos grupos.
ConclusionesSe asoció con ME ≤ 24 horas el GCS < 5, el desplazamiento de línea media, la pérdida de diferenciación córtico-subcortical, el borramiento de surcos, el borramiento completo de cisternas basales, de la cisterna supraselar y la presencia de trasudado periependimario.
Patients with endocranial hypertension progress into brain death (BD) when intracranial pressure (ICP) exceeds the systolic arterial pressure, thus leading to cerebral circulatory arrest,1–5 being the intracerebral hemorrhage (ICH) the most common etiology in Spain followed by traumatic brain injury (TBI), and subarachnoid hemorrhage (SAH).3 Donors who die under conditions of brain death are still the main source of organs for transplants,6 but over the last few years there has been fewer patients dead due to neurological criteria,7 which is a universal problem since the number of organs available for transplant drops. In Spain, BD represents 2.3% of all in-hospital deaths, and 12.4% of all deaths reported in the intensive care unit (ICU) setting.8 In our setting, a European study confirms that 7.8% of the patients hospitalized at an ICU die under conditions of BD.9
We know that most patients with severe brain injuries who progress into BD do so within the first 72 h.3 However, being able to identify what patients will progress into BD and when they’ll do so is not an easy task. Brain injuries have a varied etiology, a different pathophysiological behavior, and great clinical complexity. Several studies have identified clinical and radiological signs10–15 predictive of progression into BD. However, in the clinical practice, there is no such thing as a tool to predict it with a high degree of certainty. Being able to identify, at admission, what factors are associated with early BD can help during the therapeutic management of patients, the decision-making process such as whether to proceed with life-sustaining treatment limitation (LSTL) or during the identification of potential organ donors.16
The objective of this study was to establish the existence of differences between clinical and radiological elements seen in patients who progress into early BD defined as death occurring within the first 24 h compared to those patients who died under conditions of BD after 24 h.
Patients, material, and methodsSettingPolyvalent adult ICU of a tertiary teaching hospital.
PatientsPatients hospitalized at an ICU who progressed into BD from January 1 2015 through December 31 2017. Retrospective study.
Data miningA standard form was designed to collect all the epidemiological, clinical, and radiological variables. Similarly, data on the treatments received like barbiturates, use of neuromuscular blockers, osmotic therapy, etc were collected too. The severity scales used were the E. Glasgow Coma Scale (GCS) for TBI and ICH (in patients intubated and sedated the GCS registered by the mobile ICU ambulance or obtained at the hospital emergency unit), the Hunt and Hess score for SAH, and the NIHSS (National Institutes of Health Stroke Scale) for ischemic stroke. When the patient was intubated or in a coma and the NIHHS could not be used, it was replaced by the GCS. The radiological variables studied in the cranial CT scan at admission were volume of hematoma measured using the AxBxC/2 method adding the area of surrounding edema, measurement (in mm) of the maximum thickness of extra-axial lesions, diffuse sulcal effacement, loss of cortico-subcortical differentiation, ventricular collapse, presence or absence or suprasellar cistern, basal partial/total cistern effacement, measurement of the diameter of the optic nerve sheath 3 mm–10 mm away from the eyeball, presence of hydrocephaly, and periependymal transudation. The interpretation of the images obtained was performed by a neuro-radiologist. Early BD was defined as death occurring within the first 24 h after admission, and late BD as dead occurring under conditions of BD after the first 24 h.
Statistical analysisData were analyzed using the statistical software package SPSS 20.0 for Windows® (SPSS Inc., Chicago, IL, United States). The comparative analysis was performed using the Student t test, Mann–Whitney U test, and the chi-square test for the clinical and radiological variables. Both the univariate and multivariate analyses were performed through logistic regression. P values <.05 were considered statistically significant.
ResultsFrom 2015 through 2017, both years included, a total of 3834 patients were admitted to the ICU with a mean age of 60.3 ± 15.8 years, and an APACHE II score of 18.0 ± 7.9. The mean ICU stay was 7 ± 12.5 days. Out of all the hospitalized patients, 724 died (mortality rate, 18.8%), and 166 of these did so under conditions of BD (144 of them were organ and tissue donors). BD represented 22.9% of all deaths reported at the ICU setting, and 3.4% of all in-hospital deaths reported.
Clinical variablesOut of the 166 cases of BD, 86 were men (51.8%), and 80 (48.2%) women with a mean age of 62.75 years. Etiology of BD: 71 (42.8%) intracerebral hemorrhages, 31 (18.7%) SAHs, 29 (17.5%) TBIs, 13 (7.8%) ischemic strokes, 15 (9%) anoxias, and 7 (4.2%) other causes. The past medical history of 50% of the patients revealed the presence of AHT, 34% dyslipidemia, 33% smoking habit, 21% were on antiplatelet therapy, 19% had issues with wine abuse, 15% remained on oral anticoagulants, 15% had diabetes, and 11% atrial fibrillation. None of these variables kept a statistically significant correlation with the appearance of BD ≤ 24 h. No significant differences were reported either associated with the patients’ age, sex or diagnosis.
Regarding the severity scales, the median GCS score was 3 with statistically significant differences in the distribution of the GCS scores between both groups (P = .0001). The GCS score was 3 in 68.8% of the patients with early BD vs 38.2% of the patients with late BD. Range-based, GCS scores of 4–5 were obtained in 24.7% of all early BDs vs 27% of all late BD; GCS scores of 6–9 were obtained in 5.2% of all early BDs vs 23.6% of all late BDs; finally, GCS scores of 10–15 were obtained in 1.3% of all early BDs vs 11.1% of all late BDs. GCS scores <5 were associated with early BD (OR = 2.9, P = .002). In the 31 patients with SAH significant statistical differences were reported in the Hunt and Hess scale scores obtained between early and late BD (P = .026). The Hunt y Hess scale was 5 points in 93.8% of the patients with early BD vs 66.7% of the patients with delayed progression into BD. In the group of patients with late BD, 26.6% of the patients scored between 3 and 4, and 6.7% between 1 and 2. The mean NIHSS score of the 11 patients with ischemic stroke was 24.
Intracranial pressure was monitored in 39 patients (23.5%), in 11 of them using the Camino® catheter, and in 28 patients using an intraventricular catheter. Most of the cases monitored (89.7%) were patients with delayed progression into BD. The mean peak ICP of the group with late BD was 60.4 mmHg vs 63.7 in the group with early BD (P = .8).
TreatmentOf all the patients with aneurysmal SAH, endovascular treatment was performed in 18 patients (15 from the group with late BD vs 3 from the group with early BD). A total of 7 patients with ischemic stroke were treated with mechanical thrombectomy (6 from the group with late BD and 1 from the group with early BD). Treatment for intracranial hypertension: 69 patients (41.6%) received osmotic therapy (OR = 0.28; P = .0001), 44 patients (26%) neuromuscular blockers (OR = 0.24; P = .0001), 5 were treated with barbiturates (3%) (P = .62), and 11 patients received decompressive surgery (6.6% of the total) 10 of whom progressed into late BD and 1 ≤24 h, (OR = 0.1; P = .033). Surgery for space-occupying lesion was also performed in 10 patients. All the patients who eventually received some type of endocranial anti-hyperintensive medication, except for those who received barbiturates, kept a statistically significant correlation with progression into late BD.
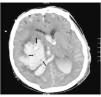
Radiological variablesVolume of hematoma: a total of 85 patients had a hematoma of supratentorial location with a mean volume of 86.5 mL, CI 0.95 [73.3–99.7], and 12 had an infratentorial hematoma with a mean volume of 23.8 mL, CI 0.95 [11.0–36.6]. No statistically significant differences were found regarding the volume of the supratentorial hematoma (90.9 mL in early BD vs 82.7 mL in late BD; P = .54) or the volume of the infratentorial hematoma (26.2 mL in early BD vs 23.0 mL in late BD: P = .83).
Extra-axial lesion: no patient with epidural hematoma was ever reported. In the series, 33 patients had subdural hematomas (16 from the in early BD group >24 h, and 17 from the BD group <24 h). The mean maximum thickness measured in mm in the first group was 9.59 compared to 16.23 in the second group; P = .053.
Brain midline shift: 10.7 mm in the early BD group vs 7.8 mm in the late BD group with a statistically significant difference (P = .045). For every increase of 1 mm in the brain midline shift, the risk of early BD goes up by 1.06 times (P = .047).
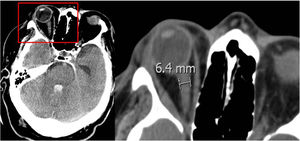
Optic nerve sheath: its diameter was measured between 3 mm and 10 mm away from the eyeball (Fig. 1). Results are shown on Table 1. No statistically significant differences were seen between the diameter of the optic nerve sheath and early BD.
Diameter of the optic nerve sheath measured in mm.
| CT scan at admission | BD ≥ 24 h | BD > 24 h | P |
|---|---|---|---|
| Left eye measured: | |||
| 3 mm away from the eyeball | 5,5 | 5,7 | .23 |
| 10 mm away from the eyeball | 4,4 | 4,4 | .70 |
| Right eye measured: | |||
| 3 mm away from the eyeball | 5,4 | 5,5 | .60 |
| 10 mm away from the eyeball | 4,3 | 4,3 | .82 |
| Mean: | |||
| 3 mm | 5,4 | 5,5 | .60 |
| 10 mm | 4,3 | 4,4 | .86 |
Hydrocephaly: a total of 91 patients had ventriculomegaly, and 38 of these showed periependymal transudation. The presence of transudation kept a statistically significant correlation with progression into early BD (OR = 2.74, P = .021).
Total basal cistern effacement (36 patients from the early BD group vs 24 patients from the late BD group) (OR = 255 and P = .005), sulcal effacement (OR = 2.28; P = .013), the loss of cortico-subcortical differentiation (OR = 3.96; P = .0001), and the lack of suprasellar cistern (OR = 2.55; P = .005) kept a statistically significant correlation with progression into early BD. The partial basal cistern effacement was not associated with early BD (Table 2).
Signs of cerebral herniation and brain swelling on the cranial CT scan.
| CT scan at admission | BD > 24 h | BD ≤ 24 h | P |
|---|---|---|---|
| N = 85 | N = 71 | ||
| Signs of cerebral herniation | |||
| Basal partial cistern effacement | 57 | 52 | .4 |
| Basal total cistern effacement | 24 | 36 | .005 |
| Tumefaction/diffuse cerebral edema | |||
| Sulcal diffuse effacement | 38 | 46 | .013 |
| Loss of cortico-subcortical differentiation | 12 | 28 | .0001 |
| Ventricular collapse | 15 | 15 | .68 |
| Absent suprasellar cistern | 41 | 48 | .015 |
When the multivariate analysis was performed progression into BD was associated with the loss of cortico-subcortical differentiation (OR = 2.81; P = .041), and GCS scores <5 (OR = 2.13; P = .05). The increase of 1 point in the GCS reduces the possibilities of progression into early BD by 1.4 times (P value = .013).
DiscussionNeurological severity scores contribute to estimate the chances of death or functional prognosis, but without a high degree of certainty,17–20 which is why studies look for other factors that may be associated with progression into BD to optimize the clinical decision-making process at admission. Some authors have come up with the concept of «imminent BD» defined as patients with catastrophic cerebral injuries, Glasgow 3, lack of 3 or more trunk reflexes, and on mechanical ventilation.21,22 However, this entity is a highly evolved clinical situation that is not useful in the routine clinical practice. Humbertjean et al.12 describe 6 determinant factors to anticipate progression into BD: Glasgow scale scores ≤6 before sedation, volume of hematoma >65 mL, signs of herniation on the CT scan, hydrocephaly, early systolic arterial blood pressure >150 mmHg, and a past medical history of alcohol abuse with which they try to identify, within the first 24 h, patients with high chances of progressing into BD. Other studies find that the determinant factors of poor progression are old age, low scores on the Glasgow scale or high NIHSS scores, infratentorial location, arterial hypertension, anticoagulation and/or coagulopathy, ventricular hemorrhage, and hydrocephaly.13,23 In our study we did not find a statistically significant correlation with the patients’ past medical history or with their age, sex or even diagnosis. Consistent with the medical literature available, we did find that the Glasgow scale score at admission was a good predictor. In our case, the GCS score was 3 in 68.8% of the patients from the early BD group vs 38.2% of the patients from the late BD group (P = .0001) whereas GCS scores <5 were clearly associated with early BD. On the other hand, patients with intracranial hypertension treated with osmotic therapy, neuromuscular blockers, and decompressive surgery progressed into late BD as expected.
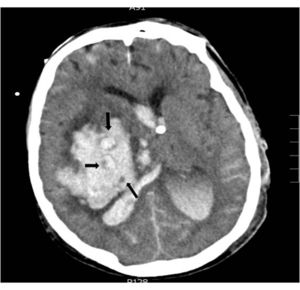
Regarding the radiological variables, traditionally we know that the presence of signs of endocranial hypertension on the CT scan is a severity indicator being the volume of the lesion one of the determinant factors of poor prognosis;14,24 regarding bleeding, several studies correlate the volume of hematoma with short- and long-term mortality.11,15,18,26–30 (Table 3). The presence of intraventricular blood and its volume, the infratentorial location of the hemorrhage or the brain midline shift are also determinant factors of poor prognosis.13,26,28,31,32 In patients with intracerebral bleeding, the lack of corneal reflex and the «whirlwind sign» seen on the CT scan (Fig. 2), indicative of active bleeding inside the hematoma, have been associated with progression into BD10 due to the greater chances of hematoma spread same as the presence of spot sign on the CCTA that is also indicative of active hemorrhage.33 In our series all patients died under situations of BD and showed signs of endocranial hypertension on the CT scan; the radiological signs that, in our setting, are significantly associated with progression into BD within the first 24 h are brain midline shift, the presence of active hydrocephaly, basal total cistern effacement, sulcal effacement, the loss of cortico-subcortical differentiation, and the lack of suprasellar cistern.
Volume of hematoma, and patient progression in the different studies.
| Study | Design | Volume of hematoma | Progression |
|---|---|---|---|
| Broderick et al.199326 | Mortality rate 71%–90% based on location | ||
| >60 cm3 | Mortality rate 60%–64% based on location | ||
| Retrospective | 30−60 cm3 | Pontine hemorrhage >5 cm3 and cerebellar hemorrhage >30 cm3; patients died after 30 days. | |
| Cohorts | <30 cm3 | Mortality rate 7%–23% based on location | |
| Inagawa et al. 200325 | Retrospective | >20 cm3 | The volume of the hematoma was the most important prognosis predictor, and was significantly associated with the 30-day mortality rate (P = .003). |
| Cohorts | 6−20 cm3 | ||
| <5 cm3 | |||
| Kim, 200928 | Retrospective | >60 cm3 | Hematomas >60 cm3 were associated with a high 30-day mortality rate. |
| Cohorts | 30−59 cm3 | Hematomas between 30 cm3 and 59 cm3 left us with inconclusive results. | |
| <30 cm3 | Hematomas <30 cm3 were associated with good functional recovery after 90 days. | ||
| Hallevi et al. 200937 | Retrospective | >30 cm3 | It increases mortality |
| Cohorts | |||
| Humbertjean et al. 201612 | Retrospective | >65 cm3 | Rapid progression into brain death. |
| Cohorts |
The optic nerve sheath accumulates cerebrospinal fluid and increases its diameter in intracranial hypertension. Although there is no unanimity in the medical literature on the measuring site or the diameter required to consider endocranial hypertension, some authors find that diameters >5 mm to 6 mm are often followed by ICP >20 mmHg.34,35 We measured the optic nerve sheath between 3 mm and 10 mm away from the eyeball and found no significant correlation with an early progression into BD; in the measurements of the optic nerve sheath taken 3 mm away from the eyeball, the mean diameter found was > 5 mm in both groups of patients (early and late BD). Some of this study limitations are its retrospective nature, the fact that it only analyzed patients who progressed into BD, and that it was conducted in a single center only.
The scarcity of organs for transplants is a global problem that has led to the implementation of strategies to increase donation. The Non-therapeutic elective ventilation (NTEV) (in the Western world), and Donation-oriented intensive care (DOIC) (in Spain) initiatives promote the ICU admission of patients with cerebral catastrophic lesions without therapeutic options to promote donation.11,16,36 However, one of the difficulties of DOIC is to predict, with a certain degree of certainty, what patients will eventually progress into BD and when will they do so. In this sense, our study is interesting despite its limitations because it provides some of the most early prognostic factors thus facilitating the intensivist’s difficult time whenmaking a decision on an ICU admission when it comes to LSTL or patient maintenance as a potential organ donor under situations of BD.
Authors/collaborationsDolores Escudero designed the study and wrote the manuscript.
Iván Astola participated in the study design, statistical analysis, and the manuscript draft and proofread.
Ángela Meilán, and Débora Vizcaino collected the radiological data, analyzed the images from the cranial CT, and reviewed the manuscript.
Salvador Balboa, Blanca Leoz, Cecilia del Busto, Brígida Quindós, Lorena Forcelledo, Lorena Martín, Estefanía Salgado, and Lucía Viña performed the data mining, and proofread and reviewed the manuscript.
FundingNone whatsoever.
Conflicts of interestNone reported.
Please cite this article as: Escudero D, Astola I, Balboa S, Leoz B, Meilan Á, del Busto C, et al. Factores clínico-radiológicos asociados con muerte encefálica precoz. Med Intensiva. 2020. https://doi.org/10.1016/j.medin.2020.06.019