No data are available on blood caspase-8 concentrations (the initiator caspase in the extrinsic apoptosis pathway) in septic patients. The present study thus describes the blood caspase-8 concentrations in survivors and non-survivors, and examines the possible association between blood caspase-8 concentrations and mortality in septic patients.
DesignA prospective observational study was carried out.
SettingThree Spanish Intensive Care Units.
PatientsSeptic patients.
InterventionsSerum caspase-8 concentrations were determined at the diagnosis of sepsis.
Main variable of interestMortality after 30 days.
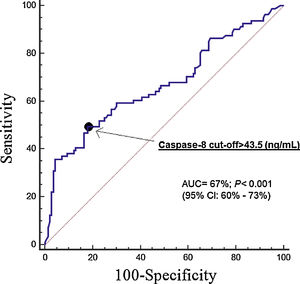
ResultsPatients not surviving at day 30 (n=81) compared to surviving patients (n=140) showed higher serum caspase-8 levels (p<0.001). Multiple logistic regression analysis found an association between serum caspase-8 levels>43.5ng/ml and mortality (OR=3.306; 95%CI=1.619–6.753; p=0.001). The area under the curve (AUC) for mortality predicted by serum caspase-8 levels was 67% (95% CI=60–73%; p<0.001).
ConclusionsThe novel findings of our study were that blood caspase-8 concentrations are higher in non-survivors than in survivors, and that there is an association between blood caspase-8 concentrations and mortality in septic patients.
No existen datos publicados sobre los niveles sanguíneos de caspasa-8 (la caspasa iniciadora en la vía extrínseca de apoptosis) en pacientes sépticos. Por lo tanto, los objetivos del estudio fueron describir los niveles sanguíneos de caspasa-8 en pacientes supervivientes y fallecidos y determinar si existe una asociación entre los niveles sanguíneos de caspasa-8 y la mortalidad de los pacientes sépticos.
DiseñoEstudio observacional y prospectivo.
ÁmbitoTres unidades de cuidados intensivos españolas.
PacientesPacientes sépticos.
IntervencionesSe determinaron las concentraciones séricas de caspasa-8 al diagnóstico de la sepsis.
Variable de interés principalMortalidad a los 30 días.
ResultadosEncontramos que los pacientes fallecidos en los primeros 30 días (n=81) comparados con los pacientes supervivientes (n=140) presentaban niveles séricos mayores de caspasa-8 (p<0,001). En el análisis de regresión logística múltiple encontramos una asociación entre los niveles séricos de caspasa-8>43,5ng/ml y la mortalidad (OR: 3,306; IC 95%: 1,619-6,753; p=0,001). El área bajo la curva para predecir la mortalidad por los niveles séricos de caspasa-8 fue del 67% (IC 95%: 60-73%; p<0,001).
ConclusionesLos nuevos hallazgos de nuestro estudio fueron que los niveles séricos mayores de caspasa-8 eran superiores en los pacientes fallecidos en los primeros 30 días, y que existe una asociación entre los niveles séricos de caspasa-8 y la mortalidad.
Sepsis contributes to many deaths and healthcare system costs.1,2 Apoptosis, the process in which cells are programmedly and actively eliminated, that occurs in physiological processes (during morphogenesis and tissue remodeling) and also in different diseases.3 In addition, in animal models has been found an increase of apoptosis.3 Apoptotic cell death occurs mainly through the extrinsic pathway (or death receptor pathway) and the intrinsic pathway (or mitochondrial pathway). The extrinsic pathway is activated when tumor necrosis factor membrane receptors superfamily (TNFRSF) binding to its ligands of the tumor necrosis factor ligand superfamily (TNFSF). The main ligands and receptors in this pathway are the ligand FasL (which bind to its Fas antigen) and the TNF-related apoptosis-inducing ligand (TRAIL) (which bind to its different receptors (TRAILR1–4). When TNFRSF and its ligand TNFSF binding is generated a death signal that adheres to pro-caspase-8 and appears active caspase-8 (initiator caspase), which activates caspase-3 (the main effector caspase) leading to cellular apoptotic changes.3
The extrinsic pathway of apoptosis in septic patients has been scarcely explored and only in studies of small sample size.4,5 In one study higher blood Fas levels in septic patients than in controls and in non-survivor than in survivor septic patients,4 and the same findings for TRAIL.5 However, there are not data on blood caspase-8 concentrations in septic patients. Increased blood caspase-8 concentrations have been found in patients with systemic lupus erythematosus6,7 and with in patients with metabolic syndrome and non-alcoholic fatty liver disease.8 We have chosen the study of blood caspase-8 concentrations in our research due to that the extrinsic pathway of apoptosis in septic patients has been scarcely explored and that caspase-8 is the initiator caspase of the extrinsic apoptosis pathway. We hypothesized that non-survivor septic patients could have higher blood caspase-8 concentrations than survivor ones. Therefore, the objectives of this study were to describe blood caspase-8 concentrations in survivor and non-survivor patients and to determine whether there is an association between blood caspase-8 concentrations and mortality in septic patients.
Material and methodsDesign and subjectsThis prospective and observational study was conducted in the polyvalent Intensive Care Unit from 3 Spanish hospitals: H. Universitario de Canarias, H. Universitario Nuestra Señora de Candelaria and H. General de la Palma. The study was approved by the Ethics Committee of all hospitals. The informed and signed consent from patients or their relatives was obtained to participate in the study.
The study was conducted between 2013 and 2014. We included patients with sepsis according to the Sepsis-3 Consensus criteria9; thus, the patients were recoded according to those sepsis criteria. We excluded patients with white solid tumor, blood cell count <1000/μl, hematological tumor, human immunodeficiency virus (HIV), immunosuppressive therapy, radiation therapy, steroid agents, breastfeeding, or pregnancy, or age <18 years.
Age, sex, and the history of chronic renal failure, ischemic heart disease, diabetes mellitus, chronic obstructive pulmonary disease, sepsis focus and appropriateness of antimicrobial therapy were registered. We also registered creatinine, leukocytes, bilirubin, activated partial thromboplastin time, international normalized ratio, platelets, lactic acid, pressure of arterial oxygen, fraction inspired of oxygen, site of infection, Acute Physiology and Chronic Health Evaluation (APACHE)-II score10 and Sepsis-related Organ Failure Assessment [SOFA] score.11 Besides, we registered the appearance of bloodstream infection and septic shock. Mortality at 30 days was considered our endpoint study.
Determination of serum caspase-8 concentrationsWe freeze patient sera at moment of sepsis diagnosis at −80°C until the determination of serum caspase-8 levels. We used the Human Caspase 8 ELISA Kit (Elabscience, Houston, Texas, United States) for the determination of serum caspase-8 concentrations. The detection limit of the assay was 0.10ng/mL, the intra-assay coefficient of variation (CV) was <6%, and the inter-assay CV was <8%.
Statistical methodsWe used medians (percentile 25–75) and frequencies (percentages) to describe continuous and categorical variables. Mann–Whitney U test and chi-square test were used to compare continuous and categorical variables between surviving and non-surviving patients. We carried out receiver operating characteristic (ROC) analyses using 30-day mortality, serum caspase-8 concentrations, SOFA and APACHE-II. Comparison of ROC curves to test the statistical significance of the difference between the areas under curves (derived from the same cases) was carried out with the method of DeLong et al.12 We reported sensitivity, specificity, positive and negative likelihood ratios, and positive and negative predictive values and its 95% confidence interval (CI) of serum caspase-8 levels cut-off selected in basis to Youden J index. We constructed Kaplan–Meier 30-day survival curves using survival at 30 days and the cut-off of serum caspase-8 concentrations estimated by Youden J index, and Hazard Ratio was calculated for this cut-off. To explore the association between serum caspase-8 concentrations and 30-day mortality controlling for possible confounds was performed a multiple logistic regression analysis; and to estimate the clinical impact of prognostic variables were reported odds ratio (OR) and its 95% confidence interval (CI). For statistical analyses were used the programs NCSS 2000 (Kaysville, Utah) and SPSS 17.0 (SPSS Inc., Chicago, IL, USA), and for the establishment of significant differences was used the point p<0.05
ResultsWe included a total of 221 patients, 140 (63.3%) surviving at 30 days and 81 (36.7%) deaths in the following 30 days. The number of patients included in each hospital were 121, 68 and 32 patients respectively. There were not significant differences in the mortality rate between hospitals (47/121 (38.8%), 22/68 (32.4%) and 12/32 (37.5%); p=0.67). We found that non-surviving patients (n=81) compared to surviving patients (n=140) had higher SOFA (p<0.001), APACHE-II (p<0.001), age (p<0.001), rate of septic shock (p<0.001), lactic acid (p<0.001), creatinine (p=0.01), aPTT (p=0.01), INR (p=0.02), and lower platelet count (p<0.001). In addition, non-surviving patients had higher serum caspase-8 levels (p<0.001) (Table 1). Patients with septic shock showed median lactate of 4.00mmol/L (percentile 25–75 of 2.70–6.35, and range of 2–20).
Comparisons between non-surviving and surviving septic patients on demographic and clinical characteristics at moment of sepsis diagnosis.
| Surviving(n=140) | Non-surviving(n=81) | p-Value | |
|---|---|---|---|
| Sex female – n (%) | 49 (35.0) | 27 (33.3) | 0.88 |
| Age – median years (p 25–75) | 55 (45–68) | 64 (56–74) | <0.001 |
| APACHE-II score – median (p 25–75) | 19 (14–23) | 23 (18–28) | <0.001 |
| SOFA score – median (p 25–75) | 9 (7–11) | 11 (9–14) | <0.001 |
| Chronic renal failure – n (%) | 8 (5.7) | 8 (9.9) | 0.29 |
| COPD – n (%) | 16 (11.4) | 13 (16.0) | 0.41 |
| Diabetes mellitus – n (%) | 38 (27.1) | 31 (38.3) | 0.10 |
| Ischemic heart disease – n (%) | 11 (7.9) | 6 (7.4) | 0.99 |
| Bilirubin (mg/dl) – median (p 25–75) | 0.90 (0.43–1.45) | 0.85 (0.50–2.17) | 0.49 |
| Leukocytes (cells/mm3) – median*103(p 25–75) | 15.0 (10.2–19.6) | 15.3 (8.5–20.7) | 0.84 |
| PaO2/FIO2ratio – median (p 25–75) | 173 (120–263) | 172 (101–239) | 0.32 |
| Creatinine (mg/dl) – median (p 25–75) | 1.10 (0.80–1.90) | 1.45 (0.93–2.95) | 0.01 |
| aPTT (seconds) – median (p 25–75) | 32 (28–40) | 36 (30–46) | 0.01 |
| INR – median (p 25–75) | 1.27 (1.10–1.54) | 1.41 (1.13–1.91) | 0.02 |
| Platelets (cells/mm3) – median*103(p 25–75) | 197 (135–296) | 136 (76–223) | <0.001 |
| Lactic acid (mmol/L) – median (p 25–75) | 1.80 (1.08–3.50) | 3.50 (1.55–6.00) | <0.001 |
| Septic shock – n (%) | 62 (44.3) | 56 (69.1) | <0.001 |
| Bloodstream infection – n (%) | 22 (15.7) | 10 (12.3) | 0.56 |
| Caspase-8 (ng/mL) – median (p 25–75) | 10.8 (4.2–35.8) | 39.7 (6.7–206.5) | <0.001 |
| Sepsis focus | 0.75 | ||
| Respiratory – n (%) | 79 (56.4) | 48 (59.3) | |
| Abdominal – n (%) | 37 (26.4) | 21 (25.9) | |
| Neurological | 2 (1.4) | 0 | |
| Urinary – n (%) | 8 (5.7) | 4 (4.9) | |
| Skin – n (%) | 8 (5.7) | 3 (3.7) | |
| Endocarditis – n (%) | 6 (4.3) | 4 (4.9) | |
| Osteomyelitis – n (%) | 0 | 1 (1.2) | |
| Empiric antimicrobial treatment adequate | 0.91 | ||
| Unknown due to negative cultures – n (%) | 74 (52.9) | 44 (54.3) | |
| Adequate – n (%) | 57 (40.7) | 30 (37.0) | |
| Inadequate – n (%) | 4 (2.9) | 3 (3.7) | |
| Unknown due to antigenuria diagnosis – n (%) | 5 (3.6) | 4 (4.9) | |
APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sepsis-related Organ Failure Assessment; COPD, Chronic Obstructive Pulmonary Disease; PaO2/FIO2, pressure of arterial oxygen/fraction inspired oxygen; aPTT, activated partial thromboplastin time; INR, international normalized ratio.
Multiple logistic regression analysis found an association between serum caspase-8 levels>43.5ng/mL (OR=3.306; 95% CI=1.619–6.753; p=0.001) after controlling for age, lactic acid, septic shock, SOFA, aPTT and INR (Table 2). The Hosmer and Lemeshow goodness test of the logistic model had a chi-square of 11.4 and a p-value of 0.18.
Multiple logistic regression analyses to predict mortality at 30 days.
| Odds ratio | 95% confidence interval | p-Value | |
|---|---|---|---|
| Age (years) | 1.034 | 1.009–1.060 | 0.01 |
| SOFA score (points) | 1.188 | 1.071–1.317 | 0.001 |
| Lactic acid (mmol/L) | 1.120 | 0.963–1.303 | 0.14 |
| Septic shock (yes vs non) | 1.483 | 0.614–3.584 | 0.38 |
| Serum caspase-8 level>43.5ng/mL (yes vs non) | 3.306 | 1.619–6.753 | 0.001 |
| aPTT (seconds) | 1.017 | 0.991–1.043 | 0.21 |
| INR | 0.879 | 0.551–1.404 | 0.59 |
SOFA, Sepsis-related Organ Failure Assessment; aPTT, activated partial thromboplastin time; INR, international normalized ratio.
The area under the curve for mortality prediction was of 67% (95% CI=60–73%; p<0.001) by serum caspase-8 levels (Fig. 1), of 69% (95% CI=63–77%; p<0.001) by SOFA, and of 71% (95% CI=65–77%; p<0.001) by APACHE-II score. No significant differences were found in the area under the curve between serum caspase-8 levels and SOFA (p=0.64), serum caspase-8 levels and APACHE-II score (p=0.38), and SOFA and APACHE-II (p=0.57). We found that the point of serum caspase-8 levels>43.5ng/mL showed a sensitivity of 49% (38–61%), specificity 82% (75–88%), positive likelihood ratio 2.8 (1.8–4.2), negative likelihood ratio 0.6 (0.5–0.8), positive predictive value 62% (51–71%) and negative predictive value 74% (69–78%) for mortality prediction.
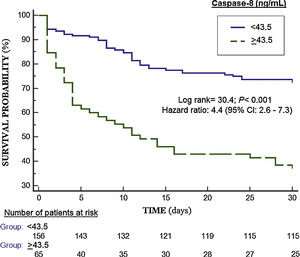
In Kaplan–Meier analysis was found that patients with serum caspase-8 levels>43.5ng/mL had a higher mortality rate (Hazard ratio=4.4; 95% CI=2.6–7.3; p<0.001) (Fig. 2).
DiscussionThat we know this is the study reporting data on blood caspase-8 concentrations in septic patients and the main novelty of our study is that blood caspase-8 concentrations could have a role in the mortality prediction of septic patients.
We found in the multiple logistic regression analysis an association between serum caspase-8 levels and mortality after controlling for age, lactic acid, septic shock, SOFA, aPTT and INR. We did not include in the regression model APACHE due to that we included SOFA, other severity score. In addition, we neither include creatinine and platelet count due that both are included in SOFA.
There are different types of programmed cell death, as apoptosis,3 autophagy,13–15 and Pyroptosis.16–18 Autophagy is induced by pro-inflammatory cytokines that produce the activation of Toll-like receptor (TLR)-2 and TLR-4, then 5′ adenosine monophosphate-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) are activated and produce the inhibition of mammalian target of rapamycin complex 1 (mTORC1) that lead to cell death by autophagy.13–15 Pyroptosis is an inflammatory form of programmed cell death, in which participate caspase-1, caspase-4, caspase-5 and caspase-11, and is characterized by the release of inflammatory cytokines as IL-1β and IL-18.16–18
Apoptotic cell death occurs through death receptor pathway or mitochondrial pathway. The apoptotic extrinsic pathway is activated when some membrane receptor superfamily binding to its ligand superfamily generating a death signal that active caspase-8. Afterwards, caspase-8 will activate caspase-3, the main effector caspase of cellular changes by apoptosis.3 Therefore, our findings could mean that the non-surviving patients showed a higher activation of apoptosis extrinsic pathway that lead to higher activation of effector caspases and finally to a higher apoptotic state.
The blockade of extrinsic apoptosis pathway in septic mice has been associated with reduction of apoptosis and improvement of survival.19–23 Treatment with small interfering RNA (siRNA) against caspase-8 or Fas increased survival and decreased active caspase-3 and apoptosis in the liver and spleen.19 Treatment with caspase-8 or caspase-3 siRNA reduced apoptosis in aortic endothelium and increased survival.20 Treatment with Fas-associated death domain (FADD) siRNA increased survival and reduced acute lung injury21 and caspase-3 activation and apoptosis in aortic endothelium.22 Treatment with a caspase-8 inhibitor increased survival, and decreased monocyte activation, induced necroptotic cell death of activated monocytes, and release of interleukin-1.23 Thus, in all those studies with septic mice has been found a reduction of apoptosis and an increase of survival decreasing extrinsic apoptosis pathway by the block of caspase-8, the main executor caspase of that apoptosis pathway. Thus, it is possible that according the results of our study (association between blood caspase-8 levels and mortality in septic patients) and those in animal models (increase of survival with the agents that block caspase-8 activation) could be interesting to research about the use of agents that block extrinsic apoptosis pathway in septic patients.
We must recognize some limitations of our study. First, we have not assessed cellular damage by apoptosis with some biochemical (Annexin V) or histological assay as terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL), and neither proinflammatory cytokines (as IL-1). Second, we have not data about compliance of sepsis bundle, nor a validation patient cohort. Third, we have not determined blood anti-caspase-8 antibodies, which have been described in patients with type 1 diabetes,24 patients with systemic sclerosis, systemic lupus erythematosus, silicosis, as well as in healthy individuals.25 Fourth, we do not know whether the mortality prognostic ability of serum caspase-8 levels is generalizable to all septic patients due to exclusion criteria. In addition, although the AUC was statistically significant; however, the AUC (67%) and the lower limit of 95% CI (60%) were low.26 Besides, there were no differences in the prediction of mortality between serum caspase-8 levels, SOFA and APACHE-II score; however, the objective of our study was to determine whether serum caspase-8 levels could add in the mortality prediction and not replace other severity scores.
ConclusionsTo the best of our knowledge, our study is the first series reporting data on blood caspase-8 concentrations in septic patients. The new findings from our study were that blood caspase-8 concentrations were higher in non-survivor than in survivor patients and there is an association between blood caspase-8 concentrations and mortality in septic patients.
Authors’ contributionsLLo conceived, designed and coordinated the study, participated in acquisition and interpretation of data, and drafted the manuscript.
MMM, RO, MM, VG, AP and MR participated in acquisition of data.
AGC and APC carried out the determinations of serum caspase-8 concentrations.
AJ participated in the interpretation of data.
All authors revised the manuscript critically for important intellectual content and made the final approval of the version to be published.
FundingThis study was supported by a grant from Instituto de Salud Carlos III (PI-18-00500) (Madrid, Spain) and co-financed with Fondo Europeo de Desarrollo Regional (FEDER).
Conflicts of interestThe authors declare that they have no competing interests.