Although several studies have established the association between antibiotics and Clostridium difficile infection (CDI), there is a lack of epidemiological studies on the incidence of CDI in European Intensive Care Units outside the context of infection outbreaks. The present study describes the incidence, patient characteristics, complications, and recurrence rates of CDI in a Spanish ICU.
DesignA retrospective study was carried out.
SettingA clinical–surgical ICU with 34 beds, a tertiary referral hospital with 1400 beds.
PatientsAll patients over 18 years of age admitted to the ICU from January 2010 to December 2011 with diarrhea for more than 48h.
InterventionsNone.
Study variablesUnderlying diseases, risk factors, fever, leukocyte count, complications, recurrence of infection.
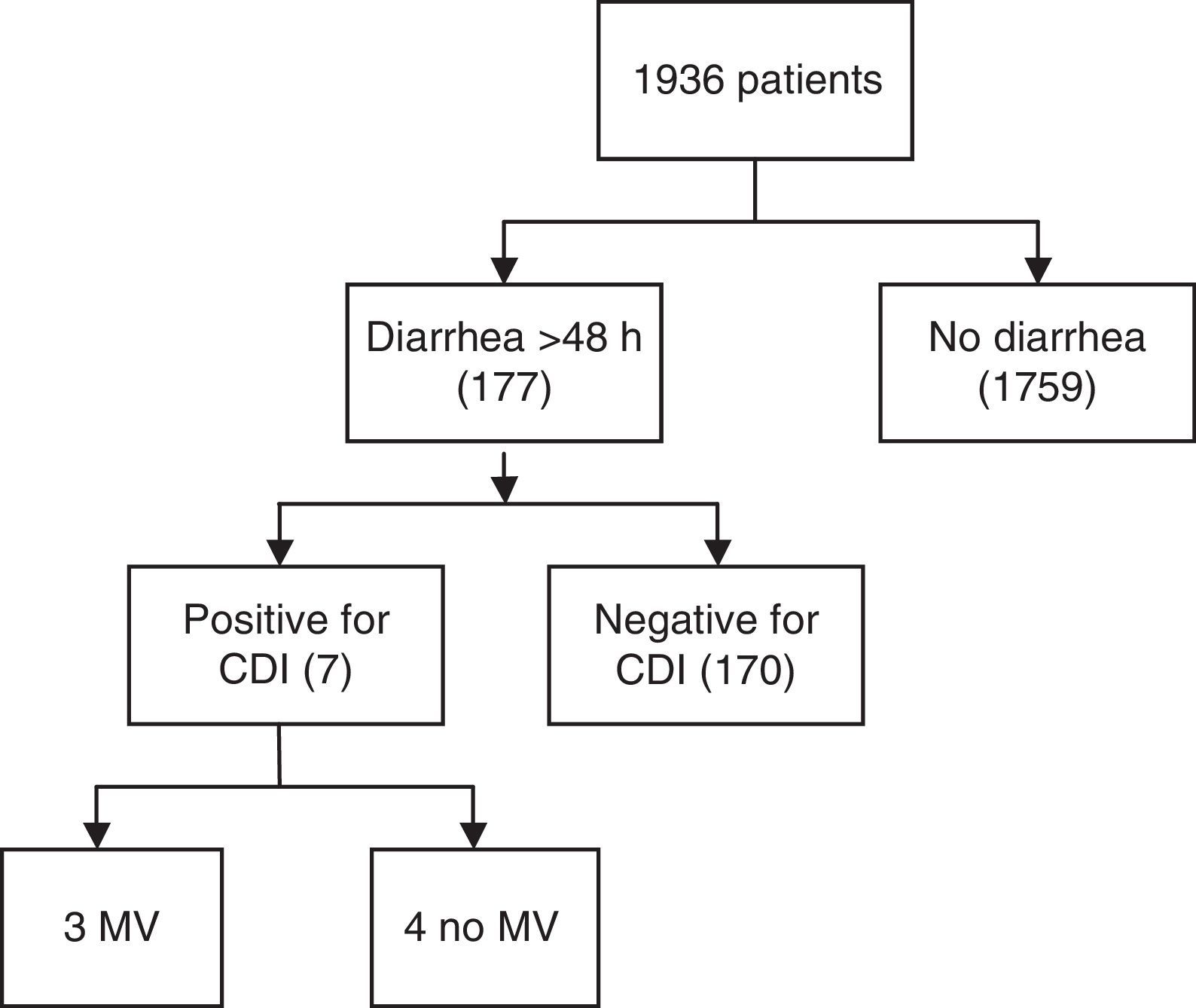
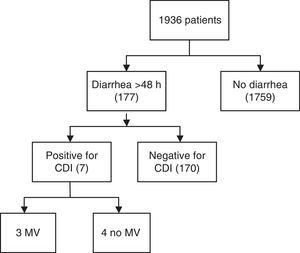
ResultsA total of 1936 adult patients were admitted. Seven patients acquired CDI (0.36%), representing an infection rate of 3.1 per 10,000 bed-days and a cumulative incidence rate of 3.6 in two years. The mean age was 61 years. Six patients showed some degree of immunosuppression. The mean APACHE II score at ICU admission was 17 (IQR 13–24). Severe sepsis was reported in 5 cases of CDI, three of which presented shock and multiorgan dysfunction. Four patients presented recurrence of CDI during hospitalization. ICU admission was prolonged for a mean of 24 days (SD 17.8) after diagnosis.
ConclusionsLess than 1% of the patients admitted to a clinical–surgical ICU in a large teaching institution in Spain developed CDI. However, a high risk of recurrence/complications was associated with prolonged ICU stay.
El objetivo de este estudio es describir la incidencia, características de los pacientes, complicaciones y la recurrencia de infección por Clostridium difficile en una UCI de un hospital universitario en un período fuera de brote.
DiseñoSerie de casos.
ÁmbitoUCI médico-quirúrgica de 34 camas en hospital de referencia de 1.400 camas.
Pacientes o participantesTodo paciente mayor de 18 años ingresado en la UCI desde enero de 2010 hasta diciembre de 2011 que tuviese diarrea durante más de 48h.
IntervencionesSin intervención.
Variables de interés principalesEnfermedades de base, factores de riesgo, recurrencia de infección.
ResultadosUn total de 1.936 pacientes fueron ingresados en el periodo de estudio, de los cuales 177 presentaron diarrea durante más de 48h y 7 cumplieron criterios de infección por Clostridium difficile (0,36%). Una tasa de infección de 3,1 pacientes por cada 10.000 camas/día y una incidencia acumulada de 3,6×1.000, en 2 años. La edad media fue de 61 años. Seis mostraron algún grado de inmunosupresión. La media del score APACHE II al ingreso fue de 17. Cinco casos presentaron sepsis grave, 3 de los cuales tuvieron shock y disfunción multiorgánica. Un paciente de los 7 (14%) falleció. Cuatro pacientes sufrieron recurrencia durante la hospitalización (57%). La estancia media se prolongó 24 días (DE 17,8) después del diagnóstico.
ConclusionesMenos del 1% de los pacientes hospitalizados en una UCI médico-quirúrgica desarrollaron infección por Clostridium difficile. Sin embargo, se asoció a un alto grado de recurrencia/complicaciones y a una larga estancia en la UCI.
Clostridium difficile infection (CDI) is an important hospital-acquired (nosocomial) complication associated with the use of antibiotics. Some patients remain asymptomatic after exposure, while in others the disease can vary from mild diarrhea to fulminant colitis.1,2 Colonization of the intestinal tract occurs via the feco-oral route by disruption of normal intestinal flora, especially related to the use of antibiotics, in immunosuppressed situations or related to the use of proton pump inhibitors. Incidence of diarrhea in hospitalized patients associated with CDI has increased fourfold from 1991 till 2002 rising from 3 to 12 per 1000 people and from 25 to 43 per 1000 people in 2003–2004. In previous studies, cases were found to be serious and refractory to therapy with significant rates of toxic megacolon requiring colectomy. Ten percent of cases required admission to an intensive care unit (ICU) and 2.5% required emergent colectomy. Mortality was 16%. The CDI increase was reported primarily in individuals over 65 years.3,4 After an outbreak in Canada, there was an increase in the incidence, severity and mortality associated with CDI in North America and Europe which was attributed to the emergence of the new strain NAP1/BI/0.27. The virulence of this new strain was defined by: increased production of A and B toxins, fluoroquinolone resistance and production of binary toxin.5,6 Antibiotic use is the most widely recognized and modifiable risk factor for CDI. Other risk factors include hospitalization, advanced age and severe underlying disease.7 Besides, gastric acid suppression, enteral feeding, gastrointestinal surgery, cancer chemotherapy and hematopoietic stem cell transplant could be considered as risk features too. Nevertheless, CDI can occur without the presence of risk factors.
Although several studies have established the association between antibiotics and CDI we lack of epidemiological studies that determine the incidence or the prevalence of CDI in European Intensive Care Units except during outbreaks and little is known about the real associated risk factors. Data on recurrence or relapse are also limited. The main objective of the study was to establish the incidence of C. difficile infection in a medical–surgical Intensive Care Unit, outside an epidemic outbreak period. Secondary objectives were to determine patient characteristics for this type of infection, to document complications, to identify the treatments used and to record the recurrences.
Patients and methodsThis study was conducted at Vall d’Hebron University Hospital, an urban tertiary referral academic center (1400 beds) in Barcelona, Spain. We included all patients admitted to the Medical/Surgical ICU (34 beds) between January 2010 and December 2011. The Vall d’Hebron University Hospital Clinical Research Ethics Committee approved the study, and the need for informed consent was waived (Number 54/2012). This retrospective study identifying ICU patients with CDI was based on information from the microbiology laboratory electronic database and a retrospective review of medical charts. All patients over 18 years old were included. Data were recorded on demographic characteristics of all patients and clinical manifestations for CDI. Previous antimicrobial exposure, immunosuppression and previous hospital admission in the last six months were also recorded. Patients included were identified by diarrhea and a positive C. difficile toxin result according to ICU protocol. Diarrhea was clinically defined as more than three watery stools per day and/or an increase in stool amount of more than 200 grams per day.1 A positive result for C difficile toxin was determined using immunochromatography methods for urgent cases (Immunocard EIA® Toxins A & B, Meridian Bioscience®, Inc., USA) and enzyme immunoassay for routine cases (Enzyme Immunoassay Premier® Toxins A & B Meridian Bioscience).
Statistical analysisThe results were described as frequency (%) in the case of discrete variables. Variables with a normal distribution were described with the mean and standard deviation (SD) and those with a non-normal distribution with the median and interquartile range (IQR).
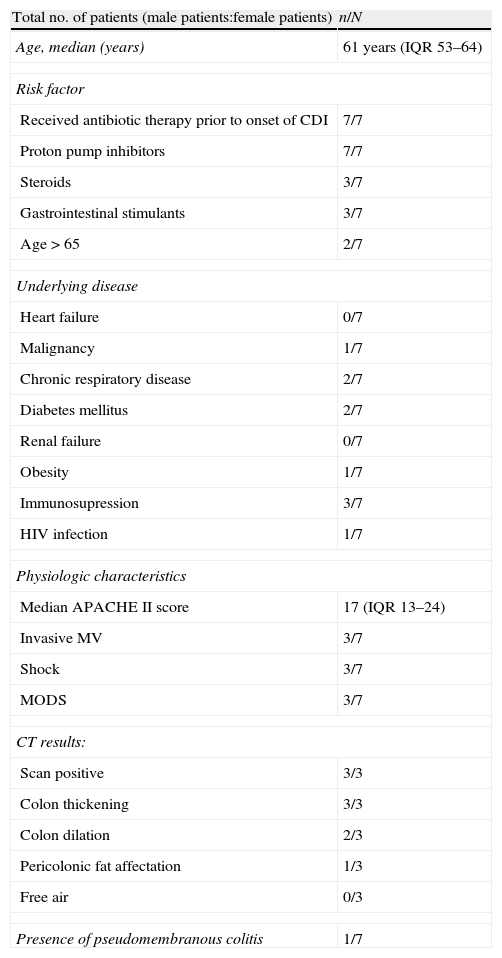
ResultsA total of 1936 adult patients were admitted to the ICU from January 2010 to December 2011. Out of 177 patients with diarrhea >48h, seven (0.36%) acquired CDI in this period (Fig. 1), all of which were diagnosed with Enzyme-Linked ImmunoSorbent Assay (ELISA) and one case was confirmed through immune-chromatography. Hence, the rate of infections per 10,000 bed-days was 3.1. The cumulative incidence rate was 3.6×1000, in two years. The median age was 61 (IQR 53–64). Four patients were men. Two of the seven patients with CDI infection had suffered gastrointestinal pathology characterized by either cancer or Helicobacter pylori infection. Six patients showed some degree of immunosuppression (86%), three secondary to pharmacology due to the treatment of organ transplantation or cystic fibrosis disease, one because of corticoid use, another one due to cancer and the last one caused by AIDS. Additionally, five of the cases were hospitalized (had past medical history of hospitalization) six months prior to CDI infection. None of the patients were from a long stay center. The reason for admission to the ICU was severe respiratory failure in three cases, shock in two and pulmonary transplant surgery also in two cases. None of the patients were admitted for elective surgery. The median time of ICU hospitalization previous to CDI infection was 33 days (IQR 4–19). APACHE II score (Acute Physiology and Chronic Health Evaluation) at ICU admission ranged between 10 and 25 with a median of 17 (IQR 13–24). Risk factors associated with CDI are shown in Table 1. Only two patients were over the age of 65. The median number of antibiotics prescribed per patient was two, the most commonly prescribed antibiotic was carbapenems (four) followed by cephalosporins (two), piperacillin/tazobactam (two) and quinolones or aminopenicillins (one). None of the cases received prior glycopeptides, metronidazole, tygecicline or linezolid. Four out of seven patients received two different antibiotics in the six months prior to the occurrence of CDI. In two cases only one kind of antibiotic was administered for CDI whereas in just one case three different antibiotics were used.
Baseline characteristics of patients with Clostridium difficile-associated diarrhea.
| Total no. of patients (male patients:female patients) | n/N |
| Age, median (years) | 61 years (IQR 53–64) |
| Risk factor | |
| Received antibiotic therapy prior to onset of CDI | 7/7 |
| Proton pump inhibitors | 7/7 |
| Steroids | 3/7 |
| Gastrointestinal stimulants | 3/7 |
| Age>65 | 2/7 |
| Underlying disease | |
| Heart failure | 0/7 |
| Malignancy | 1/7 |
| Chronic respiratory disease | 2/7 |
| Diabetes mellitus | 2/7 |
| Renal failure | 0/7 |
| Obesity | 1/7 |
| Immunosupression | 3/7 |
| HIV infection | 1/7 |
| Physiologic characteristics | |
| Median APACHE II score | 17 (IQR 13–24) |
| Invasive MV | 3/7 |
| Shock | 3/7 |
| MODS | 3/7 |
| CT results: | |
| Scan positive | 3/3 |
| Colon thickening | 3/3 |
| Colon dilation | 2/3 |
| Pericolonic fat affectation | 1/3 |
| Free air | 0/3 |
| Presence of pseudomembranous colitis | 1/7 |
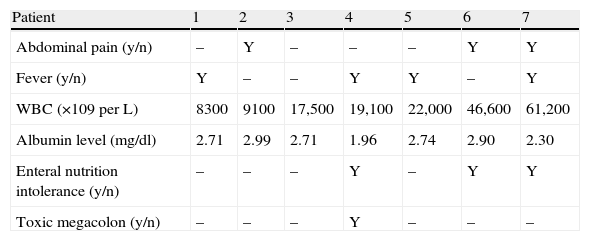
Table 2 summarizes the different clinical manifestations of the CDI. Six out of the seven positive CDI diarrheas lasted less than a week. Five patients showed an increase in white blood cell count above 15,000 (above 60,000 in one). The mean white blood cell count was 26,250 (SD 18,500).
Clinical manifestations in Clostridium difficile infection.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Abdominal pain (y/n) | – | Y | – | – | – | Y | Y |
| Fever (y/n) | Y | – | – | Y | Y | – | Y |
| WBC (×109 per L) | 8300 | 9100 | 17,500 | 19,100 | 22,000 | 46,600 | 61,200 |
| Albumin level (mg/dl) | 2.71 | 2.99 | 2.71 | 1.96 | 2.74 | 2.90 | 2.30 |
| Enteral nutrition intolerance (y/n) | – | – | – | Y | – | Y | Y |
| Toxic megacolon (y/n) | – | – | – | Y | – | – | – |
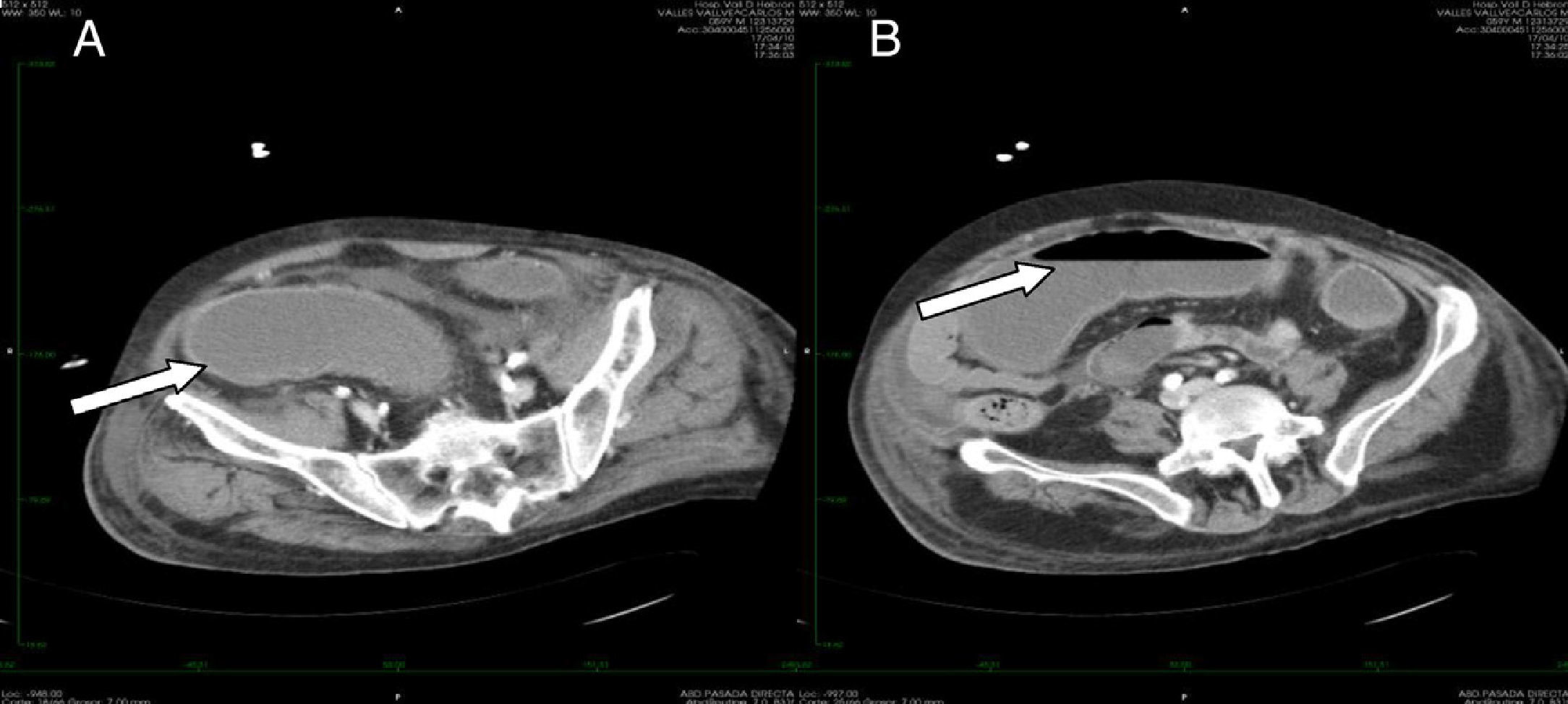
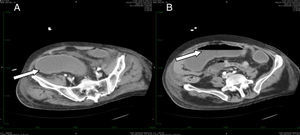
Only one patient had toxic megacolon (14%). The mean albumin level was 2.61 (SD 0.33) mg/dL. Scan tomography was performed in three cases. All the patients in whom tomography was performed showed thickening of the colonic wall. In one case involvement of the pericolonic fat was observed (Fig. 2). Severe sepsis was reported in five cases, (71%) three of whom were in shock and showed multiorgan dysfunction when diagnosed. Three cases had renal impairment with a 50% increase in creatinine levels within the first 24h. No cases of intestinal perforation were documented. All cases were treated with medical therapy and surgery was not required.
The treatment schedule, oral or intravenous, comprised metronidazole in the majority of patients. In two cases a combined treatment was used with oral vancomycin plus intravenous metronidazole. One case (n° 5) received double therapy, rectal vancomycin plus intravenous metronidazole due to the large-scale impairment of the intestinal function. The mean treatment duration was 10 days (range 7–14 days).
Four of our patients presented recurrence of the infection at least once during hospitalization and a second phase of antibiotics was necessary. Additionally, hospitalization was prolonged by a mean of 24 days (SD 17.8) after diagnosis. One of the seven patients who presented infection for C. difficile, died 30 days after discharge from the intensive care unit, secondary to multiple organ failure with limitation of therapeutic effort in the ward.
DiscussionTo the best of our knowledge, this is the first study describing the incidence, general characteristics, risk factors and complications of patients infected with C. difficile in a European medical–surgical intensive care unit outside a period of outbreak. Our findings suggest that the CDI in our ICU is not frequent (<1/100 admissions), but is associated with a significant rate of complications and a prolonged ICU and hospital stay. Only seven of all the patients admitted to the ICU were found to have an infection with C. difficile; the incidence was 0.36%, with a rate of infection of 3.1 per 10,000 bed-days in this two-year period. This incidence is low compared to other studies in the ICU which have reported incidences of up to 4%8 or 8.3 per 10,000 bed-days in a neurocritical ICU in the United Kingdom.9 Antibiotic exposure has been strongly associated with CDI, therefore emphasizing that their rational use may have a positive impact on CDI incidence in the ICU population. However, antibiotics are usually a cornerstone treatment in the ICU due to the high number of infections. In this case, the use of antibiotics like piperacillin/tazobactam when appropriate might be preferred in order to minimize the risk of CDI.8 Moreover, the prevalence of ribotype O27 is low in Spain.10
A striking finding in this study was the low median age; 61 years old (IQR 53–64) compared with the median ages of 74 years old (IQR 68–77) in a surgical ICU11 and 68.9 in a medical ICU.8 This result may be influenced by the fact that the underlying medical conditions (transplant and HIV) usually occur in younger people, but are associated with severe immunosuppression. In fact, the majority of our patients had immunosuppression secondary to their previous condition and the rates were higher than in other studies12–15 possibly due the characteristics of our hospital. Therefore, it seems that immunosuppression played a role in the development of CDI in our ICU. An important element described is the recurrence and complication of CDI. In our series, more than half of our patients (four out of seven) showed deterioration and recurrence of infection, showing a higher rate than the overall figure of 25% reported in the literature.16–19
At the time of diagnosis, six patients had a severe infection according to the score classification proposed by Zar et al.,20 presenting scores of 2 or more. In our study we did not identify differences in the rate of complications or recurrence according to this score. The extra period of mechanical ventilation (MV) after diagnosis of CDI was over twelve days. Additionally, hospitalization was prolonged by a median of 24 days after diagnosis, indicating the impact on the health care resources that CDI can cause. We must bear in mind that measures to reduce complications or recurrences may be associated with significant cost savings in an era of cost containment.21
Some limitations of our study should be acknowledged. The first is its retrospective design. Second, we evaluated only follow-up during the duration of hospitalization in ICU: we cannot comment on long term follow-up, including relapse of diarrhea outside ICU. Third, the small number of cases precludes certain statistical comparisons and may thus lead to a type II error. However, it demonstrates that CDI is unlikely out of an epidemic outbreak. Fourth, the incidence of CDI can be influenced by the diagnostic methods and criteria. Alternative methods such as enzyme glutamate dehydrogenase antigen (GDH) are described in the United Kingdom National Health System guides in 2012, as the best option in combination with enzymatic immunoassay (EIA) tests for CDI detection.22 Therefore, our microbiology laboratory is working in the implementation of this technique. Finally, the prevalence of CDI in Spain, in particular, ribotype 027 is lower than in other countries such as the United Kingdom or the United States of America. As a conclusion, fewer than 1% of patients hospitalized in a medical–surgical ICU in a tertiary referral center in Spain developed CDI, but showed a high risk of recurrence/complications associated with prolonged ICU stay. In addition, our results suggest that immunosuppression plays an important role in patients younger than 65 years old. These differences must be considered when generalizing the findings from North America to Europe.
Funding supportThis study was supported in part by an unrestricted grant from Astellas and CIBERES Network (Instituto de Salud Carlos III), Spain.
Conflict of interestDr Rello is a consultant for Astellas. The rest of the authors have no conflict of interest to declare.
The Clinical Research/Innovation in Pneumonia & Sepsis group (CRIPS).
| Roser Anglès Coll |
| Joan Balcells Ramirez |
| Elisabet Gallart Vive |
| Rosa Maria Gracia Gozalo |
| César Laborda Soriano |
| Joan Ramon Masclans Enviz |
| Mercedes Palomar Martínez |
| Marcos Pérez Carrasco |
| Teresa Pont Castellana |
| Isabel Porta Pampalona |
| Maria Alba Riera Badia |
| Judith Sacanell Lacasa |
| Bárbara Borgatta |
| Melisa Fernandez Pinto |
| Alejandra García Roche |
| Simone Gattarello |
| Elisabeth Papiol Gallofré |
| Ana Parra Castillo |
| Purificación Perez Terán |
| Laura Ruano Burgos |
| Anna Sánchez Corral |
| Jessica Souto Higueras. |
The Clinical Research/Innovation in Pneumonia & Sepsis group (CRIPS). List of CRIPS Investigators in detailed at Appendix A.