Hemodynamic monitoring offers valuable information on cardiovascular performance in the critically ill, and has become a fundamental tool in the diagnostic approach and in the therapy guidance of those patients presenting with tissue hypoperfusion. From introduction of the pulmonary artery catheter to the latest less invasive technologies, hemodynamic monitoring has been surrounded by many questions regarding its usefulness and its ultimate impact on patient prognosis. The Cardiological Intensive Care and CPR Working Group (GTCIC-RCP) of the Spanish Society of Intensive Care and Coronary Units (SEMICYUC) has recently impulsed the development of an updating series in hemodynamic monitoring. Now, a final series of recommendations are presented in order to analyze essential issues in hemodynamics, with the purpose of becoming a useful tool for residents and critical care practitioners involved in the daily management of critically ill patients.
La monitorización hemodinámica nos permite obtener información sobre el funcionalismo cardiovascular del paciente crítico, por lo que constituye una pieza fundamental en la aproximación diagnóstica y en la guía terapéutica del paciente con hipoperfusión tisular. Desde la aparición del catéter de arteria pulmonar hasta el desarrollo reciente de tecnologías mínimamente invasivas, la monitorización hemodinámica se ha rodeado de interrogantes en cuanto a su utilidad y su impacto final sobre el pronóstico de nuestros pacientes. El Grupo de Trabajo de Cuidados Intensivos Cardiológicos y RCP (GTCIC y RCP) de la SEMICYUC ha impulsado recientemente la realización de la serie de «Puesta al día en monitorización hemodinámica» y ha querido además desarrollar unas recomendaciones que pretenden analizar cuestiones fundamentales en la valoración cardiovascular del paciente crítico, con la intención final de ser una herramienta útil para residentes, intensivistas y otros profesionales que afrontan el manejo diario de estos pacientes.
The study of cardiovascular function is a fundamental aspect in critical patient care. Hemodynamic monitoring allows us to obtain information on cardiocirculatory physiopathology that will help in establishing a diagnosis and in orienting patient management in situations of hemodynamic instability. The pulmonary artery catheter (PAC) has been the most widely used technique since its introduction over 40 years ago. Although its contribution to the in-depth knowledge of cardiovascular function is undeniable, the use of the PAC has decreased because of controversy regarding its indications and its limitations. This in turn has intensified the search for new monitoring methods. At present, a range of technological advances offer us many systems that can be used to explore the most important aspects of hemodynamics (preload, ventricular function, hemodynamic resuscitation targets or goals, etc.). In the same way as PAC, these systems have advantages and limitations that must be known before they are used in clinical practice.1,2 Echocardiography, while not a continuous monitoring system in the strict sense, offers anatomical and functional information that can be enormously useful for the hemodynamic assessment of the critically ill patient.3,4
The Cardiological Intensive Care and CPR Working Group (GTCIC-RCP) of the Spanish Society of Intensive Care and Coronary Units (SEMICYUC) has impulsed an “Update in hemodynamic monitoring” series,5 composed of different chapters that offer a review of the most relevant aspects in the field. The series has recently been published in this journal. On the other hand, it has been the aim of the Working Group to develop and publish a series of recommendations on specific issues in hemodynamic monitoring and resuscitation, essentially based on the contents of the mentioned chapters and on the respective supporting literature searches. The objective of these recommendations is to afford a guide that is useful in clinical practice. The concrete issues or questions raised are the following: (1) What are the objectives of hemodynamic resuscitation? (2) How do we evaluate the factors determining cardiac yield? (3) Initial basic hemodynamic monitoring. Continuous hemodynamic monitoring. When and with what? (4) What role does echocardiography play in hemodynamic resuscitation? (5) What evidence is there of the usefulness of hemodynamic monitoring in the critical patient? Each of these five issues was addressed by a group composed of several of the professionals who participated in the drafting of the recommendations–all of them experts in hemodynamic monitoring and/or echocardiography in the critical patient. The final document was discussed, and consensus was established among all the participants. In addition, the document was forwarded to the members of the Working Group for due assessment and approval. The document has received the scientific endorsement of the SEMICYUC.
The level of recommendation and the quality of the evidence have been defined according to the criteria of the GRADE system,6 which scores evidence as high (grade A), moderate (grade B), low (grade C) or very low (grade D), according to factors that include the methodology of the studies and the consistency and precision of the results, among other aspects. The GRADE system classifies the recommendations as strong (L1) or weak (L2), on the basis of factors such as the balance between benefit and risk, the quality of the evidence, the costs, and resource utilization.
Definition of the scenario. Type of patients and professionals to whom the recommendations are addressedThe recommendations refer to patients with systemic hypoperfusion, independently of the underlying cause, and run parallel to the specific measures applicable to each disease condition (e.g., septic drainage, coronary vessel opening, and fibrinolysis in cases of pulmonary thromboembolism). The recommendations are addressed to all intensivists, residents in training and other professionals who care for critically ill patients in their daily practice.
Question 1. What are the objectives of hemodynamic resuscitation?An obligate first step in the initial evaluation of the critical patient is to determine tissue perfusion status. The presence and/or persistence of cellular oxygenation problems is a key factor in the development of organic damage, multiorgan failure and, eventually, patient death. What we commonly call hemodynamic instability usually refers to the presence of clinical signs suggestive of hypoperfusion (sensory alterations, poor capillary filling, etc.), and especially to arterial hypotension. In this regard, in recent years the evidence that the presence of hypoperfusion even in the absence of hypotension and/or of the abovementioned clinical signs–a condition known as occult or compensated shock–is also associated to significantly increased morbidity-mortality7 has led to intensified effort to detect such situations of hypoperfusion.
In the critical patient, we speak of shock or cardiovascular insufficiency when there is evidence of tissue hypoperfusion. The incapacity to maintain adequate tissue perfusion causes an increase in peripheral (microcirculatory) oxygen extraction, together with the intervention of anaerobic pathways in order to maintain cellular respiration. In clinical practice, we refer to shock when the venous oxygen levels are seen to decrease and/or the serum lactate concentrations rise, beyond the simple presence or not of arterial hypotension.8
The main determinants of oxygen supply to the tissues are (a) perfusion pressure and (b) global oxygen transport. Hemodynamic resuscitation, based on the manipulation of these pressure and flow variables, seeks to restore the balance between oxygen transport (DO2) and consumption (VO2) by the tissues, with the consequent reversion of anaerobiosis. The correction of dysoxia should be achieved as quickly as possible, since its duration conditions organ failure, with direct consequences in relation to the patient prognosis.
Arterial pressureWe use mean arterial pressure (MAP) as an estimation of tissue perfusion pressure. Since from the physiological perspective the vascular system loses its autoregulating capacity at MAP values of less than 60–65mmHg, most studies have proposed a target MAP of 65mmHg. In septic patients, for example, a MAP cutoff value of 65mmHg during the first 48h of admission was found to be the value that best discriminated between survivors and non-survivors.9 Furthermore, the achievement of higher MAP values did not result in better outcomes. However, it should be mentioned that recent capillary videomicroscopic studies have shown that the microcirculation of certain patients may indeed benefit from higher MAP values,10 thus suggesting the possibility of individualizing MAP according to its effect upon the microcirculation. However, we lack prospective studies on the prognostic impact of the individualized optimization of MAP according to microcirculatory parameters.
Two special situations require mention in the management of MAP in acute critical illness: (a) uncontrollable bleeding in trauma patients and (b) patients with severe traumatic brain injury in the absence of systemic bleeding.11 In the first situation, it is advisable to maintain a MAP level of 40mmHg until surgical control of the bleeding is achieved. In situations of severe traumatic brain injury with neurological impairment but no evidence of systemic bleeding, since we do not know the brain perfusion pressure, it is advisable to maintain a MAP level of 90mmHg. Once intracranial pressure is monitored, we adjust the MAP level to ensure brain perfusion.12
Oxygen transport variablesIn addition, to ensure correct tissue perfusion, we must adapt DO2 to the metabolic needs. Our aim in this regard is to increase DO2. Although hemodynamic resuscitation is fundamentally based on optimization of the flow variables (ensuring arterial O2 content), as a general rule we do not seek any predetermined values but rather the adaptation of these variables to secure normalization of the parameters that reflect the global oxygen supply-consumption balance. Accordingly, after discarding or correcting arterial hypoxemia, it does not seem logical to seek concrete values referred to DO2, cardiac output (CO) or hemoglobin, but to adjust them according to the global condition of tissue oxygenation.
Special mention should be made of the resuscitation of high surgical risk patients–a population in which there is enough evidence to recommend the use of certain levels of DO2 (>600mlO2/min/m2) as a target for hemodynamic optimization before, during and after surgery.13 However, it seems reasonable to assume that the use of variables which inform us about the balance between DO2 and VO2 offers advantages compared with the isolated use of DO2 variables, with a view to avoiding both over- and under-resuscitation (with the problems this entails).
Global hypoperfusion markersAs we have mentioned, beyond the achievement of predetermined DO2 or flow values, hemodynamic resuscitation seeks to normalize the global perfusion markers. In clinical practice we fundamentally have two extremely useful variables for this purpose: venous oxygen saturations and the lactate levels.
Venous oxygen saturationsMixed venous oxygen saturation (SvO2), recorded in the pulmonary artery, is probably the best indicator of the adequacy of DO2. In different critical situations, central venous oxygen saturation (SvcO2), recorded in the right atrium, has shown good correlation to SvO2 (though with over-estimations of about 5%), as well as consistent parallelism with changes in the latter parameter.14 A decrease in CO and/or an increase in the metabolic needs leads to a compensatory increase in oxygen extraction, with the consequent lowering of the venous saturations. This decrease occurs early, and can even precede the rise in serum lactate concentration. The incorporation of venous saturation as an end metabolic target in the resuscitation process has been shown to benefit the prognosis of different critical patient populations.15 However, in certain situations of distributive shock, the presence of SvcO2 elevation has also been associated with increased mortality.16 This could be attributed to different mechanisms such as shunt phenomena, heterogeneous flow, or alterations in oxygen extraction. It is therefore essential to know the limitations of this variable and, within an adequate clinical context, to make use of other parameters that inform us about the tissue perfusion status of the patient.
LactateIn general, elevation of the blood lactate concentration indicates the presence of tissue hypoxia and anaerobic metabolism. The magnitude of this increase in lactate levels has been directly correlated to the prognosis of the patient with acute critical disease.17 Regarding its usefulness in guiding the resuscitation process, the monitoring of lactate clearance in response to treatment interventions has not been found to be inferior to resuscitation guided by SvcO2.18
In situations of tissue hypoxia, and in addition to the production of lactate, anion elevations secondary to anaerobiosis are observed, as well as defects in CO2 clearance from the body. In this respect, the determination of standard base excess and the CO2 arteriovenous difference (P(v−a)CO2) may be of help in evaluating the global tissue oxygenation status. Although the prognostic usefulness of alterations in the initial standard base excess values has been shown to be similar to that of lactate, their evolution over time is influenced by a range of factors other than cellular hypoxia. The use of standard base excess is therefore not recommended as an independent parameter in guiding resuscitation.19 Regarding P(v−a)CO2 (whether central or mixed), different studies have found its value to be inversely correlated to the cardiac index. Levels of P(v−a)CO2>6mmHg have been shown to be useful in detecting persistent hypoperfusion despite the normalization of SvcO220 – though the incorporation of this variable to resuscitation algorithms has not been evaluated to date.
Regional circulation and/or microcirculation markersAlthough in recent years there has been growing evidence of the prognostic value of different microcirculatory parameters, no studies have evaluated their usefulness as targets or goals in the hemodynamic resuscitation process.8 Thus, although very promising, it is presently not possible to recommend the incorporation of these markers to clinical practice as guiding parameters in the reversion of shock.
- 1.
Shock is a life-threatening situation characterized by an alteration in DO2and/or in the capacity to use oxygen, giving rise to tissue dysoxia.
- 2.
The presence of arterial hypotension (MAP<65mmHg) is not a prerequisite for shock.
- 3.
In the presence of a suggestive clinical condition, the alteration of a tissue perfusion marker (lactate and/or venous oxygen saturation) serves to define shock, whether accompanied by arterial hypotension or not.
- 1.
Hemodynamic resuscitation measures should be adopted immediately, and the established management goals should be reached as quickly as possible (ideally in the first 6h). (L1; B)
- 2.
The first step in hemodynamic resuscitation is to quickly reach and maintain minimum acceptable tissue perfusion pressure, defined as MAP≥65mmHg. (L1; B)
- 3.
Once perfusion pressure has been secured, we must correct the tissue dysoxia, defined as the restoration of normal global hypoxia tissue marker values: SvcO2≥70% (or SvO2≥65%), and/or normalize the lactate levels. (L1; A)
- 4.
The guiding of hemodynamic resuscitation based on the monitoring of lactate clearance has not been shown to be inferior to the monitoring of SvcO2. (L1; B)
- 5.
In high-risk surgical patients we can aim to optimize DO2to values ≥600ml O2/min/m2in order to avoid tissue hypoperfusion. (L1; A)
- 6.
In situations of SvcO2≥70%, a high arteriovenous CO2gradient may indicate the persistence of hypoperfusion in certain territories; we thus could aim to optimize DO2to P(v−a)CO2values <6mmHg. (L2; B)
- 7.
At present, the use of technologies for evaluating the microcirculation or regional circulation has not been explored in the context of the resuscitation process; its routine incorporation to clinical practice is therefore not recommended. (L1; B)
- 8.
Integration of the different objectives or goals in early resuscitation algorithms or bundles will afford improved patient prognosis. (L1; A)
Cardiac output (CO) is considered a marker or evaluator of global cardiac function. It moreover also offers data on the cause of shock and of organ failure. CO is therefore a fundamental parameter in the hemodynamic evaluation of the critical patient. Nevertheless, the value of CO must be integrated with other hemodynamic variables (measures of preload, contractility, postload), biological signs and tissue oxygenation parameters, in order to obtain full information with which to guide our treatment decisions.
There is little evidence in support of the systematic monitoring of CO in critical patients.1,2,11,21,22 In many cases the situation of hemodynamic instability can be resolved through more simple assessment and monitoring (physical examination, diuresis, blood pressure [BP], estimation of preload and volume response parameters, etc.), without having to resort to additional measures or procedures. However, some patients continue to show signs of hypoperfusion 3–6h after the start of treatment. In these cases it may be useful to use more exhaustive monitoring producing more detailed information on cardiovascular function, and which may allow us to understand the reason for initial management failure and thus more adequately guide the resuscitation measures. Such monitoring, which must include CO, should be introduced early once the patient is found to be refractory to initial treatment.2,23
In patients with severe initial hypoxemia and suspected heart failure, or in patients with complex cardiopulmonary problems, it seems reasonable to monitor CO from earlier stages, since the initial resuscitation measures (volume expansion, mechanical ventilation, etc.) can worsen cardiac and respiratory function. On the other hand, in cardiogenic shock, correct and early monitoring of CO is particularly important not only in terms of the diagnosis but also in guiding posterior treatment.1,2,21
Lastly, and as commented above, when dealing with high-risk surgical patients, the optimization of CO during the operation and in the hours immediately after surgery has a direct impact upon the patient prognosis.13
Many methods are currently available for monitoring CO, with important differences among them. Information can be found regarding the existing devices in the chapter dedicated to the estimation of CO of the “Update in hemodynamic monitoring” series.23 These monitoring systems can be classified according to their invasiveness. In this regard we have invasive devices (PAC), semi-invasive systems (transpulmonary thermodilution, lithium dilution, pulse wave analysis, esophageal Doppler ultrasound, etc.) and noninvasive techniques (ultrasound, bioreactance, Doppler technology, etc.).
Cardiac output obtained by thermodilution with the PAC has been regarded as the gold standard for the measurement of CO since its introduction in 1970.24,25 Most of the methods for estimating CO have been evaluated by comparison with the data afforded by thermodilution with the PAC, despite the fact that this technique has limitations and might not be the best comparator in this sense. The PAC moreover allows us to obtain relevant hemodynamic parameters such as pulmonary artery pressure (PAP), pulmonary artery occlusion (wedge) pressure (PAOP), and parameters referred to DO2 and VO2. However, the use of PAC has decreased because of its invasiveness and controversy regarding its possible complications and its indications.25,26
The semi-invasive methods for estimating CO in turn include transpulmonary thermodilution, lithium dilution and pulse wave analysis.
Transpulmonary thermodilution is a variant of the thermodilution method in which injection of the saline bolus is made through a central venous catheter, and the temperature change is detected by a sensor placed in an artery (femoral or axillary)–the CO value being calculated from a modification of the Stewart-Hamilton equation. Its use is subject to debate in situations characterized by important body temperature variations, the use of extracorporeal filtration systems, and intracardiac shunts. A number of studies have validated this technique in different critical patient populations.2,23,27,28
Lithium dilution is based on the use of lithium chloride as a tracer for calculating CO. Calibration is performed by injecting a lithium chloride bolus into a central or peripheral venous line, and an electrode placed in an artery detects the lithium concentration in arterial blood. Cardiac output is calculated using the area under the concentration–time curve (AUC). The technique is contraindicated in patients receiving treatment with lithium or non-depolarizing muscle relaxants, and in patients with intracardiac shunts. Lithium dilution has also been found to be useful in different studies conducted in Intensive Care Units (ICUs) and in the surgical setting.29
Many devices offer us the possibility of obtaining CO on a continuous basis using arterial pulse wave analysis. The systems currently found on the market are PiCCO® (Pulsion), PulseCO® (LiDCO), Modelflow® (TNO/BMI), Most Care® (Vygon) and FloTrac®/Vigileo (Edwards Lifesciences). They differ in the way of transforming the information provided by the blood pressure wave profile in beat-to-beat stroke volume (SV), in the algorithms used in each case, in the form of calibration (since some require manual calibration while others do not), the arterial catheterization site, the parameters analyzed, and the accuracy with which they are able to determine CO.30–33 Most of the techniques at the same time generate continuous information referred to preload, postload and contractility, and also allow calculation of percentage pulse pressure variation (PPV) or stroke volume variation (SVV), used to guide fluid therapy and analyze its effects. The main importance of these devices is that they allow us to calculate CO in a continuous and scantly invasive manner. On the other hand, there are resuscitation protocols based on hemodynamic parameters obtained with these systems that have been shown to reduce morbidity among postsurgical and critical patients.32,34 Their main disadvantage is that they lose precision and reliability in situations characterized by important changes in volemia and changes in vascular tone – these aspects being particularly important in the case of systems without external calibration.
Esophageal Doppler ultrasound estimates blood flow in the descending aorta and allows us to calculate SV. The limitations of this technique include operator dependency and the fact that the probe is poorly tolerated in non-ventilated patients.2,23,35 It should be noted that this system has been shown to be useful for fluid optimization in high-risk surgical patients, resulting in shorter hospital stay and fewer postoperative complications.
Among the noninvasive methods used to monitor CO, mention should be made of bioreactance, transthoracic Doppler ultrasound and echocardiography.
Bioreactance, used by the NICOM® system (Cheetah Medical), is based on analysis of the phase shift that takes place in the high-frequency electric impulses targeted to the thorax, produced by changes in blood volume. Promising results have been obtained in heart surgery patients, though there are still not enough studies on its usefulness and reliability in broader critical patient populations.2,23,36 Transthoracic Doppler ultrasound in turn consists of applying a blind Doppler probe over different thoracic areas to measure flow at different levels of the cardiovascular system. This technique has a fast learning curve, with no need for calibration, but is operator dependent. The most widely used system, and which is supported by the largest number of studies in the literature, is the USCOM® monitor (Pty Ltd.). Despite its purported advantages, however, the literature comparing its utilization with the PAC in the intensive care setting is scarce.2,23,37
Echocardiography allows us to determine CO in a noninvasive (transthoracic echocardiography, TTE) or minimally invasive manner (transesophageal echocardiography, TEE), and moreover affords abundant hemodynamic information. Despite its multiple applications and the rapid spread of its use in the ICU, the technique requires adequate training in order to guarantee the quality and reliability of the measurements.3,4
The choice of one technique or the other in estimating CO is influenced by a number of factors, some of which are related to the device itself (e.g., its advantages and limitations), while others may be of an institutional nature or related to the patient. On the other hand, it should be taken into account that the use of a less invasive system may be preferable if it is able to quickly and easily offer us information, even if such information is slightly less exact–particularly in situations in which rapid assessment of the patient condition is required. In turn, the comparability of the techniques for the monitoring of changes and trends in CO may be more relevant in clinical practice than the degree of concordance or agreement among the absolute values. In many cases, the choice of hemodynamic monitoring device depends not only on the technique for estimating CO but also on additional parameters provided by the system, the severity of the patient condition, and the etiology underlying shock.1,21,22 The indication and type of continuous monitoring will be addressed in another section of these recommendations.
Estimation of preload and cardiovascular response to volume expansionVolume expansion constitutes the first line therapy in most situations of hemodynamic instability, despite the fact that only 50% of the patients admitted to the ICU respond to fluid administration by increasing SV and CO.38–40 Recent data suggest that resuscitation with early and “aggressive” volume administration can limit or revert tissue hypoxia, progression to organ failure, and improve the prognosis.15,41 On the other hand, a clear association has been found between the accumulated water balance and mortality among critical patients.42 These findings underscore how important it is for the hemodynamic parameters proposed in deciding volume administration to be able to identify those patients who will benefit from volume expansion by increasing SV (responders) and those who will not (non-responders)–with a view to avoiding useless and potentially harmful treatments.
The parameters traditionally used to decide fluid administration are the preload estimators known as static parameters: filling pressures (central venous pressure [CVP] and PAOP), and the volumes and areas (global end-diastolic volume, right ventricle end-diastolic volume and left ventricle end-diastolic area). Central venous pressure, in particular, and also PAOP are still used in daily practice as routine tools for deciding when to administer volume. In this respect, a recently published German registry has shown that over 92% of all intensivists and anesthetists use CVP for monitoring patient resuscitation following heart surgery.43 Likewise, the guidelines of the Surviving Sepsis Campaign44 recommend the use of CVP within an early resuscitation protocol, based on the results of the study of Rivers et al.15 However, failure of these parameters to predict volume response has been evidenced by many studies.40,45–47 No significant differences have been found in the baseline CVP or PAOP values between patients who respond and patients who do not respond to volume expansion. Other preload estimators such as the end-diastolic area obtained by echocardiography or the volumes obtained with PAC or PiCCO® likewise have not been identified as good predictors of cardiovascular response to volume administration. Nevertheless, it is accepted that very low CVP or PAOP values (<5mmHg) could be predictive of a favorable response.47 The reasons why the static parameters are not reliable estimators of volume response include technical measurement aspects and the possibility of inadequate estimation of transmural pressures secondary to the effect of positive end-expiratory pressure (PEEP), abdominal hypertension, or alterations of ventricular distensibility. Moreover, from the purely physiological point of view, the knowledge of a concrete preload value does not allow us to know the preload reserve of a ventricle, due on one hand to the fact that the Frank–Starling curve or ventricular function curve is curvilinear (ascending segment: dependence on preload; descending segment: independence of preload), and on the other hand to the fact that there is no single ventricular function curve but rather a whole family of curves that relate preload and SV according to the changes in postload or contractility. For this reason, and for a given preload value, the increase in SV will depend on the part of the Frank–Starling curve on which both ventricles are operating.
The use of dynamic parameters based on a “functional” hemodynamic evaluation has recently been proposed. This approach consists of introducing a reversible and transient change in cardiac preload, followed by observation of the effects upon SV or CO. The most widely studied dynamic parameters in patients subjected to mechanical ventilation are those obtained from the analysis of the changes in SV and blood pressure during a mechanical respiratory cycle, based on heart–lung interaction.38,39,47–51 The main hemodynamic effect of an increase in intrathoracic pressure is a decrease in venous return and in right ventricle ejection, which may give rise to a drop in preload and in SV of the left ventricle. The magnitude of the respiratory changes of SV depends on the position of both ventricles on the ventricular function curve. Thus, when both ventricles are operating on the ascending part of the curve (the zone of preload dependence), a change in preload induced by positive intrathoracic pressure gives rise to a significant change in SV and in pulse pressure (since the latter is directly proportional to SV). Many studies have found that PPV values ≥13% [(maximum pulse pressure−minimum pulse pressure)/(maximum pulse pressure+minimum pulse pressure)/2×100] and SVV≥10% [(SV maximum−SV minimum)/(SV maximum+SV minimum)/2×100] predict the response to volume expansion with high sensitivity and specificity in different critical patient populations. Furthermore, it repeatedly has been shown that the dynamic parameters are better predictors than the static parameters. In this sense, a recent systematic review of 29 studies involving 685 patients referred to the predictive capacity of dynamic parameters derived from the blood pressure curve showed the areas under the curve (ROC) for PPV and SVV to be 0.94 and 0.84, respectively, while in comparison the areas of the static parameters were 0.55 for CVP; 0.56 for the indexed global end-diastolic volume; and 0.64 for the left ventricle end-diastolic area.50 Other methods based on the same physiological concept, such as the variation in aortic flow velocity as determined by esophageal Doppler and the variation in peak velocity or the velocity–time integral of aortic flow determined by echocardiography, are also useful for predicting the response to volume expansion. Analysis of the amplitude of the plethysmographic signal in pulsoxymetry and the plethysmographic variability index has even been proposed.52–55 Likewise, other indices based on heart–lung interaction, such as variations of the superior and inferior vena cava, have been validated as reliable and precise predictors in patients subjected to mechanical ventilation.56,57
Despite the undeniable usefulness of these parameters in the evaluation of patient response to volume expansion, it is important to know their limitations. Firstly, the patient must be subjected to controlled mechanical ventilation, without spontaneous respiratory activity. On the other hand, these parameters have not been validated in the presence of cardiac arrhythmias, and their predictive value is lower in patients ventilated with tidal volumes below 8ml/kg.48,58
Raising the legs of the patient can also be useful in predicting the response to volume administration. Raising the legs to an angle of 45° with respect to the bed for at least 1min reversibly produces the cardiovascular effects of the administration of a volume of 300ml. The hemodynamic response to this maneuver–regarded as a test rather than a treatment–can be used in patients subjected to mechanical ventilation and with spontaneous breathing. Its predictive capacity has also been demonstrated in patients with arrhythmias. An increase in SV>10–12% estimated by echocardiography, PiCCO® system, esophageal Doppler or other devices during the maneuver is able to predict an increase in SV>15% following the administration of volume, with high sensitivity and specificity. A recent metaanalysis including the results of 8 studies confirms the excellent value of the leg-raising maneuver in predicting volume response in critical patients, with an area under the curve (ROC) of 0.95.59,60
In patients with spontaneous breathing, some studies have examined different maneuvers that could be useful in clinical practice, though confirmatory studies are still needed: the variation in arterial pulse pressure during the Valsalva maneuver, the variation in blood pressure with end-expiratory occlusion maneuvering, and the variation in right atrial pressure.40
Lastly, the evaluation of CO response to the administration of a certain volume of CO (fluid challenge) has been used for many years in clinical practice to assess the efficacy and safety of volume expansion therapy. This maneuver should be used in cases in which none of the above described parameters can be evaluated.61
How do we evaluate contractility and postload in critical patients?Contractility can be defined as the capacity of the heart to generate external work independently of the loading conditions. Cardiac dysfunction is mainly caused by failure of the ventricular pump, which is unable to afford sufficient hydraulic energy to maintain effective circulation. Most of the indices used to evaluate contractility at experimental or clinical level are partially dependent upon preload or postload–a fact that may complicate the evaluations. The slope of the end-systolic ventricular pressure–volume ratio, known as end-systolic elastance (Ees), is regarded as the reference contractility index, due to its relative independence of the loading conditions and its sensitivity to changes in inotropism. The generation of complete systolic and diastolic data requires invasive left ventricle instrumentation for the simultaneous estimation of measures of pressure and volume, which complicates determination,62–65 though echocardiography could partially facilitate the recording of Ees at the patient bedside in a noninvasive manner. However, it is not possible to determine Ees in unstable patients through occlusion of the inferior vena cava and the administration of vasodilator or vasoactive drugs, since the pre- and postload alterations could trigger a life-threatening situation. A number of methods have been proposed for recording Ees through the estimation of a single heart beat, without the need to modify the loading conditions, though there is not enough clinical evidence in critical patients to apply such methods on a systematic basis.66
Traditionally, the parameter most widely used in the ICU for assessing ventricular function has been CO. However, it must be remembered that this hemodynamic parameter depends not only on contractility but also on preload and postload. The joint utilization of CO and filling pressures (CVP, PAOP) yields a series of hemodynamic patterns or references that can be very useful in clinical practice. In this sense, severe left heart failure is characterized by low CO and elevated PAOP. The most characteristic example of the usefulness of these patterns is the Forrester classification of acute myocardial infarction.67 However, the data obtained from the combination of CO and filling pressures does not afford sufficient information to know the mechanisms (alteration of ventricular distensibility, decreased contractility, increased postload, etc.) underlying the clinical and hemodynamic findings of the patient. Another parameter classically used in the ICU for the evaluation of contractility at the patient bedside is stroke work (SW), defined as the product of SV and the difference between MAP and PAOP. This index also depends on preload, but is independent of postload; as a result, the observation of low SW could serve to identify diminished contractile function in situations in which volume expansion has been adequate. Many studies in the fields of heart failure, ischemic heart disease and sepsis have used this parameter as an indicator of altered contractility, and it has even been related to patient prognosis.64,68
The heart may be regarded as a mechanical pump capable of generating hydraulic energy. Accordingly, the pumping capacity of the heart can be expressed as cardiac power (CP), defined as the product of the flow and pressure generated by the heart. Thus, CP is the product of CO and MAP determined simultaneously. Cardiac power is not significantly influenced by postload, though it can change with variations in preload. For this reason, we must make sure that the heart of the patient is not dependent upon preload before affirming that CP–and therefore heart pumping function–is diminished. In recent years, the maximum CP recorded after pharmacological stimulation or exercise has been used to evaluate patients with acute or chronic heart failure. In this regard, different studies have shown that the maximum CP reached is a more powerful predictor of the evolution of patients with chronic heart failure than VO2, CO or left ventricular ejection fraction (LVEF).69 These results have been confirmed in critical patients in situations of cardiogenic shock. Tan and Littler70 found the hemodynamic evaluation of cardiac reserve (difference between maximum CP maxima and basal CP) through dobutamine stimulation to clearly distinguish between survivors and non-survivors. Patients with maximum CP<1W (normal basal value for the healthy adult) or SW index <0.25j/m2 died, while all those with higher values survived for over one year. A posterior confirmation of the prognostic value of CP has recently been published in the same population of patients.71 For the time being, no data have been published referred to broader critical patient populations.
Echocardiography plays a key role in the evaluation of contractility, since it offers us a range of parameters that can be useful in the ICU for assessing contractility. The left ventricular ejection fraction is the parameter most widely used to evaluate contractility in patients with heart disease.3,72,73 In this regard, LVEF is considered fundamental in patients with heart failure not only because of its prognostic importance (the lower the LVEF, the poorer the patient prognosis) but also because most studies select patients based on their LVEF.72 At the time of its interpretation and evaluation in critical patients, we must take into account that LVEF is also dependent upon preload, heart rate and, fundamentally, postload. This dependence would be particularly significant in situations characterized by an important increase or decrease in postload, as occurs in sepsis. Furthermore, echocardiography offers us other parameters of known usefulness in the evaluation of contractility, such as the Tei index, dP/dt max, systolic tricuspid annular displacement, and the maximum velocity of the S-wave in mitral or tricuspid tissue Doppler imaging (TDI), which show greater or lesser sensitivity to changes in inotropism and loading conditions.74
At present, the new monitoring systems offer other contractility-estimating parameters such as the cardiac function index and global ejection fraction, obtained by transpulmonary thermodilution, though there are still not enough studies to allow us to recommend their use on a routine basis. Even the measurement of natriuretic peptides could play a role in the noninvasive assessment of ventricular dysfunction and in the evaluation of left ventricle filling pressures–though the existing data are not yet conclusive.64
Postload, defined as the “load” or opposition which the heart must overcome in order to expel its stroke volume, is an important determinant of CO for a given set of contractility and preload conditions. Although aortic pressure is one of the main components of ventricular postload, the parameter best able to estimate the latter remains the subject of debate. A form of assessment could be the estimation of systolic ventricular wall stress according to the equation of Laplace. Echocardiography can facilitate the determination of this parameter.
The estimation of postload in the ICU has been a traditional practice, despite its limitations, based on the determination of systemic and pulmonary vascular resistances using PAC. The current technological advances allow us to use other devices, including ultrasound, to obtain this same parameter in a less invasive manner.
It has been suggested that the ratio between PPV and SVV, called dynamic elastance by Monge Garcia et al.,75 could be of help in estimating vascular tone and, consequently, might be useful in hemodynamic resuscitation algorithms–though there is still not enough literature support in this respect.63,64,75,76
In conclusion, the new technological resources allow the combination of different hemodynamic monitoring techniques, affording information or a different approach to the same problems, with more precise evaluation of the contractility and postload alterations that may be found in critical patients.
- 1.
Routine and systematic monitoring of CO is not recommended in patients with hemodynamic instability. (L1; B)
- 1.
The measures of preload, such as the intravascular pressures (CVP or PAOP), volumes or areas, are unable to offer a reliable prediction of the response to volume expansion (L1; B), though low preload values (CVP, PAOP<5mmHg) can be associated to a positive response to the administration of volume. (L2; C)
- 2.
SVV or its related parameters (pulse pressure variation, aortic flow variation, variation of peak aortic flow velocity, etc.) have been found to be good predictors of volume response in critical patients subjected to controlled mechanical ventilation, without spontaneous breathing effort, and with sinus node rhythm (L1; A). The respiratory variation of the diameter of the inferior vena cava and the collapsibility index of the superior vena cava allow us to predict volume response in the same population of patients. (L1; B)
- 3.
The passive leg raising maneuver (monitored by pulse wave profile, echocardiography or esophageal Doppler) allows very reliable identification of those patients who respond to the administration of fluids. The predictive capacity of this maneuver is not affected in cases of atrial fibrillation, low tidal volumes or spontaneous breathing. (L1; B)
- 4.
A fluid challenge test is recommended in clinical situations in which the static and dynamic predictive parameters cannot be used. (L1; C)
- 1.
Despite its relative dependence upon the loading conditions, LVEF estimated by echocardiography is the fundamental parameter for estimating contractility in clinical practice. (L1; C)
- 2.
The parameters obtained by PAC (SW, vascular resistance, CO and filling pressures, among others) and other echocardiographic parameters are useful for estimating contractility/postload at the patient bedside. (L2; B)
- 3.
The information obtained with the new monitoring systems and with the determination of natriuretic peptides could allow the assessment of contractility, though confirmatory studies are needed. (L2; C)
Hemodynamic monitoring aims to be the support and guide to the global tissue O2 supply optimization process, based on the premise that detection, knowledge and understanding of the physiopathological disorders of critical illness must result in better patient treatment and recovery. It is important to stress that no hemodynamic monitoring system in itself is able to improve the prognosis. In order to secure clinical benefit, (1) the data obtained from the monitoring system must be sufficiently precise to be able to influence therapeutic decision making; (2) the data must be clinically relevant to the patient; and (3) the treatment provided, guided by interpretation of the data obtained, must have a favorable impact upon the prognosis of the patient.
It should be mentioned that the hemodynamic monitoring process can yield two fundamental types of variables: (a) those which could be defined as targets, objectives or goals of the resuscitation process and (b) those which we regard as hemodynamic evaluation tools of potential usefulness in decision making. The former type of variables comprises those parameters already mentioned in these recommendations, such as MAP, lactate level and venous oxygen saturation.8 Examples of the second type of variables would be all those parameters that explore preload dependency, such as PPV and SVV, and which are also analyzed in detail in these recommendations.40 The combination of these two types of variables allows us to create algorithms or to systematize the hemodynamic resuscitation process, with the ultimate aim of securing faster and better recovery, as demonstrated by Rivers in his early goal-directed therapy (EGDT) protocol.15 Lastly, and as occurs with all algorithms, good understanding of the underlying physiopathological bases, as well as of the limitations and inconveniences of the variables used, will allow better management of the hemodynamic resuscitation process.
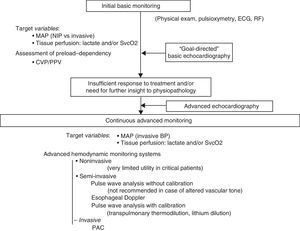
Initial basic monitoringInitial management of shock patients comprises adequate anamnesis and physical examination, together with electrocardiographic (heart rate and ECG) and pulsoxymetric monitoring (SpO2)–not only to assess possible cardiovascular failure, but also as necessary complements in diagnosing the clinical condition.
Initial basic hemodynamic monitoring of patients with potentially critical acute disease is determined by the definition of shock itself.8 Thus, the measurement of blood pressure and of at least one variable informing of global tissue oxygenation, such as serum lactate or SvcO2, is essential. The importance of monitoring MAP as an indicator of tissue perfusion pressure has been considered in detail in another section. Different studies have shown that the duration of hypotension in the first hours of shock exerts an accumulative effect upon the development of organ failure9–thus pointing to the need to establish frequent and precise blood pressure measurements. On the other hand, the monitoring of blood pressure using noninvasive systems loses precision in situations of shock. It therefore seems reasonable to indicate invasive and continuous blood pressure monitoring in the critical patient. In any case, in the early stages, the monitoring of blood pressure could be carried out on a noninvasive basis during admission to the hospital ward or in the emergency service.
The clinical detection of tissue hypoperfusion (with or without arterial hypotension) implies the need to adopt measures to improve DO2. In this dysoxia reversion or hemodynamic resuscitation process, the first step always includes the need to optimize volemia. Consequently, and in addition to the abovementioned goals of the resuscitation process, it is advisable from the start to monitor some parameter estimating preload dependency and which can be of help in making initial resuscitation decisions. However, the possibilities at the time of initial monitoring are limited, fundamentally when the patient has not been admitted to the ICU. In addition to an anamnesis and clinical exploration suggesting hypovolemia, the measurement of CVP by inserting a venous catheter with the distal tip positioned in the right atrium or superior vena cava will be the main parameter offering information on volemia, despite its limited capacity to predict the response to volume expansion. Rather than a specific parameter of volemia, or of preload dependency, CVP could be viewed as a marker of minimum circulating volemia. In this respect, extremely low CVP values are probably associated to insufficient volemia, though elevated CVP values are entirely nonspecific in predicting the response to volume expansion.39 Despite all its limitations, different multicenter studies have found initial volume expansion with CVP safety limits of 8mmHg (12mmHg in the case of patients subjected to mechanical ventilation), within the setting of a bundle of measures, to be associated with improved patient prognosis.15,44 Furthermore, if an invasive arterial catheter is available, we can use PPV as an estimator of the response to volume expansion in patients subjected to mechanical ventilation and with sinus rhythm.
Echocardiography currently plays a relevant role in the early diagnosis and management of patients with hemodynamic instability, as will be commented in the following section of these recommendations.3,77
Continuous hemodynamic monitoring. When and with what?In general, adequate initial management based on the information obtained from the clinical history, the physical examination and basic monitoring may suffice to secure a favorable outcome. However, in some patients, despite adequate initial resuscitation, persistent shock may be observed, or new complications related to the initial disease process or with the adopted treatments may develop. As has already been mentioned in the section referred to the estimation of CO, in patients with an insufficient response to the measures applied in the first 3–6h, or in patients with organ failure and/or comorbidities capable of causing interferences or of worsening during the resuscitation process, we should consider a greater degree of continuous hemodynamic monitoring to help optimize our interventions, quantify their effects and avoid complications resulting from the applied treatments.1,2,21–23
Continuous hemodynamic monitoring should provide information on CO and its determinants: preload/preload dependency, contractility and postload. Thus, in addition to monitoring of the variables used as goals or targets–whether MAP and venous O2 saturation and/or lactate clearance–an intensive resuscitation process will require the incorporation of technologies that allow the continuous evaluation of these parameters, with a view to correcting them. Monitoring of the mentioned target variables will remain essential, since they will mark the end of the resuscitation process. It will be necessary to measure these variables repeatedly after the treatment interventions to confirm that they remain normalized over time.
When choosing a monitoring system, and in addition to factors inherent to the patient, we also must take other elements into account, such as the technological resources available in the center, the experience of the team with each system, the place where monitoring and resuscitation will be carried out (emergency service, ICU, operating room, etc.), and cost-effectiveness criteria. The ideal hemodynamic monitoring system is simple, safe, relatively versatile, easy to use, operator-independent, cost-effective, reliable and precise, and should yield variables and information capable of guiding patient treatment.21,22 No system is presently able to satisfy all these conditions. Nevertheless, the following principles can help us choose a continuous hemodynamic monitoring system:
- -
Monitoring has been shown to be particularly useful in the early phases of hemodynamic resuscitation, and proves less useful once organ failure has become established.
- -
Although less invasive systems are preferable, they cannot always be used, since in complex situations we need full, precise and reliable information that is not always obtainable with less invasive techniques.
- -
Noninvasive systems could be used in the hospital ward or emergency service to confirm a preliminary diagnosis, to assess the course in lesser risk patients, or for monitoring prior to admission to the ICU. For the time being, the use of such systems is not recommended in application to more critically ill patients. In general, the greater the severity and complexity of the disease condition, the greater the need for intensive treatments and increased precision in the measures obtained–and such conditions are currently associated with increased invasiveness of the systems used.21
- -
Monitoring of hemodynamic changes in short periods of time is important (e.g., volume administration or the use of inotropic drugs). Moreover, continuous monitoring of the hemodynamic variables and the beat-to-beat estimation of CO can be particularly useful.1
- -
The PAC can be useful for the management of complex circulatory conditions in which it is particularly important to know PAP, PAOP and tissue oxygenation parameters (e.g., acute left- and right-side failure, pulmonary hypertension, difficult weaning, patients subjected to heart surgery or pending heart transplantation).1,21,78
- -
The transpulmonary dilution techniques that determine intrathoracic volumes and extravascular lung water can be regarded as the methods of choice in guiding hemodynamic management and the application of mechanical ventilation in patients with acute lung injury or acute respiratory distress syndrome. In these cases, PAC could also be used.2
- -
The systems that determine CO through pulse wave analysis would be indicated in surgical areas, in situations of septic shock, or in other clinical scenarios without serious respiratory complications. Devices without external calibration lose reliability in the presence of important alterations in vascular tone.2,21
- 1.
Complete physical examination, including ECG and SpO2. (L1; C)
- 2.
Initial and frequent measurement of blood pressure (preferably on an invasive basis) in patients with conditions suggestive of cardiovascular failure. (L1; C)
- 3.
Initial measurement of a DO2/VO2equilibrium metabolic marker, fundamentally serum lactate. (L1; A)
- 4.
Repeated measurement of a DO2/VO2equilibrium metabolic marker, serum lactate or venous O2saturation, during the resuscitation process. (L1; A)
- 5.
In the initial management of shock, and in the absence of other variables assessing preload dependency and/or CO, the monitoring of CVP may prove useful in the decision-making process. (L1; B)
- 1.
Repeated measurement is recommended of a DO2/VO2equilibrium metabolic marker, serum lactate or venous O2saturation, during the resuscitation process. (L1; A)
- 2.
Continuous hemodynamic monitoring is recommended in patients who continue to show signs of tissue hypoperfusion 3–6h after the start of treatment and/or in those patients in whom more in-depth assessment of the physiopathology of the disease process is required. (L1; B)
- 3.
The PAC would be indicated in complex circulatory conditions in which the knowledge of PAP, PAOP and tissue oxygenation parameters is considered to be particularly important. (L1; C)
- 4.
Noninvasive systems are presently not recommended for the hemodynamic monitoring of critically ill patients in the ICU. Such systems should be reserved for patients with less serious conditions and/or for patients admitted to the hospital ward or emergency service. (L2; C)
Echocardiography is a useful tool for evaluating cardiovascular function in the critical patient, since it offers real-time imaging at the patient bedside, and in a noninvasive (transthoracic echocardiography [TTE]) or minimally invasive manner (transesophageal echocardiography [TEE]). The information obtained is interpreted and immediately integrated in the global assessment of the patient. The main indication of echocardiography in the ICU is the study of cardiocirculatory function in shock, since it allows us to obtain information on the underlying cause, and can be of great help as a guide and for monitoring the treatment provided.3,77 The latest American cardiological guides on the use of echocardiography consider the technique to have grade A recommendation (appropriate use: the test is generally acceptable and is a reasonable procedure for that indication) in situations of hypotension or hemodynamic instability.79 On the other hand, the guides on acute and chronic heart failure recommend TTE for the evaluation of cardiac structure and function, for establishing the diagnosis, and in planning and monitoring the treatment, as well as for obtaining prognostic information (recommendation grade I C).73
Although no randomized studies have analyzed the impact of echocardiography upon the treatment and prognosis of the critical patient, several studies in the ICU setting have found that the information obtained by TEE or TTE leads to changes in therapeutic and diagnostic management in 30–60% of the patients.80 This technique moreover allows us to discard structural anomalies such as valve disease and left ventricle outflow tract obstruction that cannot be detected by any other hemodynamic monitoring system. Most critical patients can be adequately studied with TTE, thanks to technological advances that have served to improve ultrasound quality (second harmonic, digital acquisition, etc.). However, TEE is preferable in situations in which we may have difficulties in acquiring optimum images, such as for example when applying mechanical ventilation with positive pressure, in the presence of edemas or obesity, etc. Furthermore, TEE should be regarded as the technique of choice in the following situations: aortic dissection, endocarditis, intracavitary thrombi, study of the thoracic aorta, and the assessment of valve prostheses.
The growing incorporation of ultrasound to the ICU has caused many national Intensive Care societies throughout the world to promote the learning of this technique. On one hand, basic training in echocardiography is advocated for intensivists, adopting a goal-directed approach, i.e., focused on solving specific issues in Critical Care, while on the other hand advanced training is advised only for intensivists with a specific interest in furthering their knowledge of echocardiography. Joint statements have been published by the American and French societies regarding the competencies in ultrasound in the ICU.4 These recommendations define the skills and competencies required for the different levels of knowledge in echocardiography and in other ultrasound techniques in the ICU, such as thoracic, abdominal and vascular ultrasound. Likewise, the European Society of Intensive Care Medicine, together with representatives of other societies, has recently proposed a series of standard training requirements in ultrasound.81
In relation to the basic level, emphasis is placed on the need for echocardiographic examination to be qualitative, dynamic and goal oriented. It should include basic assessment of left and right ventricular function, as well as the assessment of cardiac tamponade, the estimation of volume response, and evaluation of massive valve regurgitation. Evaluation should aim to answer a limited series of specific questions regarding situations of hemodynamic instability (Is left ventricle function normal? Is the right ventricle dilated? What about right ventricle function? Are there echocardiographic signs of cardiac tamponade? Are there signs of severe hypovolemia? Is there serious valve disease?). More complete and detailed information in turn would form part of an advanced level of training in echocardiography. The skills at this advanced level in the ICU emphasize specific knowledge of aspects related to hemodynamic assessment of the critical patient, such as volume response indices, filling pressures, CO, the impact of mechanical ventilation upon right ventricle function, etc.
Basic echocardiography should be applied in the initial assessment of shock, since it allows us to quickly detect the characteristic causal conditions of shock: severe left ventricle failure, right ventricle failure generally secondary to pulmonary thromboembolism, cardiac tamponade, massive valve insufficiency, and hypovolemia. At this point of the evaluation, before the adoption of any treatment measure (pharmacological or surgical), the diagnostic yield of the technique is maximum. Posteriorly, a more complete echocardiographic evaluation should be made in the event of insufficient treatment response or if further insight into the physiopathology of the process is required. This more exhaustive exploration requires training in advanced echocardiography, affording reliable, detailed and more in-depth information about relevant aspects of cardiovascular function in the hemodynamic management of the critical patient. Discontinuous but repeated echocardiographic evaluations contribute to further hemodynamic assessment and to evaluate and guide treatment. On the other hand, general ultrasound is also very useful for the global evaluation of shock patients, and can help identify a non-cardiogenic origin of hemodynamic instability. Clearly, the information afforded by ultrasound exploration must always be accompanied by other clinical assessment elements in critical patients such as the case history and initial examination, imaging and laboratory tests, and the data obtained with other hemodynamic monitoring systems (Fig. 1).3,4,77
- 1.
In the initial phase of the evaluation of shock, basic echocardiography is extremely useful for obtaining information on the underlying cause, and can be of great help as a guide and for monitoring the treatment provided. (L1; B)
- 2.
In situations of shock characterized by insufficient treatment response, or if further insight into the physiopathology of the process is required, advanced level echocardiography is indicated. (L1; B)
Algorithm for the evaluation of cardiovascular function and hemodynamic monitoring in situations of shock. PAC: pulmonary artery catheter; ECG: electrocardiogram; RF: respiratory frequency; BP: blood pressure; MAP: mean arterial pressure; NIP: noninvasive pressure; CVP: central venous pressure; SvcO2: central venous oxygen saturation; PPV: pulse pressure variation.
As we have commented above, hemodynamic monitoring is based on the premise that the detection and treatment of the physiopathological alterations of critical disease should result in improved patient outcomes. Despite the logic of this affirmation, the concept of “hemodynamic monitoring” has been subject to endless discussion due to the lack of studies demonstrating that monitoring in itself is able to improve the patient prognosis. This attitude may be wrong, however, since the use of certain variables (such as venous oxygen saturation) in cardiovascular resuscitation algorithms has indeed shown a beneficial impact upon the prognosis of different disease conditions. The most evident example of this can be found in recent studies in septic patients, such as the work of Rivers et al.,15 in which resuscitation guided by previously defined hemodynamic objectives or goals had a strong impact upon the survival of these patients.
On the other hand, demonstrating the isolated prognostic value of the many existing hemodynamic variables will be very complicated. This would be the case, for example, of PPV and/or SVV as predictors of the response to volume expansion in certain patients. Despite their undeniable physiological meaning and the fact that they have been shown to be clearly superior to the cardiac filling pressures, the lack of randomized studies specifically demonstrating that the isolated use of such dynamic variables is directly correlated to improved prognosis has caused them to be excluded from the different recommendations and management guides–while in contrast the mentioned cardiac filling pressures have been maintained.
Thus, the impact that hemodynamic monitoring will have upon our patients depends not only on the reliability of the systems used, but also on our knowledge of their limitations, as well as on adequate understanding of the physiological principles and correct interpretation of the variables obtained. The adequate use of these variables, as a goal or tool in decision making, is the factor ultimately deciding the beneficial impact upon patient outcome. The reasoning, therefore, is that hemodynamic monitoring will only result in an improved patient prognosis when it is associated to a treatment that has been shown to afford benefits.82
Conflicts of interestAna Ochagavia and Javier Maynar: payment for conferences, consultants (Pulsion): Ignacio Monge: payment for conferences, consultants (Edwards).
Please cite this article as: Ochagavía A, Baigorri F, Mesquida J, Ayuela JM, Ferrándiz A, García X, et al. Monitorización hemodinámica en el paciente crítico. Recomendaciones del Grupo de Trabajo de Cuidados Intensivos Cardiológicos y RCP de la Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias. Med Intensiva. 2014;38:154–169.