Diseases due to SARS-CoV-2 is characterized by cytokine storm mediated by the overproduction of proinflammatory cytokines that can be expressed with a higher level of interleukin 6 (IL-6). On the other hand, infection can cause pneumonia, and eventually progress into acute respiratory distress syndrome being one of the leading causes of death in these patients.1,2
The basis of treatment of critically ill patients with pneumonia due to SARS-CoV-2 is symptomatic and supportive: only the use of corticoids has proven capable of reducing mortality3 while the effects of tocilizumab as a recombinant humanized monoclonal antibody antagonizing the IL-6 receptor have been confirmed.4
During this pandemic a great number of patients have received a great variety of therapies in all sorts of combinations. Some of these combinations—based on the premises described before—have been the combined use of tocilizumab and corticoids.
We wanted to analyze the impact that a combined therapy of tocilizumab and corticoids in this context has on the short-term survival (28 days) of patients admitted to an intensive care unit (ICU) due to SARS-CoV-2. Therefore, we conducted a retrospective study that included cases hospitalized due to SARS-CoV-2 infection admitted from March 10, 2020 through December 5, 2020. Patients were categorized into 4 groups based on the different combinations used: group A: did not receive corticoids or tocilizumab; group B: received combined therapy with corticoids and tocilizumab; group C: received tocilizumab only; group D: received corticoids only.
Tocilizumab was used in patients with disease progression with a PaO2/FiO2 ratio <300, and D-dimer levels >1500 ng/mL (or in gradual increase) or IL-6 levels >40 pg/mL or elevated ferritin levels. The dose administered in patients of >75 kg of weight was a single dose of 600 mg, and in patients of <74 kg of weight, the dose administered was 1 single dose of 400 mg. Five patients received a second dose of tocilizumab, 6 patients received 3 doses of tocilizumab, and 2 patients received 4 doses of tocilizumab.
The use of corticoids was registered as a binary variable (Yes or No) if patients received, at least, 40 mg of methylprednisolone or its equivalence for, at least, 3 days to treat inflammation associated with viral pneumonia.
A descriptive analysis of the sample was initially conducted and then followed by Cox regression and a 28-day survival analysis using the Kaplan-Meier method for the variable of time of death. Survival curves were compared using the log-rank test.
In the study period a total of 254 patients were admitted. A total of 46 patients were excluded from the study due to lack of data or no confirmation of the presence of SARS-CoV-2. A total of 208 patients were analyzed. The main characteristics of the 4 groups of patients are shown on Table 1.
Main differences between patients treated with tocilizumab and corticoids of the sample studied.
| Variables | Group A (n = 72) | Group B (n = 49) | Group C (n = 29) | Group D (n = 58) | P |
|---|---|---|---|---|---|
| Age, mean (SD) | 64 (13) | 67 (11) | 57 (18) | 65 (8) | <.05 |
| Sex | |||||
| Men, n (%) | 53 (74) | 38 (77) | 20 (69) | 50 (86) | .93 |
| Comorbidities | |||||
| AHT, n (%) | 38 (53) | 27 (55) | 14 (48) | 33 (57) | .94 |
| DM, n (%) | 17 (24) | 14 (28) | 5 (17) | 13 (22) | .63 |
| Obesity, n (%) | 8 (11) | 12 (24) | 6 (21) | 19 (32) | .07 |
| Dyslipidemia, n (%) | 29 (40) | 21 (43) | 8 (27) | 25 (43) | .63 |
| Smoker, n (%) | 18 (25) | 15 (31) | 11 (38) | 25 (43) | .36 |
| SOFA score, median (SD) | 5 (3) | 5 (4) | 7 (3) | 4 (2) | .12 |
| PaO2/FiO2 ratio at the ICU admission, mean (SD) | 155 (92) | 150 (63) | 106 (40) | 146 (58) | <.05 |
| Lab data at the ICU admission | |||||
| CK, mean (SD) | 549 (1032) | 199 (287) | 983 (1246) | 156 (562) | <.05 |
| DD, mean (SD) | 8959(19975) | 7347(25248) | 13061(28630) | 6634(19335) | <.05 |
| IL-6, mean (SD) | 57 (51) | 92 (131) | 148 (168) | 88 (132) | <.05 |
| CRP, mean (SD) | 27(11) | 22 (14) | 20 (7) | 29 (8) | .37 |
| LDH, mean (SD) | 469 (512) | 369 (114) | 643 (536) | 395 (141) | <.05 |
| Ferritin, mean (SD) | 1299 (1140) | 1203 (814) | 1312 (775) | 1112 (947) | .41 |
| Decubitus position | 36 (50) | 32 (65) | 23 (79) | 29 (50) | .35 |
| HFNO, n (%) | 24 (33) | 22 (45) | 7 (24) | 39 (67) | <.05 |
| IMV, n (%) | 48 (66) | 42 (85) | 28 (96) | 48 (82) | <.05 |
| Need for vasopressors, n (%) | 33 (46) | 22 (45) | 20 (69) | 13 (22) | <.05 |
| ECMO, n (%) | 0 (-) | 1 (0.2) | 1 (0.3) | 0 (-) | – |
| iNO, n (%) | 3 (0.4) | 0 (-) | 3 (0.1) | 3 (0.5) | – |
| Need for RRT, n (%) | 6 (8.3) | 1 (2) | 3 (10) | 2 (3.4) | <.05 |
| Nosocomial over-infection, n (%) | 20 (28) | 17 (35) | 18 (62) | 14 (24) | <.05 |
| VAT/VAP, n (%) | 15 (21) | 13 (26) | 15 (52) | 5 (9) | <.05 |
| Germs | |||||
| Aspergillus, n (%) | 2 (10) | 2 (12) | 1 (5) | – | – |
| Candida, n (%) | 1 (5) | 2 (12) | 3 (15) | 3 (21) | <.05 |
| E. coli, n (%) | 1 (5) | 5 (29) | – | 2 (14) | – |
| Pseudomonas, n (%) | 3 (15) | 1 (6) | 2 (10) | 3 (21) | <.05 |
| MRSA, n (%) | 1 (5) | 2 (12) | 3 (15) | – | |
| Stay at the ICU, mean (p25–p75) | 11 (3−17) | 14 (6−28) | 17 (9−33) | 11 (5−18) | <.05 |
| Mortality at the ICU setting, n (%) | 27 (37) | 7 (14) | 8 (27) | 10 (17) | .16 |
AHT, arterial hypertension; CK, creatine kinase; CRP, C-reactive protein; DD, D-dimer; DM, diabetes mellitus; ECMO, extracorporeal membrane oxygenation; HFNO, high flow nasal oxygen; IL-6, interleukin-6; IMV, invasive mechanical ventilation; iNO, inhaled nitric oxide; LDH, lactate dehydrogenase; MRSA, methicillin-resistant Staphylococcus aureus; RRT, renal replacement therapies; VAP, ventilator-associated pneumonia; VAT, ventilator-associated tracheobronchitis.
Group A: did not receive corticoids or tocilizumab; group B: received combined therapy with corticoids and tocilizumab; group C: received tocilizumab only; group D: received corticoids only.
Reference values: CK (46−171 U/L); DD (0–500 (ng/mL); IL6 (<5 pg/L); CRP (<0.5 g/dL); LDH (120−246 U/L); ferritin (22−322 ng/mL).
The table shows the OR values with its corresponding 95% confidence interval.
Using Cox regression analysis (method: intro; state: dead at ICU; covariates used: age, sex, patients’ comorbidities, therapies involved in the management of these patients, the SOFA score, the PaO2/FiO2 ratio at admission, and the analytical values and ventilatory support therapies). The PaO2/FiO2 ratio at the ICU admission behaved as a protector factor against mortality (HR, 0.98; 95%CI, 0.97−0.99; P = .01). Out of all the therapies analyzed, the combined use of corticoids and tocilizumab was associated with a better survival rate at the ICU setting (HR, 9.623; 95%CI, 2.39–38.69; P = .001). No significant differences were seen with the remaining variables analyzed.
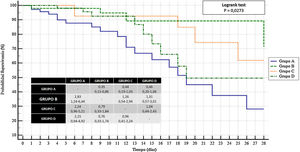
In the 28-day survival analysis, the group on a combined therapy of tocilizumab and corticoids had more chances of survival after 28 days (OR, 2.83; 95%CI, 1.24–6.44) compared to the nonuse of tocilizumab and corticoids. This significant difference was not observed compared to the other 2 groups analyzed: OR, 1.26; 95%CI, 0.54–2.94 vs therapy with tocilizumab; OR, 1.31; 95%CI, 0.57–3.01 vs corticoid therapy (Fig. 1).
Currently, the most consistent and recent studies that assessed tocilizumab in monotherapy against COVID-19 revealed no beneficial effects whatsoever.5–7 This data proves that blocking one single cytokine is not enough to have a significant clinical impact in these patients.
Similarly, it could be interpreted that one single fixed dose of steroids regardless of disease severity, the cytokine storm released or the presence of acute respiratory distress syndrome would be insufficient in many of these patients.8
Our results are consistent with those reported in the REMAP-CAP study9 that analyzed the use of tocilizumab in a population of patients admitted to the ICU for organ support. This study confirmed that the addition of tocilizumab to the use of dexamethasone improved the mortality of the sample. Consistent with this, the study conducted by Mikulska et al. confirmed that the negative impact of the immune response to COVID-19 might mitigate through the early administration of corticoids plus tocilizumab.10
On the other hand, we wish to mention that the group that only received tocilizumab had worse oxygen levels at admission, required more vasopressor drugs and renal replacement therapies during the ICU admission. Also, they developed more nosocomial over-infections, among them, ventilator-associated tracheobronchitis and pneumonia (VAT/VAP). In this sense, according to the medical literature it is a known fact that upper airway infections are the most common side effects of tocilizumab.11,12
Our study has several limitations. it is a relatively small series of patients from a single hospital. On the other hand, the results were obtained in the middle of a pandemic with saturated hospital units and, often with non-expert medical and nursing personnel. None of the treatments were implemented randomly, but according t the health professionals’ best clinical judgement. Also, we should mention that there are specific contraindications for the use of tocilizumab like the presence of hepatic failure or previous infections that may lead to aprioristic patient selection bias.
Currently, the indication for a combined therapy of tocilizumab plus corticoids could be considered premature meaning that new randomized clinical trials would be needed before establishing better options of treatment, duration, and limitations.
FundingNone whatsoever.
Conflicts of interestNone.
Please cite this article as: González-Castro A, Cuenca Fito E, Fernandez A, Escudero Acha P, Rodríguez Borregán JC, Peñasco Y. Terapia combinada de tocilizumab y corticoides en la enfermedad grave por SARS-CoV-2. Med Intensiva. 2022;46:285–287.