To describe sedation with continuous perfusion of propofol in critically ill children.
DesignA retrospective, descriptive observational study was carried out.
SettingA pediatric Intensive Care Unit.
PatientsPediatric patients requiring sedoanalgesia between October 1, 2009 and September 30, 2010.
InterventionsNone.
Data collectedDemographic, clinical and laboratory test variables, diagnosis, treatment, complications and evolution in each patient. In addition, the potential adverse effects associated with propofol administration were analyzed.
ResultsMidazolam, fentanyl and propofol were the most commonly used sedative and analgesic drugs. Seventy-one out of 222 patients (32%) received propofol in continuous infusion. The average dose was 2.1mg/kg/h (SD 1.3, range: 0.5–6), and the average duration of treatment was of 6.7 days (SD 8.5 range 0.5–40). Fifty-two percent were male, and the mean patient age was 45.8 months (median: 24; interquartile range: 7–65). No patient developed propofol infusion syndrome or other serious drug-related adverse effects. Patients treated with propofol showed more abnormal laboratory test findings, although no relationship to drug administration could be demonstrated. There were no significant differences in lactate level or in the incidence of infection in either group.
ConclusionsPropofol at a dose of 1–4mg/kg/h is a safe alternative for sustained sedation in critically ill children. However, further studies are needed to assess its effects and safety profile.
describir la sedación con perfusión continua de propofol en niños en estado crítico.
DiseñoEstudio observacional descriptivo retrospectivo.
ÁmbitoUnidad de cuidados intensivos pediátricos.
PacientesPacientes que requirieron sedoanalgesia entre el 1 de Octubre del 2009 y el 30 de Septiembre del 2010.
Intervencionesninguna.
Variables recogidasDemográficas, clínicas, de laboratorio, diagnóstico, tratamiento, complicaciones y evolución de cada paciente. Se analizaron los posibles efectos adversos asociados a la administración de propofol, comparando el grupo de pacientes a los que se les administró con el resto de los niños críticos.
ResultadosRecibieron propofol en perfusión continua 71 de los 222 pacientes recogidos (32%). Los fármacos sedoanalgésicos más utilizados fueron el midazolam, seguido del fentanilo y del propofol. La dosis media de propofol fue de 2,1mg/kg/h [desviación estándar (DE) 1,3, rango: 0,5-6)] y la duración media de 6,7 días (DE 8,5; rango: 0,5-40). La edad media fue de 45,8 meses (mediana 24; rango intercuartil: 7-65), siendo el 52% varones. Ningún paciente presentó síndrome de infusión por propofol ni otros efectos adversos graves. Los pacientes tratados con propofol presentaron con mayor frecuencia algunas alteraciones analíticas que el resto, pero no se demostró relación causa efecto con la administración del fármaco. No existieron diferencias significativas en los niveles de lactato ni en la incidencia de infecciones entre ambos grupos.
ConclusiónEl propofol a una dosis de 1 a 4mg/kg/h puede utilizarse como un fármaco alternativo para la sedación de mantenimiento en los niños críticamente enfermos. Sin embargo son necesarios más estudios que valoren su eficacia y seguridad.
Sedation and analgesia are essential elements in the management of critically ill children.1 One of the sedatives used in the Intensive Care Unit (ICU) is propofol, an alkylphenol solubilized in different lipids and at concentrations of 1% and 2%. At low doses it produces conscious sedation, while at higher doses it produces deep sedation and anesthesia.2 The main characteristic of propofol is its rapid action and disappearance of the effects after suspending administration, with quick awakening of the patient.3,4
As a result of these properties, propofol is widely used in both children and adults for the induction and maintenance of anesthesia and for sedation in the context of brief interventions.5–8
The most frequent adverse effects of propofol are arterial hypotension in patients with intravascular volume depletion (hypovolemia), bradycardia due to anomalies in cardiac conduction, hypertriglyceridemia, and pain at the injection site.
The use of propofol for the prolonged maintenance of sedation in critically ill children has been disadvised by some authors due to the risk of propofol infusion syndrome (PIS) when high doses are employed.9 This syndrome is a serious condition characterized by metabolic acidosis, cardiac dysfunction (diminished myocardial contractility and conduction disorders), and at least one of the following signs: rhabdomyolysis, hypertriglyceridemia and renal failure.10
As a result of this risk, very few studies have been made of the continuous perfusion of propofol in critically ill children.11 The present study describes our experience with the prolonged continuous infusion of propofol at moderate doses in critically ill children, and examines its side effects compared with patients who do not receive this drug.
Patients and methodsA retrospective observational study was made involving a review of the case histories of all patients administered with sedoanalgesia in the Pediatric Intensive Care Unit (PICU) between 1 October 2009 and 30 September 2010. We collected demographic, clinical and laboratory test data and documented the diagnosis upon admission, treatment and complications, and the outcome of each patient. The sample was divided into two groups according to whether continuous propofol perfusion had been administered or not, with comparison of the presence of adverse effects between the two groups. In addition, we reviewed all the incidents recorded in the pharmacovigilance registry of the Department of Pharmacy during the period of the study.
The statistical study was carried out using the SPSS version 16.0 statistical package (SPSS, Chicago, IL, USA). Frequency tables were used for the qualitative variables, and central tendency and dispersion measures for the quantitative variables. The Student t-test and Mann–Whitney U-test were used to compare quantitative variables between the two groups, while qualitative variables were compared using the chi-squared test with Yates correction. Statistical significance was accepted for p<0.05.
ResultsA total of 222 patients were studied, of which 71 (32%) received propofol in continuous perfusion. Of the patients who received propofol, 52% were male, and the mean age was 45.8 months (median 24; interquartile range 7–65). The most frequent age range was between 2 and 12 months (34.3%). There were no significant differences between the two groups in terms of age, weight or gender.
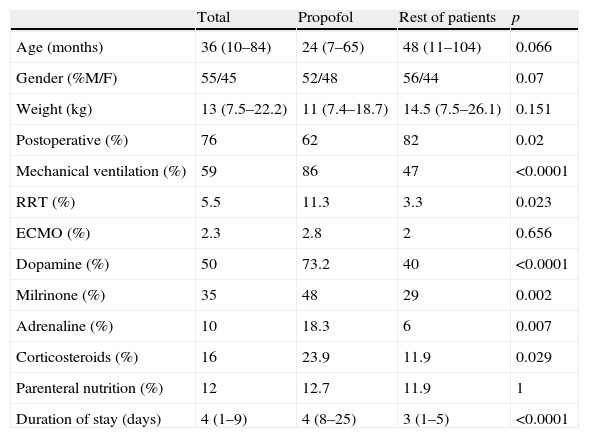
The reason for admission among the patients who received propofol was post-operative recovery in 62% of the cases, while in the group without propofol this percentage reached 82% (p=0.02). In 44% of the cases the patients were recovering from heart surgery. Mechanical ventilation was needed in 86% of the patients treated with propofol, while renal replacement therapy in the form of continuous venovenous hemodiafiltration proved necessary in 11%, and extracorporeal membrane oxygenation (ECMO) in 2.8%. The duration of stay in the PICU among the patients who received propofol was significantly longer than in the rest of the cases (p<0.0001), and four patients died (5.5%) (Table 1).
Characteristics and treatment of the patients. Comparison between those who received propofol and the rest of the patients.
| Total | Propofol | Rest of patients | p | |
| Age (months) | 36 (10–84) | 24 (7–65) | 48 (11–104) | 0.066 |
| Gender (%M/F) | 55/45 | 52/48 | 56/44 | 0.07 |
| Weight (kg) | 13 (7.5–22.2) | 11 (7.4–18.7) | 14.5 (7.5–26.1) | 0.151 |
| Postoperative (%) | 76 | 62 | 82 | 0.02 |
| Mechanical ventilation (%) | 59 | 86 | 47 | <0.0001 |
| RRT (%) | 5.5 | 11.3 | 3.3 | 0.023 |
| ECMO (%) | 2.3 | 2.8 | 2 | 0.656 |
| Dopamine (%) | 50 | 73.2 | 40 | <0.0001 |
| Milrinone (%) | 35 | 48 | 29 | 0.002 |
| Adrenaline (%) | 10 | 18.3 | 6 | 0.007 |
| Corticosteroids (%) | 16 | 23.9 | 11.9 | 0.029 |
| Parenteral nutrition (%) | 12 | 12.7 | 11.9 | 1 |
| Duration of stay (days) | 4 (1–9) | 4 (8–25) | 3 (1–5) | <0.0001 |
The quantitative variables are expressed as median and interquartile range (in parentheses).
ECMO: extracorporeal membrane oxygenation; RRT: renal replacement therapy.
The vasoactive drugs most commonly used together with propofol were dopamine (73%) and milrinone (48%). Total parenteral nutrition (TPN) was provided in 9 patients (14%), with a mean duration of 20.7 days (range 3–34 days). The mean lipid administration in TPN was 1g/kg (range 0.3–2.0g/kg).
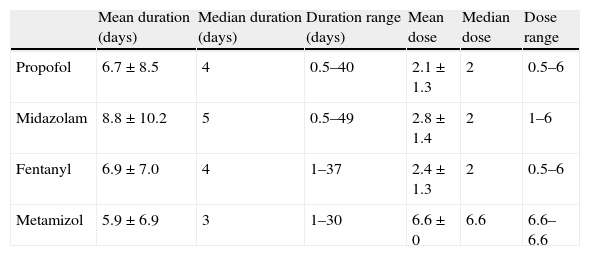
The mean propofol dose was 2.1mg/kg/h [standard deviation (SD) 1.3; range 0.5–6], and the mean duration of infusion was 6.7 days (SD 8.5; range 0.5–40). The duration of administration was over three days in 53% of the cases and over 7 days in 25%. Some patents required several analgesics and sedatives either simultaneously or on a rotational basis. The most commonly used sedoanalgesic agents were midazolam, fentanyl, metamizol and propofol (Tables 2 and 3).
Duration and doses of the sedoanalgesic drugs used in the group of patients treated with propofol.
| Mean duration (days) | Median duration (days) | Duration range (days) | Mean dose | Median dose | Dose range | |
| Propofol | 6.7±8.5 | 4 | 0.5–40 | 2.1±1.3 | 2 | 0.5–6 |
| Midazolam | 8.8±10.2 | 5 | 0.5–49 | 2.8±1.4 | 2 | 1–6 |
| Fentanyl | 6.9±7.0 | 4 | 1–37 | 2.4±1.3 | 2 | 0.5–6 |
| Metamizol | 5.9±6.9 | 3 | 1–30 | 6.6±0 | 6.6 | 6.6–6.6 |
Propofol and metamizol dosage: mg/kg/h; midazolam: μg/kg/min; fentanyl: μg/kg/h.
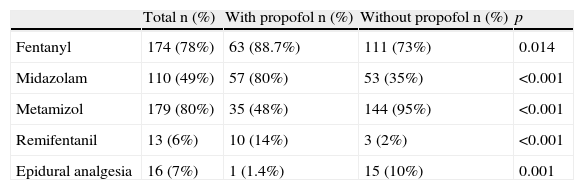
Sedoanalgesic drugs used in the two groups of patients.
| Total n (%) | With propofol n (%) | Without propofol n (%) | p | |
| Fentanyl | 174 (78%) | 63 (88.7%) | 111 (73%) | 0.014 |
| Midazolam | 110 (49%) | 57 (80%) | 53 (35%) | <0.001 |
| Metamizol | 179 (80%) | 35 (48%) | 144 (95%) | <0.001 |
| Remifentanil | 13 (6%) | 10 (14%) | 3 (2%) | <0.001 |
| Epidural analgesia | 16 (7%) | 1 (1.4%) | 15 (10%) | 0.001 |
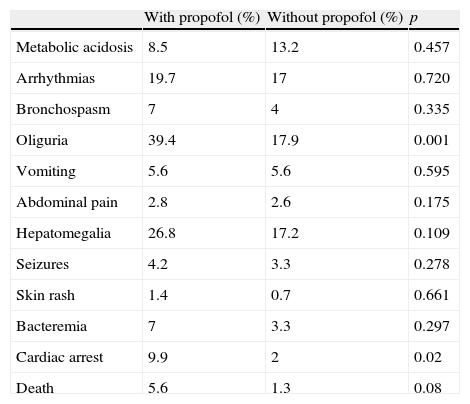
No incidents were recorded by the pharmacovigilance registry related to the administration of propofol. No patients suffered propofol infusion syndrome or other serious adverse effects related to the drug. The patients treated with propofol had a higher frequency of oliguria, cardiac arrest and death than those who did not receive the infusion (Table 4).
Comparison of the complications between the two groups of patients.
| With propofol (%) | Without propofol (%) | p | |
| Metabolic acidosis | 8.5 | 13.2 | 0.457 |
| Arrhythmias | 19.7 | 17 | 0.720 |
| Bronchospasm | 7 | 4 | 0.335 |
| Oliguria | 39.4 | 17.9 | 0.001 |
| Vomiting | 5.6 | 5.6 | 0.595 |
| Abdominal pain | 2.8 | 2.6 | 0.175 |
| Hepatomegalia | 26.8 | 17.2 | 0.109 |
| Seizures | 4.2 | 3.3 | 0.278 |
| Skin rash | 1.4 | 0.7 | 0.661 |
| Bacteremia | 7 | 3.3 | 0.297 |
| Cardiac arrest | 9.9 | 2 | 0.02 |
| Death | 5.6 | 1.3 | 0.08 |
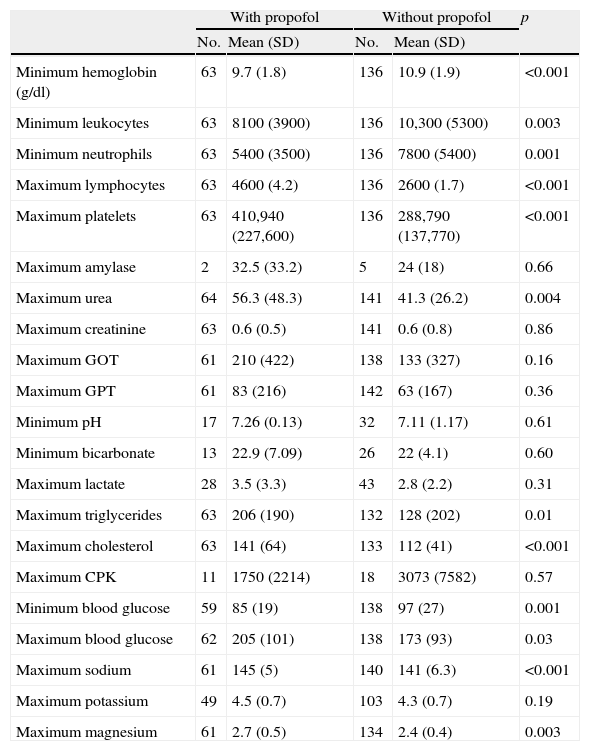
Table 5 compares the laboratory test parameters of the patients who received propofol versus the rest of the critically ill children. Significant differences were observed in some of the parameters, including hemoglobin concentration, leukocyte count, urea, cholesterol and triglyceride levels. There were no significant differences in lactate concentration between the two groups.
Laboratory test values. Comparison between the two groups of patients.
| With propofol | Without propofol | p | |||
| No. | Mean (SD) | No. | Mean (SD) | ||
| Minimum hemoglobin (g/dl) | 63 | 9.7 (1.8) | 136 | 10.9 (1.9) | <0.001 |
| Minimum leukocytes | 63 | 8100 (3900) | 136 | 10,300 (5300) | 0.003 |
| Minimum neutrophils | 63 | 5400 (3500) | 136 | 7800 (5400) | 0.001 |
| Maximum lymphocytes | 63 | 4600 (4.2) | 136 | 2600 (1.7) | <0.001 |
| Maximum platelets | 63 | 410,940 (227,600) | 136 | 288,790 (137,770) | <0.001 |
| Maximum amylase | 2 | 32.5 (33.2) | 5 | 24 (18) | 0.66 |
| Maximum urea | 64 | 56.3 (48.3) | 141 | 41.3 (26.2) | 0.004 |
| Maximum creatinine | 63 | 0.6 (0.5) | 141 | 0.6 (0.8) | 0.86 |
| Maximum GOT | 61 | 210 (422) | 138 | 133 (327) | 0.16 |
| Maximum GPT | 61 | 83 (216) | 142 | 63 (167) | 0.36 |
| Minimum pH | 17 | 7.26 (0.13) | 32 | 7.11 (1.17) | 0.61 |
| Minimum bicarbonate | 13 | 22.9 (7.09) | 26 | 22 (4.1) | 0.60 |
| Maximum lactate | 28 | 3.5 (3.3) | 43 | 2.8 (2.2) | 0.31 |
| Maximum triglycerides | 63 | 206 (190) | 132 | 128 (202) | 0.01 |
| Maximum cholesterol | 63 | 141 (64) | 133 | 112 (41) | <0.001 |
| Maximum CPK | 11 | 1750 (2214) | 18 | 3073 (7582) | 0.57 |
| Minimum blood glucose | 59 | 85 (19) | 138 | 97 (27) | 0.001 |
| Maximum blood glucose | 62 | 205 (101) | 138 | 173 (93) | 0.03 |
| Maximum sodium | 61 | 145 (5) | 140 | 141 (6.3) | <0.001 |
| Maximum potassium | 49 | 4.5 (0.7) | 103 | 4.3 (0.7) | 0.19 |
| Maximum magnesium | 61 | 2.7 (0.5) | 134 | 2.4 (0.4) | 0.003 |
SD: standard deviation.
The present study is one of the first to analyze the use of propofol in continuous perfusion for the prolonged sedation of critically ill children.11 The sedoanalgesia protocol in our PICU contemplates propofol in continuous perfusion as a second-level sedative, administered in replacement of (or as a coadjuvant to) first-level treatment (midazolam and fentanyl). The maximum propofol dose used is 4mg/kg/h. During the study period, administration was not limited by either age or duration. As a result, some patients received propofol for a very long time (up to 40 days), without evident tolerance or adverse effects–in coincidence with the findings of Cornfield et al.11 At present, we rotate sedoanalgesic drugs; as a result, the duration within each cycle is generally a maximum of four consecutive days.
Despite the good sedative performance of propofol in critically ill children, its use in continuous infusion has been limited due to the risk of propofol infusion syndrome (PIS). The main risk factors underlying PIS are the infusion for over 48h of doses in excess of 4–5mg/kg/h.12 However, there have also been reports of PIS at lower doses and even with single dosing. An individual susceptibility factor is therefore probably present in PIS.13 Other risk factors described in the literature are upper airway infection and association of the drug to vasopressors and corticosteroids.13
Since first described by Parke et al.14 in the year 1992, the incidence of PIS has been highly variable.15 In March 2001 the drug company AstraZeneca, which produced propofol (Diprivan®), issued a report on a randomized study including 327 children sedated in the PICU, in which mortality proved significantly higher among the patients who received propofol–without being able to demonstrate the existence of other underlying causes.16 Following this report, the United States Food and Drug Administration (FDA) and the Committee on Safety of Medicines (CSM) in the United Kingdom decided to disadvise the infusion of propofol in critically ill children for periods of over 24h.17
Roberts et al.,12 in a multicenter prospective study of 1017 adults, documented 9 cases of clinical manifestations consistent with this syndrome. Only 1% of the subjects presented PIS when receiving the drug for more than 24h. PIS also appeared with low doses of propofol. Most of the patients survived, and none developed rhabdomyolysis.12
Cornfield et al., in a study of 142 critically ill children administered propofol in continuous perfusion, recorded no side effects and thus defended cautious use of the drug for the maintenance of sedoanalgesia in critically ill children.11 On the other hand, Crawford et al.18 reported their experience with propofol used in approximately 100,000 pediatric for sedation and general anesthesia, describing a very low incidence of PIS. According to these authors, it is very unlikely for the syndrome to occur when administering propofol in bolus doses or at low doses. Nevertheless, these indications have been criticized by other authors,19 who even disadvise propofol use in pediatric patients as an anesthetic and sedative in brief interventions.20
The latest international pediatric sedoanalgesia guides do not recommend prolonged propofol administration in children, though they recognize its potential usefulness in short duration techniques and as a bridge to extubation, replacing other drugs during 6–12h.21–23 Despite these recommendations, different surveys have shown that propofol is used in many PICUs in different countries.24–26 In Spain, a sedoanalgesia survey conducted in 36 PICUs found propofol to be used in 22% of the patients requiring mechanical ventilation during more than 24h.27
In our series we recorded no cases of PIS with a dosage similar to that published by Cornfield et al.11 Furthermore, in our more than 15 years of experience with the utilization of propofol in continuous perfusion in over 1000 patients, we have never observed this syndrome. However, close monitoring is needed, and the drug should be suspended upon the appearance of signs such as metabolic acidosis with hyperlactacidemia of indeterminate cause.
On the other hand, although the frequency of medical complications and laboratory test alterations in our study was greater among the patients treated with propofol than in the rest of the critically ill children, none of these problems were directly associated to administration of the drug. Probably, the mentioned alterations were secondary to the patient background disease, since propofol in accordance with our protocol is used as a second-line sedative in patients requiring high doses of midazolam and fentanyl, or who fail to achieve adequate sedation with the latter substances, and who generally are the most seriously ill individuals. The longer duration of admission and increased mortality among the patients treated with propofol support this idea–though no firm conclusions can be drawn, since ours is a retrospective study, and no patient severity score measurements were made.
We likewise observed no increased incidence of serious adverse effects secondary to propofol administration in the literature, on establishing comparisons versus the group not administered propofol. These data were confirmed by the pharmacovigilance registry, which during this time did not record any serious effects resulting from the use of propofol.
One adverse effect of the administration of propofol is arterial hypotension, resulting from a decrease in peripheral resistance and cardiac inotropism.4,28 This effect has been related to administration of the drug in the form of boluses, particularly in hypovolemic patients. Our experience coincides with the observations of other authors29,30 who recorded no increased incidence of arterial hypotension in patients treated with propofol–despite the fact that most of our admissions correspond to cardiac postsurgery cases in which the patients usually present initial hemodynamic instability.
There have also been reports of increases in transaminases (GOT, GPT), gamma-glutamyl transpeptidase (GGT), amylase, triglycerides and lipase in the immediate postoperative period,31 as well as an increase in nosocomial infections, respiratory depression, epileptiform movements, headache, greenish colored urine, thrombosis, phlebitis, pancreatitis and postoperative fever. Among the effects reported in under 1% of the cases, mention should be made of amblyopia, restlessness, sialorrhea, hypomagnesemia, blistering or necrosis secondary to accidental extravasation, laryngeal spasms, cardiac arrhythmias and lung edema.13 In our study these side effects were not more frequent in the children treated with propofol than in the rest of the patients.
The main limitation of our study is its retrospective design and, as commented above, the fact that we cannot distinguish between the effects caused by the drug and those attributable to the patient background disease. On the other hand, although the study offers important safety information, it does not allow us to evaluate the efficacy of propofol for the prolonged sedation of critically ill children.
We conclude that propofol in continuous perfusion at doses of under 4mg/kg/h is safe and can be used as an alternative for the prolonged maintenance of sedation in critically ill children. However, careful monitoring of signs suggesting the appearance of propofol infusion syndrome is required. Large prospective studies are needed to analyze the risk factors underlying propofol infusion syndrome, and to assess the effectiveness and safety of low-dose propofol perfusion.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Agudelo SC, et al. Propofol en perfusión continua en niños en estado crítico. Med Intensiva. 2012;36:410–5.