Moderate traumatic brain injury (MTBI) represents 20% of all brain injuries, and includes patients with a Glasgow coma score of 9–13. The associated mortality rate is 15%, and close to 50% of all patients suffer sequelae.1,2 Moderate traumatic brain injury is very heterogeneous in terms of its severity and clinical course.1,2 It affects young adults as well as elderly people, and one-third suffer intracranial injuries.1,2 In such cases pain and agitation are more common as a result of neurological damage, trauma in other body regions, and the consumption of alcohol or drugs of abuse.1,2 Agitation can moreover magnify brain damage through different mechanisms: increased metabolism and oxygen consumption, increased intracranial pressure (ICP), lowered cerebral perfusion pressure (CPP), increased cardiovascular stress, and altered ventilation and oxygenation.3 Likewise, agitation complicates physical examination and diagnostic and therapeutic interventions.3 Adequate sedoanalgesia is essential in this respect, since both over- and under-sedation can contribute to secondary brain damage.4
In contrast to severe traumatic brain injury (STBI), where different clinical guides have established recommendations referred to sedoanalgesia,5 no such recommendations have been made in the case of MTBI. In this case, the recommendations established for STBI are commonly used. These recommendations usually comprise high-dose midazolam, remifentanil and propofol – i.e., drugs that alter patient level of consciousness, complicate neurological exploration and lead to the indication of invasive mechanical ventilation.4,5 Dexmedetomidine (DEX), a sedating and analgesic agent that acts by stimulating the α2-adrenergic receptors within the locus coeruleus and spinal cord, makes it possible to administer what is known as “cooperative or conscious sedation”–defined as sedation in which the patient is calm and comfortable, and is able to respond adequately and immediately to tactile and verbal stimuli. This makes it possible to assess the neurological condition, with preservation of the defensive reflexes of the airway and spontaneous ventilation, and systemic hemodynamic stability.6,7 Dexmedetomidine can cause arterial hypotension and bradycardia secondary to sympathicolysis; these effects can be avoided by suppressing the loading dose, titrating the drug dosage, and maintaining strict normovolemia.6,7 The drug does not alter ICP or cerebral oxygenation, and exerts neuroprotective effects.6–10
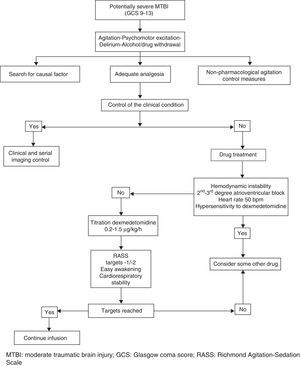
We present the preliminary results of a cooperative sedoanalgesia protocol (Fig. 1) based on the use of DEX in patients with MTBI, no associated lesions, and with a pathological Marshall computed tomography classification score admitted to the Intensive Care Unit of San Juan Bautista Hospital (Catamarca, Argentina) during the period November 2014 – May 2015. Informed consent was obtained, together with approval from the local ethics committee.
The protocol is based on assessment of the causal factor (trauma, factor, urinary retention), basic measures (verbal management, bladder catheter, adequate environment), analgesia and pharmacological treatment of agitation. Pain control is monitored on the basis of clinical parameters, and starts with nonsteroidal antiinflammatory drugs (diclofenac, ketorolac) administered via the intravenous (i.v.) route. In the event of contraindication of these drugs (gastritis, gastroduodenal ulcer, renal dysfunction, coagulation disorders), allergy, or active bleeding, or if the maximum doses have been reached without achieving pain control, we administer opioids. The drug of choice in this regard is intravenous morphine (2–4mg/2–4h). In the presence of hemodynamic instability, we use intermittent fentanyl at the recommended doses.4Table 1 shows the main clinical data of the patients included in the study.
Demographic and clinical data of the study population.
| Case | Age | Sex | Trauma mechanism | GCS | CT (Marshall) | COANA | Surgery | RASS B/A | MBP (mmHg) B/A | HR (bpm) B/A | RF (rpm) B/A | PaO2/FiO2 B/A | ICP B/A | Maximum dose DEX (μg/kg/h) | Days DEX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | F | Motorcycle | 12 | II | DF | No | 3/−1 | 91/89 | 75/68 | 19/16 | 482/477 | – | 0.7 (1.8)a | 3 |
| 2b | 19 | M | Motorcycle | 9 | SOL NE | MOR | Yes | 4/−2 | 90/84 | 87/71 | 18/16 | 432/428 | – | 0.3 | 5 |
| 3 | 80 | M | Fall | 11 | II | DF | No | 3/−2 | 129/104 | 107/72 | 22/16 | 417/416 | – | 0.5 | 5 |
| 4b | 56 | M | Fall | 9 | SOL NE | MOR | Yes | 4/−2 | 145/107 | 121/86 | 24/17 | 454/452 | 3/7 | 0.2 | 6 |
| 5 | 46 | M | Motorcycle | 10 | II | MOR | No | 3/−1 | 88/84 | 107/89 | 22/17 | 381/378 | – | 0.3 | 4 |
| 6 | 47 | F | Fall | 9 | III | MOR | No | 3/0 | 92/88 | 112/82 | 18/18 | 418/419 | – | 0.5 | 4 |
| 7b | 21 | M | Motorcycle | 9 | III | MOR | Yes | 4/0 | 87/84 | 92/83 | 26/20 | 401/404 | 9/11 | 0.4 | 3 |
B: before; COANA: coadjuvant analgesia; A: after 24h of infusion; DEX: dexmedetomidine; DF: diclofenac; F: female; HR: heart rate; RF: respiratory frequency; GCS: Glasgow coma score; h: hour; SOL NE: space-occupying lesion not evacuated at time of evaluation; M: male; MOR: morphine; ICP: intracranial pressure; RASS: Richmond Agitation-Sedation Scale; MBP: mean blood pressure; CT: initial Marshall computed tomography classification score upon admission; μg: micrograms.
1.8-fold maximum dose reached by mistake; breach in protocol. Transient arterial hypotension corrected by dose adjustment and infusion of 1000ml saline.
Patients 2 and 4 underwent surgery due to the following reasons: patient 2 developed late epidural hematoma; patient 4 presented subdural hematoma initially managed on a conservative basis. In turn, patient 7 initially presented type III diffuse axonal injury (cistern obliteration), together with bifrontal contusions<25ml, which subsequently required decompressive craniectomy due to refractory endocranial hypertension.
We do not administer a DEX loading dose, since it may cause arterial hypotension, which is deleterious in such patients.5–7 Dexmedetomidine exerts its effects within 15–30min, with peak plasma concentrations being reached after one hour. For this reason, we initially combine other drugs to help control agitation.7 In our series, we used low-dose midazolam (2mg i.v.) and chlorpromazine (25mg via the intramuscular route) in three and four patients, respectively.
Sixty minutes after starting DEX, all the patients showed a decrease of two or more points on the Richmond Agitation-Sedation Scale (RASS), while 71% of the patients reached adequate sedation and analgesia levels after 120min (RASS 0). Under these conditions the patients were calm and cooperative, under spontaneous ventilation, and serial neurological assessment could be carried out even in the postoperative period. The time course of the RASS scores is shown in Table 2. In order to avoid confounding effects, we used no other sedating agents and did not introduce drug combinations during the infusion of DEX–particularly involving drugs capable of interfering with neurological evaluation and/or which might depress patient ventilation. Coadjuvant analgesics were used in all cases at conventional doses (Table 1). On the other hand, we observed no adverse hemodynamic or respiratory effects, except in one case where DEX was used at doses far beyond the recommended ranges (breach in protocol). This iatrogenic complication required dose correction and the infusion of saline solution, followed by rapid resolution (Table 1).
Evolution of the scores on the Richmond Agitation-Sedation Scale (RASS).
| Patient | Before DEX | Start | 1h | 2h | 6h | 12h | 24h | 36h | 48h | Without DEX |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 3 | 3 | 1 | 0 | 0 | 0 | −1 | −2 | −2 | 0 |
| P2 | 4 | 4 | 1 | 1 | 0 | 0 | −2 | −2 | −2 | 0 |
| P3 | 3 | 3 | 1 | 0 | 0 | −1 | −2 | −2 | −2 | 0 |
| P4 | 4 | 3 | 1 | 0 | 0 | −1 | −2 | −2 | −2 | 0 |
| P5 | 4 | 4 | 0 | 0 | 0 | 0 | −1 | 0 | 1 | 1 |
| P6 | 3 | 3 | 1 | 1 | 1 | −1 | 0 | 0 | 1 | 1 |
| P7 | 3 | 3 | 1 | 0 | 0 | −2 | −2 | −1 | 0 | 0 |
DEX: dexmedetomidine; h: hour.
Vasopressor and inotropic drugs were not required. Likewise, in those cases where ICP was monitored after surgery, DEX did not alter the pressure values, and indeed may have contributed to pressure stabilization, since the ICP and CPP values remained within normal limits throughout the postoperative period. Dexmedetomidine was used for an average of 4.2 days. Once the patient clinical condition had stabilized, infusion of the drug was suspended on a gradual basis in all cases (20% of the dose every 6h), with oral risperidone (1.5–6mg/day) and clonazepam (1–4mg/day) having been started 24h before–coadjuvant analgesia being maintained in all patients. We recorded no rebound effects with DEX (hypertension, tachycardia) in this protocol.
To the best of our knowledge, this is the first description of the use of DEX in patients with MTBI and a pathological Marshall computed tomography classification score. Although the small sample size is admittedly a limiting factor, the preliminary data obtained indicate that DEX is effective and safe, affording adequate analgesia (with coadjuvant analgesics) and sedation during a period of 3–6 days, without problems on suspending the drug in a gradual and controlled manner. Therefore, although larger series are needed to confirm our results, we feel that DEX may be an option to be taken into account in the integral management of patients of this kind.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Godoy DA, Tolosa K, Lubillo-Montenegro S, Murillo-Cabezas F. Sedación cooperativa: opción para el manejo de la agitación en el traumatismo craneoencefálico moderado. Med Intensiva. 2017;41:193–196.