Critical pregnancy at high altitudes increases morbidity and mortality from 2500 m above sea level. In addition to altitude, there are other influential factors such as social inequalities, cultural, prehospital barriers, and lack the appropriate development of healthcare infrastructure. The most frequent causes of critical pregnancy leading to admission to Intensive Care Units are pregnancy hypertensive disorders (native residents seem to be more protected), hemorrhages and infection/sepsis. In Latin America, there are 32 Intensive Care Units above 2500 m above sea level. Arterial blood gases at altitude are affected by changes in barometric pressure. The analysis of their values provides very useful information for the management of obstetric emergencies at very high altitude, especially respiratory and metabolic pathologies.
El embarazo crítico en la alta altitud aumenta su morbimortalidad a partir de los 2.500 metros sobre el nivel del mar. Además de la altitud, existen otros factores influyentes como las desigualdades sociales, las barreras culturales, prehospitalarias y falta de desarrollo adecuado de las infraestructura sanitarias. Las causa más frecuentes del embarazo crítico que motivan el ingreso en las Unidades de Medicina Intensiva son los trastornos hipertensivos del embarazo (las residentes nativas parecen estar más protegidas), las hemorragias y la infección/sepsis. En Latinoamérica hay 32 Unidades de Medicina Intensiva a partir de los 2.500 msnm. La gasometría arterial en la altitud se ve afectada por los cambios de la presión barométrica. El análisis de sus valores proporciona una información muy útil para el manejo de las emergencias obstétricas a muy alta altitud, especialmente de las patologías respiratorias y metabólicas.
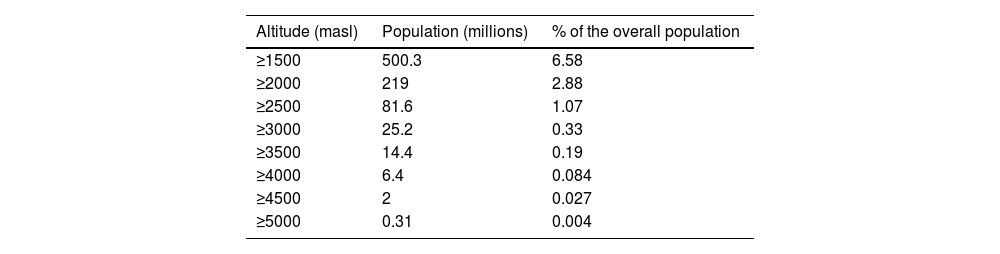
We call altitude to 1500 m above sea level (masl)1 because it has been observed that from 1500 masl, the first adaptations and effects on physical performance derived from the progressive drop of barometric pressure (BP) due to ascent to altitude can be observed. In Table 1, the millions of inhabitants living at altitude2 are exposed. Asia and South America have considerable populations residing 4000 masl, and it is in Asia where populations living > 5000 masl3 are found, although in Bolivia, there is a mining population that has been living at 5000 masl4 for over 100 years. These figures have been increasing in the last decade according to statistical projections.
Population living in high altitude.1
| Altitude (masl) | Population (millions) | % of the overall population |
|---|---|---|
| ≥1500 | 500.3 | 6.58 |
| ≥2000 | 219 | 2.88 |
| ≥2500 | 81.6 | 1.07 |
| ≥3000 | 25.2 | 0.33 |
| ≥3500 | 14.4 | 0.19 |
| ≥4000 | 6.4 | 0.084 |
| ≥4500 | 2 | 0.027 |
| ≥5000 | 0.31 | 0.004 |
masl, meters above sea level; %, percentage.
The life cycle begins with embryogenesis in a hypoxic environment within the first 10 weeks of pregnancy because the oxygen tension within the placenta is much lower (15 mmHg–20 mmHg).5 Subsequently, it increases up to 40 mmHg–80 mmHg and remains in this range within the second and third trimesters.6
Normal arterial oxygen partial pressures (PaO2) in individuals living at different altitudes vary from 75 mmHg to 100 mmHg at sea level down to 50 mmHg at 4000 masl, and to 19 mmHg–30 mmHg at much higher mountain altitudes. Although low birth weight babies, smaller placental size, and higher rates of preeclampsia (PE) have been found in mothers living at high altitudes, there is no direct evidence that their fetuses are more hypoxic than those of mothers living at sea level.7
Pregnancy at high altitude (HA), especially above 2500 masl,8 poses a challenge to oxygen transport, which is magnified because of the decrease in BP leading to limited O2 availability with a drop in arterial oxygen saturation (SaO2) and establishment of hypoxemia, causing hypoxic stress. Hypoxia consequent to altitude is known as hypobaric hypoxia (HH).9
During pregnancy at HA, a series of dynamic physiological responses begin to compensate for metabolic demands. One of the greatest physiological challenges is to maintain adequate oxygenated blood supply in the uteroplacental circulation to facilitate fetal development. Although more research is needed to reveal the mechanisms involved in vascular dysfunction, these mechanisms are related to increased hypertension (H) during pregnancy, intrauterine growth restriction (IUGR), preeclampsia (PE), postpartum hemorrhage, etc.10 Additionally, many risks associated with low and medium economic income in Latin America, which can increase morbidity and mortality of pregnant women at high altitudes should not be overlooked.11
The objective of this work is to review the problem of critical maternal diseases at high altitudes (>2500 masl) mainly focused on hypertensive disorders of pregnancy in Latin America.
Hypobaric hypoxia and pregnancyIn the HH of HA, environmental changes occur to which pregnant women are exposed, such as increased radiation, decreased temperature, lack of humidity, air dryness, and reduction of BP,12 the latter being considered the most important factor for pregnancy development, as it affects the ambient pressure of O2 and, therefore, oxygen availability for the mother and fetus.13 Hypoxic stress during pregnancy has maternal, placental, fetal, and perinatal repercussions.12
Relationship between hypoxia and the hypertensive disorders of pregnancyThe first recognition that chronic hypoxia was part of the etiology of PE occurred at high altitude. Retrospective data collected in Colorado, United States revealed that pregnancy-induced hypertension was more common at 3100 masl (12%) than at 2410 masl (4%) or 1600 masl (3%). Proteinuria and edema in the upper extremities were also more frequent at 3100 masl than at 1600 masl.14 However, in most cases, HTA during pregnancy at high altitudes is probably related to chronic hypoxia rather than classical PE.10,15 In fact, pregnant women at high altitudes lack the physiological drop in blood pressure at the beginning of the second trimester.14,15 One possible explanation is that chronic hypoxia decreases the vasodilator effect of nitric oxide (NO), while the sympathetic nervous system (α1-/α2-adrenergic receptor) is activated.5 In addition, potent vasoconstrictors such as endothelin-1 (ET-1) and hypoxia-inducible factor (HIF) are stimulated early in pregnancy due to excessive generation of reactive oxygen species (ROS).16
We know that, although pregnant women living at high altitudes (>2500 masl)10 are more susceptible to vascular complications of pregnancy, such as PE and IUGR,10 the genetic attributes of highland ancestry confer relative protection against vascular disorders of pregnancy at high altitudes.17 Andean ancestry, for example, protects vs altitude induced IUGR, provides better uteroplacental blood flow, and oxygen supply during pregnancy.18 This is one kind of protection that has not been observed in women of European descent who were born and raised at high altitudes.19 Julian CG et al.20 postulated the hypothesis, and subsequently demonstrated, that the expression of ion channels with known function as vasodilators or promoters of placental function was altered in women living at high altitudes with PE vs normotensive controls; and that the positive expression of these channels is associated with Amerindian ancestry (Bolivians and Peruvians) at high altitude, being negative in European ancestry.
Hypoxia, placental insufficiency, and birth weightExposure to high altitude develops placental insufficiency that affects natural birth weight, surpassing other risk factors such as low maternal weight, smoking, primiparity, history of preeclampsia, etc.21 A 1000 masl increase in altitude results in a natural mean decrease in birth weight of 100 g.5 Exposure to high altitude, which seems to be associated with a higher risk of PE, can further contribute to low birth weight in high-altitude populations.10 Ultrasound Doppler studies at 3600 m in Bolivia found differences in uterine artery flow from gestation week 20.18 Using the same methodology, we have been able to determine that the difference in fetal size between Cerro de Pasco (4320 masl) and Lima (150 masl) is observed from gestation weeks 25–29.22
Hypoxia and maternal effectsCardiovascular response to HA has been proposed as the main cause of pregnancy complications at altitude, such as systemic hypertension, bleeding, oligoamnios, placental insufficiency, preterm labor, and IUGR.10
The presence of maternal blood pressure is high, increasing in term pregnancies, doubling gestational hypertension and pregnant women at HA having up to 30% more hypertensive disorders than to similar populations.8 However, discrepancies in its prevalence have been observed, and these may be due to ethnic differences in the studied population, such as Andean natives adapted to HA and non-Andean population.12 We have found that Andean pregnant women from the Andes, Chile vs non-Andean women, have higher levels of progesterone, cortisol, estrone, 17β estradiol, at on pregnancy week 36 as a result of increased aromatase activity, and lower cortisol levels and higher antioxidant capacity. Lower cortisol, higher estriol, trends to higher levels of progesterone and 17β-estradiol, and greater antioxidant capacity were associated with larger uterine artery diameters and greater blood flow at HA, possible protective factors against pregnancy hypertensive disorders.23,24 Based on this information, it appears that hypertensive disorders during pregnancy are more common at high altitude, but multigenerational residents appear to be relatively more protected than newcomers are.12 Maternal mortality also increases with altitude, being the mortality range in women with severe PE and gestational hypertension at high altitude, 34.1% vs 11.8% at low altitude.9
Hypoxia and fetal effectsThe most frequent fetal finding in pregnancies at HA is IUGR, with marked decreases in fetal size and weight compared to lowland pregnancies. However, there are also differences between natives and European residents at HA.12 Several studies show that Andean ancestry favors higher birth weight12,21,25 because of increased blood flow in the uterine artery, which in turn facilitates oxygenation and nutrient exchange between the maternal and fetal sides of the placenta.20,26
Regarding late fetal mortality at HA, considered as death on gestation weeks ≥ 28, this risk is 4.82 times higher than at sea level.27
Acclimatization and maternal adaptation to high altitudeClassification of altitude in the management of critically ill patientsThe Expert Committee on Altitude Critical Care Medicine of the Pan-American and Iberian Federation of Critical Care Medicine and Intensive Therapy (CEMCA-FEPIMCTI) has worked on the consensus of these definitions and come to the conclusion that, since adaptive processes start at 1500 masl and intensive care units located at higher altitudes in the world are those in Cerro de Pasco, Peru, at 4380 masl, and El Alto, Bolivia, at 4150 masl, from a practical point of view and adapting the Barry and Pollard scale,28 altitude has been proposed to be categorized into 3 levels based on critical patient care29:
- •
Moderate altitude: from 1500 masl up to 2500 masl.
- •
High altitude: from 2500 masl up to 3500 masl.
- •
Very high altitude: from 3500 masl up to 5800 masl.
Possibly, it was the highland inhabitants of Ethiopia who first lived at high altitude (>3500 masl). Tibetan indigenous populations have been living at high altitude for a maximum of 25 000 years, and Andean populations for up to 11 000 years.30,31
The term acclimatization refers to the normal process by which humans from sea level or low altitude respond to hypoxic stress due to HH when exposed to a higher altitude (>1500 masl) compared to their natural habitat. In general, acclimatization to high altitude involves physiological changes that begin within seconds, while others will take minutes, hours, weeks, or even months.32 Acclimatization is not permanent and is lost when the subject returns to the usual habitat, so with each new ascent, the body has to restart the acclimatization process.
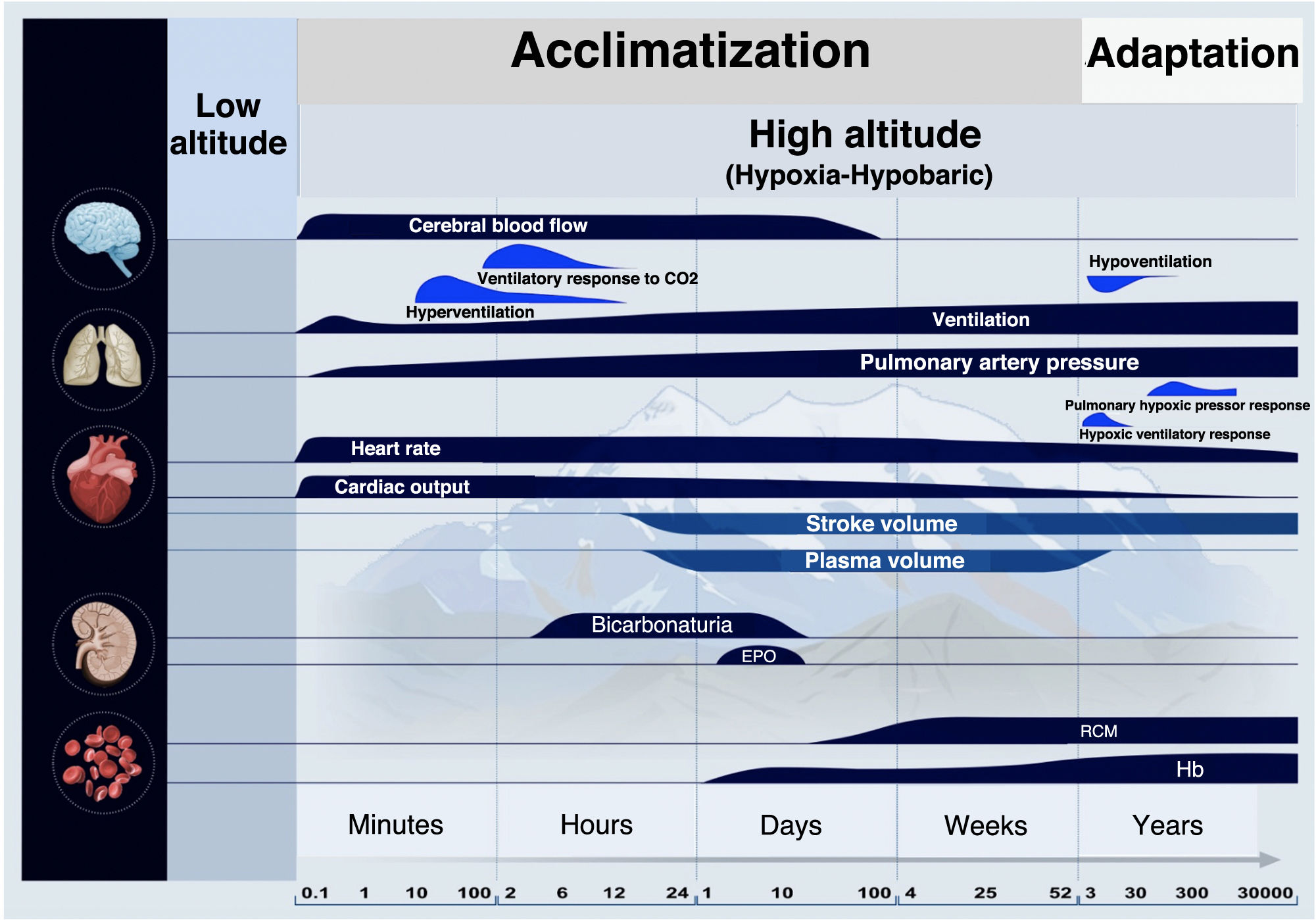
Adaptation is a long-term evolutionary response. It is a biological tool to live at altitude permanently, harmoniously, and with reproductive success associated with the transmission of genetic traits. Unlike acclimatization, it involves thousands of years and many generations, as shown by studies conducted among the Andean population.33Fig. 1 illustrates the evolution, over time, of the acclimatization and adaptation process.34–37
The main responses to ascent to high altitude involve the brain, lungs, heart, kidneys, and blood. Over time, qualitative changes are shown on a logarithmic scale. During acclimatization, these physiological changes range from minutes to weeks of stay at high altitude. Adaptation is an evolutionary response that involves thousands of years and many generations. Based on the original by Peacock34 and its subsequent and successive changes and adaptations by Imray et al.,35 Luks et al.,36 and Quispe-Cornejo et al.37
At high altitudes, there are various types of populations, classified according to the CEMCA-FEPIMCTI and based on their origin and length of stay. Based on their origin: a) atives: individuals born and raised at high altitudes, who have developed natural acclimatization (physiological adaptation) to hypoxia, enabling them to live and function efficiently. b) Non-natives or immigrants: individuals not born at high altitudes but migrated there. Based on their length of stay: a) Residents: individuals who permanently reside at an altitude of ≥1500 masl for, at least, 1 year or transiently for variable and intermittent periods of time (at least, 2 weeks per month for a 1 whole year). Although they may initially experience a few altitude-derived effects, they tend to acclimatize over time. b) Non-residents or visitors: individuals who do not live at high altitudes and spend very limited time in high-altitude areas, at risk of suffering from altitude-related illnesses due to lack of acclimatization.29
Adaptation to altitude and hypoxia-inducible factorThe hypoxia-inducible factor (HIF), especially the hypoxia-inducible factor 1α (HIF-1α), is the main factor in maternal vascular adaptation in multigenerational resident groups at HA.9 In the last 20 years, genetic studies of adaptation to HA have exponentially increased, concluding that the HIF pathway is the key mechanism through which hypoxia activates numerous genes related to blood vessel formation (VEGF, PDGF, FLT), red blood cell production (EPO), tumor necrosis factor (TNF), inflammation (IL6), vasodilation or vasoconstriction (EDN, NOS, PRKAA1), glucose metabolism (IGFBP, PRKAA1), and others that affect oxygen transport.38,39 There are certain genes, such as EGLN1 and EPAS1 that play a significant role in determining the variability of the response to HH and have been associated with polycythemia and associated abnormalities.40 For instance, the increased frequency of a causal variant of the EGLN1 gene in the Peruvian Quechua ethnic group enhances oxygen consumption and performance at altitude.41
Significance of the main physiological changes during pregnancy derived from altitude adaptationMaternal arterial oxygenation is a crucial determinant of newborn weight at very high altitudes and depends on numerous factors, such as the availability of environmental oxygen, ventilatory sensitivity, respiratory function, diffusion capacity, and oxygen transport capacity.13
The Andean population adapted to HA is characterized by higher lung volumes, decreased gradients between alveolar oxygen pressure and PaO2, reduced vasoconstrictor response to hypoxia, increased blood flow in the uterine artery during pregnancy, increased birth weight, increased head circumference, and higher oxygen consumption compared witj lowland inhabitants unadapted to HA.42–44
During pregnancy at HA, ventilatory sensitivity to hypoxia increases up to threefold, along with resting ventilation (RV), PaO2, and SatO2. This hyperventilation increases arterial oxygen content (CaO2). Andean ancestry, identified by 81 genetic markers, correlates with increased respiratory rate (RR) and reduced tidal volume (Vt). Additionally, there is a 40% increase in plasma volume, with no change in red blood cell mass, resulting in decreased hemoglobin levels. This hemoglobin decrease is compensated by an increased RV and SatO2, resulting in increased CaO2 to levels similar to those before pregnancy, which serves as protective changes for both the mother and the fetus.45
Studies show that several genes play a key and specific role in regulating HIF and are responsible for adaptation to very high altitude in Andeans and Tibetans. Multiple different chromosomal regions are involved in the presumed selection response.46 Andeans have low levels of ET-1, a potent HIF-1-induced vasoconstrictor during pregnancies at low or very high altitude. However, European mothers significantly increased plasma ET-1 levels at HA. Significant associations of single nucleotide polymorphisms (SNPs), genomic variants at a single DNA base position, have been identified with birth weight and vascular protection. These adaptations likely occurred within the last 10 000 up to 15 000 years in human populations living on the Andean plateau.47
In conclusion, maternal responses to achieve a successful pregnancy at HA depend on altitude ancestry characteristics and individual adaptation capacity.
Obstetric emergencies and Intensive Care Units in Latin AmericaEpidemiologyAdmission to an Intensive Care Unit (ICU) during pregnancy is uncommon. However, it is associated with a very high maternal and fetal morbidity and mortality. The incidence of ICU admission varies widely, ranging from 0.7 up to 13.5 per 1000 births.48,49 Although there are no major differences in the profile of ICU admission between developing and developed countries, there are differences in mortality rates, which range from 0% up to 14% in developed countries vs 2% up to 43% in developing ones.50,51
Data regarding Latin America are very scattered and inconclusive. In a study conducted in La Paz, Bolivia, the mortality rate is 3.6%, which is considered relatively low.52 A different study conducted in Argentina reported a rate of 11.2%,53 while another study stated rates of 2.6%.54 However, data on reasons for ICU admission have much more homogeneous results, with hypertensive disorders of pregnancy being more predominant in Latin American and less developed countries,50,52,53 obstetric hemorrhage being more frequent in developed countries or private hospitals in less developed countries,48,51,54 and sepsis/infection.48–57 A study conducted in Bolivia, La Paz on 401 cases of obstetric emergencies showed that 47% of cases were associated with severe hemorrhage and 46% with hypertensive disorders.52 Undoubtedly, this study, which represents 75% of hospital births and capturing most complicated pregnancies and births in the area, is biased because it is located in a large city where prehospital barriers are lower due to easy accessibility to health care. Still, a different study conducted in this setting with free and accessible maternal health care showed that 75% of women were in critical condition upon arrival.58 The main prehospital barriers found were associated with social, geographical, and cultural inequalities, lack of resources, and distrust in the system. Another example of a barrier is the significant deficit of specialists and ICU beds in countries like Bolivia. Before the COVID-19 pandemic hit, Intensive Care Units located 2500 masl had a total of 255 beds, which has now increased up to 291. In El Alto (4150 masl), with just over a million inhabitants, the total number of beds is 60. It is estimated that Bolivia only has 40% of the beds it needs, with 60% in the private sector, and the total number of intensivists barely exceeds 200.59
Intensive Care Units at altitudeTable 2 lists the polyvalent units of Latin American countries that have units located 2500 masl based on their location and altitude where they are located. These data have been reported by CEMCA-FEPIMCTI. The number of obstetric emergency rooms (OER) ranges from 5 up to 15%, and from 5% up to 20% required mechanical ventilation. This OER figure is striking because these are polyvalent units with a mean number of 10 beds. In a study conducted in a unit of a tertiary obstetric care center with 76 beds, including 4 ICU beds, the number of hypertensive ERs, hemorrhage, and sepsis/infection was 21.08% of all ICU admissions.49
Location and altitude of Intensive Care Units in Latin America at 2500 masl.
| Bolivia |
| El Alto: 4150 masl |
| Potosí: 4090 masl |
| Oruro: 3706 masl |
| La Paz 1: 3640 masl |
| La Paz 2: 3200 masl |
| Sucre: 2690 masl |
| Cochabamba: 2570 masl |
| Colombia |
| Ipiales: 2898 masl |
| Tunja: 2782 masl |
| Bogotá: 2625 masl |
| ChÍa: 2562 masl |
| Chiquinquirá: 2556 masl |
| San Juan de Pasto: 2527 masl |
| Ecuador |
| Tulcán: 2980 masl |
| Latacunga: 2860 |
| Quito: 2850 masl |
| Riobamba: 2764 masl |
| Cuenca: 2560 masl |
| Ambato: 2500masl |
| Mexico |
| Toluca: 2663 masl |
| Metepec: 2610 masl |
| Ciudad Serdán: 2526 masl |
| Peru |
| Cajamarca: 2720 masl |
| Ayacucho: 2781 masl |
| Andahuaylas: 2926 masl |
| Huaraz: 3052 masl |
| Huancayo: 3250 masl |
| Cusco: 3399 masl |
| Huancavelica: 3676 masl |
| Juliaca: 3824 masl |
| Puno: 3827 masl |
| Cerro de Pasco: 4380 masl |
masl, meters above sea level.
The application of intensive care principles during pregnancy at high altitudes is based on understanding the physiological changes related to pregnancy and altitude, as well as the unique conditions and diseases associated with obstetric patients. An essential aspect of intensive care at altitude setting it appart is the presence of pulmonary hypertension and chronic mountain sickness. Physiological cardiovascular changes during pregnancy can potentially exacerbate cardiac and pulmonary conditions.60 In the Andes, pulmonary hypertension and chronic mountain sickness, or Monge's disease, develop in 5% up to 18% of its inhabitants.61 Monge's disease, prevalent among Bolivian Andeans of mixed or entirely European genetic ancestry, is characterized by excessive erythrocytosis and hypoxemia and is often associated with moderate-to-severe pulmonary hypertension that can progress to cor pulmonale and eventually to congestive heart failure,62 complicating obstetric emergencies at HA.
Another crucial point relates to arterial gas changes. Normal gasometric values at altitude differ from those at sea level because they are conditioned by changes to BP with altitude and its influence on the progressive decrease in environmental oxygen pressure, inspiratory oxygen pressure, alveolar oxygen pressure, and arterial oxygen pressure. Altitude residents increase their minute ventilation, leading to greater CO2 elimination and causing respiratory alkalosis, which is compensated by a concomitant decrease in bicarbonate. This adaptation process results in normal values of arterial gas tests for altitude residents, corresponding to lower PaO2, PaCO2, SaO2, and CO3HNa with respect to sea level, while maintaining pH. Despite the decrease in PaO2, the oxygen cascade, which continues to function effectively in altitude-residing populations, ensures oxygen delivery to cells and mitochondrial function, regardless of inspired PO2.63
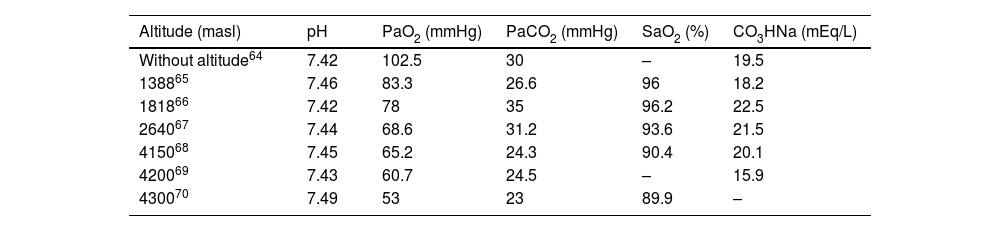
Pregnant women have a higher risk of developing acute respiratory distress syndrome vs non-pregnant women,56 which may be caused by processes similar to those described in non-pregnant patients.64 The analysis of impaired arterial blood gas test values provides valuable information about the respiratory and metabolic pathology of pregnant women. Table 364–70 shows the variation in altitude-related mean arterial blood gas test values reported within the 3rd trimester of pregnancy. Arterial blood gas test performed with caution provides valuable information on the management of obstetric emergencies at very high altitudes.68 The lessons learned in ICUs at altitude during the COVID-19 pandemic have greatly aided in understanding and managing respiratory disorders.71
Evolution of mean arterial blood gas test values reported for the 3rd trimester of pregnancy from sea level up to 4300 masl.
| Altitude (masl) | pH | PaO2 (mmHg) | PaCO2 (mmHg) | SaO2 (%) | CO3HNa (mEq/L) |
|---|---|---|---|---|---|
| Without altitude64 | 7.42 | 102.5 | 30 | – | 19.5 |
| 138865 | 7.46 | 83.3 | 26.6 | 96 | 18.2 |
| 181866 | 7.42 | 78 | 35 | 96.2 | 22.5 |
| 264067 | 7.44 | 68.6 | 31.2 | 93.6 | 21.5 |
| 415068 | 7.45 | 65.2 | 24.3 | 90.4 | 20.1 |
| 420069 | 7.43 | 60.7 | 24.5 | – | 15.9 |
| 430070 | 7.49 | 53 | 23 | 89.9 | – |
masl, meters above sea level; mmHg, millimeters of mercury; %, percentage; mEq/L, milliequivalents/liter.
Mechanical ventilation must be consistent with anatomical and cardiopulmonary changes and with the metabolic demands of the fetoplacental unit during pregnancy. The goal at sea level is to maintain maternal PaO2 levels between 65 mmHg and 70 mmHg, or higher, to guarantee adequate oxygen supply to the fetus.60 At altitude, hyperoxia should be avoided since adapted patients live in chronic hypoxia due to their residence at altitude. Arterial blood gas tests can serve as a guide to achieving values similar to those at different altitudes of residence (Table 3). Permissive hypercapnia is not a safe option during pregnancy from either a fetal or maternal perspective.72 Mechanical ventilation, in addition to ensuring adequate gas exchange, should minimize the risk of lung injury, aiming to be protective, with tidal volumes between 6 mL/kg and 8 mL/kg, avoiding excessive alveolar distension pressure (<14 cmH2O), with a plateau pressure (<30 cmH2O), and adjusting CO2 to values considered normal at the geographical altitude where the patient is located.60,72
ConclusionsCritical pregnancies at altitude increase morbidity and mortality 2500 masl. Native residents appear to be more protected against hypertensive disorders. There are also other influencing factors such as social inequalities, prehospital barriers, and the development of health care infrastructures. Arterial blood gas test at altitude is affected by changes in BP. Analysis of its values provides very useful information for the management of obstetric emergencies at very high altitudes, especially for respiratory and metabolic conditions.
FundingNone declared.
Conflicts of interestNone declared.
Authors’ contributionsA. Avila-Hilari and ML Avellanas-Chavala conducted the literature review, collected data from the ICU located 2500 m above sea level, and drafted the manuscript. All authors read, reviewed, and approved the manuscript.