Edited by: Federico Gordo. Intensive Care Service of Hospital of Henares, Madrid, Spain
Last update: April 2025
More infoIntensive Care Units (ICUs) have undergone enhancements in patient safety, and artificial intelligence (AI) emerges as a disruptive technology offering novel opportunities. While the published evidence is limited and presents methodological issues, certain areas show promise, such as decision support systems, detection of adverse events, and prescription error identification. The application of AI in safety may pursue predictive or diagnostic objectives. Implementing AI-based systems necessitates procedures to ensure secure assistance, addressing challenges including trust in such systems, biases, data quality, scalability, and ethical and confidentiality considerations.
The development and application of AI demand thorough testing, encompassing retrospective data assessments, real-time validation with prospective cohorts, and efficacy demonstration in clinical trials. Algorithmic transparency and explainability are essential, with active involvement of clinical professionals being crucial in the implementation process.
Las unidades de cuidados intensivos (UCI) han sido objeto de mejoras en la seguridad del paciente y la inteligencia artificial (IA) se presenta como una tecnología disruptiva que ofrece nuevas oportunidades. Aunque la evidencia publicada es limitada y presenta problemas metodológicos algunas áreas resultan prometedoras como los sistemas de ayuda a la decisión, detección de eventos adversos o errores de prescripción. El uso de la IA en seguridad puede tener un objetivo diagnóstico o predictivo. La implementación de sistemas basados en IA requiere procedimientos para garantizar la asistencia segura, enfrentando desafíos como la confianza en dichos sistemas, sesgos, calidad de los mismos, escalabilidad y consideraciones éticas y de confidencialidad.
El desarrollo y la aplicación de la IA demandan pruebas exhaustivas, incluyendo testeo sobre datos retrospectivos, validación con cohortes prospectivas en tiempo real y demostración de eficacia en ensayos clínicos. La transparencia y explicabilidad algorítmica resultan esenciales siendo la participación activa de profesionales clínicos en la implementación es crucial.
Artificial Intelligence (AI) is neither a new nor recent concept. The first time this term was used was back in the 1950s.1 However, it has been in recent years that its large-scale implementation has become possible due to exponential technological growth.2 AI is perceived as a powerful tool with enormous potential to change the way we live; however, AI is no stranger to controversy.3 Among all types of AI, those capable of reaching higher levels of predictive or even creative skills are based on deep learning models.4 These models are nonlinear and rely on a large number of mathematical operations, which complicate their interpretation.5,6 It is precisely this type of AI that generates more concern and distrust in society and is being analyzed from ethical and legal perspectives.7
While the introduction of AI requires a risk-benefit analysis in any scenario, in the health care field, this analysis must be deep and comprehensive. It has been proposed as a solution to many of the problems of current health care systems, contributing to decreasing mortality, shortening the length of stay, or preventing the occurrence of adverse events.8–16
Despite recent efforts to increase the culture of safety in health care systems, adverse events still significantly contribute to morbidity, mortality, and health care spending. The addition of new technologies emerges as a promising strategy in this regard. The critical care setting is an ideal scenario for the implementation of such technologies. Extremely ill patients undergoing numerous procedures and highly complex treatments on a daily basis, which happen to be prone to errors coexist in this context. Additionally, there is pressure for quick decision-making as it is a typical area for time-dependent conditions. The volume of information generated in an ICU can be overwhelming. In fact, it has been suggested that it exceeds the capacity of an expert clinician.17 Thus, the increase in available information in different formats (images, lab test results, genetics, invasive physiological monitoring, etc.) is not always associated with better decisions. Therefore, the addition of AI-derived technologies in critical care areas could help clinicians increase diagnostic or therapeutic capabilities and contribute to improving outcomes by facilitating better integration of information.18 There is growing controversy due to the proliferation of AI publications with poor methodological quality and questionable validity, which limits its implementation. A recent systematic review of more than 400 studies reporting models developed in the critical care setting found that more than 20% were associated with preventing the occurrence of adverse events. However, the authors highlight that the methodological quality of the articles was very poor, most of them being retrospective (96.4%) and highly biased.19
For all these reasons, the aim of this manuscript is to review the potential contribution of AI to patient safety in the critical care setting by summarizing technical aspects and providing examples. Secondly, aspects of safety related to the implementation processes of AI-derived technologies, which will undoubtedly be part of the future of our units, have also been reviewed.
Main applications of AI in patient safetyDecision support - clinical decision support systems (CDSS)Technological innovations allow for the implementation of CDSS aimed at assisting physicians in decision-making by quickly identifying patterns of potential problems (not easily perceptible by humans) and suggesting optimal treatment plans. These tools can analyze and synthesize large sets of clinical data quickly (health records, vital signs, or imaging).20 CDSS are not new. Their development dates back to the first advances made in computing in the 1970s.21 As noted, ICUs are a niche for CDSS due to their inherent characteristics (high availability of data, monitoring, clinical complexity), as well as new AI and Machine Learning (ML)-based technological implementations.
Why can CDSS be useful, and what role can they play?The implementation of CDSS seeks to improve quality in all dimensions, especially safety. Although their utility has been demonstrated in certain medical disciplines, their optimal role is still under discussion.22 They are useful for various reasons: 1- Addition of individualized medicine through the use of ML-based models, which have proven equal or even better than experienced professionals in various scenarios: mortality prediction, readmissions, renal failure, sepsis, and respiratory distress, among others. 2- Generation of therapeutic plans on demand. 3- Reduction of information overload, allowing teams to make better decisions based on the vast amount of available data (Table 1).21
Possible functionalities of CDSS, potential risks, and risk mitigation strategies.
| Functions and utilities of CDSS | Potential harms of CDSS | Risk mitigation strategies | Explanation |
|---|---|---|---|
| Patient safety | Alert fatigue | Prioritize critical alerts, minimize use of disruptive alerts for non-critical indications. | Alert fatigue could be minimized by prioritizing and selecting critically important alerts, having the greatest impact, and customizing alerts according to clinical scenarios. |
| Minimize the incidence of errors and adverse events. | Occurs when too many insignificant alerts are presented. There's a risk of disregarding alarms regardless of their importance. | ||
| Clinical management | Negative impact on user skills | Avoid systematic prescriptiveness in system design. Continuously evaluate system impact. | Systems should be implemented to be useful to clinicians without compromising autonomy or being overly 'prescriptive' and definitive. |
| Favor compliance with clinical practice guidelines, reminders for follow-up and treatment, etc. | An example is the dependency or excessive trust in the accuracy of a system. | ||
| Conflict with physician autonomy | |||
| Administrative function | Challenges in maintaining the system and its content | 2 strategies can be implemented here: | (1) Facilitate scheduled review, methods for acquiring and implementing new knowledge. Implement physician feedback measures on the system, and train users on proper data input. |
| Selection of diagnostic codes, automated documentation, and note auto-completion. | As practices change, there may be difficulties in keeping the content and knowledge rules that drive the CDSS up to date. | (1) Manage established knowledge, with a focus on translation to CDSS. | (2) It is important to identify changes in performance and usage over time. |
| (2) System for evolutionary performance measurement and analysis of CDSS. | |||
| Diagnostic support | User distrust towards CDSS | Include scientific references in messages when appropriate. | Provide a verifiable source of information to the user about why the recommendation exists. |
| Suggest diagnoses based on patient data and automate test result output. | Disagreement with the guidance provided by the CDSS. | In addition to increasing confidence, this can provide guidance to users to update their knowledge. | |
| Decision support for patients | Dependence on user computer literacy | (1) Adapt to existing functionality. | (1) Maintain consistency with the existing system user interface (if any) is crucial to ensure users do not have a long learning curve to use the system. |
| Assist patients in decision-making through personal health records and other systems. | CDSS may require a high level of technological competence for their use. | (2) Provide adequate training available at launch. | (2) Adequate and easily accessible training should be available for users. |
Adapted from Sutton R.21 CDSS: Clinical Decision Support System.
Until a few years ago, CDSS were based on prior knowledge using rules of the type “if A - then B,” which simplified medical practice. AI-based CDSS allow for the storage and processing of very variable patient-specific data sources, finally proposing recommendations with which we can provide feedback to the system. This mitigates the simplification of medical practice by previous CDSS.23 It is crucial for the ICU to facilitate access to a constant flow of data, allowing for specific analyses (time series) that can evaluate trends and potentially anticipate problems.24
Is it easy to implement CDSS in the routine clinical practice?The clinical implementation of CDSS is still limited, with some key elements we should take into consideration25:
- •
Trust: both patients and clinicians must trust the AI models used. There is also concern about clinicians' overconfidence as these systems require human supervision throughout the entire process.
- •
Bias: datasets used to train AI models may contain biases depending on their origin, epidemiological context, or data treatment.
- •
Scalability: the implementation of CDSS must be easily scalable and adaptable to a variety of clinical environments. Implementation requires a gradual process along with feedback between developers and health professionals. Improvement in data quality, numerous iterations and adjustments, and workflow optimization are also necessary.
- •
Deployment: CDSS face regulatory challenges because of the access to highly sensitive personal data involved, and the low reproducibility of results. However, in the case of CDSS, interest may lie in exploiting local characteristics, with data reevaluations and periodic refinements.26,27
- •
Ethical considerations: the implementation of these systems may pose a cultural and ethical challenge, which may impact how we see the autonomy of physicians and patients based on the suggestions made by these CDSS.28
- •
Clinicians' perspective: evidence shows that clinicians are favorable to the integration of CDSS, especially for certain clinical questions such as the probability of readmissions (Fig. 1). They also seek to understand those factors that contribute to predictions. Therefore, the development of algorithmic explainability (XAI, eXplainable Artificial Intelligence) is of special interest.29
Figure 1.Represents the transmission of information and generation of recommendations. The patient generates data that are clinically utilized by the physician, but which can also be leveraged for digital assessment. The increasing amount of data, especially in complex patient cases and environments with temporal pressure, can lead to biased decision-making. Therefore, processing the multiple sources of biomedical information from the patient (medical history, vital signs, radiological tests, laboratory results, or drus) can be transferred to systems that automatically store and process this information, upon which algorithms are applied. After applying algorithms, we obtain a recommendation, which can then be fed back into the system.
Recently, the use of AI has been proposed in the main domains of adverse events (AE), highlighting prediction, prevention, and early detection of patients at risk of deterioration. In this way, AI - based on the automation of records and the use of ML - offers new strategies to mitigate the occurrence of AE.30 Currently, there are algorithms that use real-time patient data accumulating information to personalize treatment during their admission. These tools, for example, would allow initiating or discontinuing antithrombotic therapy based on the risk of bleeding at a specific time during admission.31 Other algorithms allow summarizing all the information about a patient regarding an event of interest. By condensing it, they facilitate comparison with other patients, as well as the creation of patient cohorts with comparable risk profiles for a specific event of interest (mortality).32 There are multiple clinical settings in which results have been published in this regard, highlighting: 1- prediction and stratification of the risk of readmission, helping in patient flow management and avoiding AE associated with unplanned readmissions.33,34 2- prediction of kidney failure-related AE.35,36 3- prediction of unplanned extubation allowing the implementation of preventive measures and reducing the workload for the health care personnel.37 Although its implementation may focus on mitigating adverse events in ICU patients, it should not be limited to the ICU setting. This technology can be used anywhere in the health care system to predict early clinical deterioration or immediate transfers to the ICU that would allow early treatment initiation and resource organization.38,39 The effectiveness of an algorithm may be limited outside its original environment, as the data it uses reflect the culture and specific practices of each ICU. Consequently, what works in one ICU setting may not be applicable to another, especially if working conditions and staff ratios vary, thus affecting outcomes. The application areas of AI regarding safety are varied, whether inside or outside the ICU sttting (wall-less ICU strategy), and more and more innovative algorithms are becoming available that allow capturing and better adapting the information obtained from patient data to make more precise predictions.
Prescription and drug-related adverse eventsDrug-related incidents remain among the most frequent adverse events.40 Up to 25% of adverse events are considered preventable.41 Furthermore, intensive care units (ICUs), due to their technical complexity and association with time-dependent diseases, are more susceptible to prescription errors.42 The applications of AI in this area are diverse, including risk prediction models for the development of adverse reactions, detection of polypharmacy-related events, development of in silico interaction and allergy models, application of Clinical Decision Support Systems (CDSS), and exploitation of electronic health records for the detection of unintended adverse reactions.43 Depending on when these models are applied, they can help predict risk, reducing their incidence (prevention strategies), or subsequently contribute to early detection, reducing severity and duration (damage mitigation strategies).44
Regarding predictive use cases, various strategies and examples are found in the literature. The most common is the prediction of patients at high risk of adverse drug reactions. The most studied event groups in this regard are renal, cardiovascular, and opioid-related overdoses.44 A second group consists of predicting therapeutic response, which could prevent the use of drugs in non-responsive patients, which is particularly relevant in pharmaceutical groups with a high incidence of adverse events and poor tolerance, such as antineoplastics or some antivirals.45 Predicting the optimal dose is also an area of interest, with suitable drugs for these purposes being anticoagulants or antineoplastics.31,46,47 We should mention that these models integrate information from the patient’s entire medical history, including past health records, current free-text history, or lab test results. Although promising, we should mention that the addition of genetic results into the models has only marginally improved predictive capability.45 From a technical standpoint, the most widely used models are decision trees, natural language processing techniques, and neural networks, although both the variety and variability of these techniques used are extensive.
In the early incident detection block, we distinguish between detecting reactions and detecting medication errors that have already occurred (inappropriate prescription, interactions, and duplications). Undoubtedly, one of the most interesting areas is related to prescription errors. From a safety standpoint, although these occur after an individual action (prescriber, administrator, or consumer), they are considered system failures. Some authors even argue that they should be considered failures of clinical information systems.48 In recent years, some CDSS with ML algorithms have been tested to aid in real-time prescription error detection.49 These models add electronic health record information and subsequently detect prescriptions considered atypical by the model. These atypicalities can be due to infrequent prescriptions (contraceptive for an infant), discordance with the medical history (antidiabetic for a patient without a history of diabetes), or uncommon dosages. These systems can intervene in 2 different way, by generating synchronous (at the time of prescription) or asynchronous alerts (during follow-up when the patient's clinical situation changes). The main types of alerts and examples are described in Table 2.
Types of most common CDSS-generated alerts based on models.
| Alerttype | Definition | Examples |
|---|---|---|
| Time-dependent (synchronous) | Existing data in the patient's profile makes the prescribed medication inappropriate or dangerous. | Antihypertensive in a patient in septic shock. |
| Clinical atypicality | Prescription does not fit the patient's clinical profile. | Hypoglycemic drug to a patient without a diagnosis of diabetes mellitus or indicative results of such disease. |
| Dose atypicality | The dose of a certain drug is considered an outlier value with respect to the dose distribution learned by the model for that drug in the population and/or the patient's past medical history. | Rare doses, unusual dosing units, uncommon frequency, uncommon route. |
| Overlapping prescription | An alert signaled when there is simultaneous treatment with 2 drugs from the same group. | Duplicate prescription of noradrenaline infusions with different formulations. |
| Time-dependent (asynchronous) | An alert signaled when changes occur in the patient's profile after the prescription, making the prescription inappropriate or dangerous to continue. | When blood pressure decreases, and continuation of antihypertensive drugs is inappropriate. |
CDSS, Clinical Decision Support System.
One of the most developed systems in this regard is the MedAware software, which has been prospectively validated. In a validation on over 78 000 prescriptions, the alert rate was low (0.40%). Of these, 40% were synchronous alerts, with time-dependent alerts being the most frequent (64.80%). Of the generated alerts, 89% were considered appropriate, and 43% resulted in prescription changes. These data were superior to rule-based CDSS, which presented a high alert burden (37.10%) and low clinical significance (5.30% prescription changes).50
Safety in the implementation processes of AI-based toolsAs previously mentioned, AI can be useful in various aspects at the ICU setting. However, it also poses challenges, both medical and ethical, as well as technological. Therefore, it is relevant not only to analyze the utility of AI regarding patient safety, but also establish theoretical frameworks for the safe use and implementation processes of AI. After reviewing the possible contributions of this technology to safety, we will now delve into the risks it presents, the possible solutions regarding adverse events it may generate, and the safe implementation of these algorithms.
Generating safe ML-based AIPrediction algorithms employing supervised machine learning rely on learning from examples. Through them, a system is modeled capable of associating new events with learned data and generate a prediction. It is evident that the data used in learning will be a key point for the model's success. Therefore, data collection is essential, making sure that data accurately represent the target population. The following are among the most cited issues on this regard51:
- •
Imbalanced populations/cases: this occurs when not all groups are equally represented. If not considered during training, there's a risk of favoring predictions towards one group simply because it includes more cases.
- •
Non-generalization: this occurs when the population selection for training does not include cases from the entire target population. In this case, if the system goes into production, it will fail to generate predictions for these groups.
- •
Underrepresentation of a group: as in the previous case, a group is excluded from the training set. This is not a problem in population selection as much as an underrepresentation of this group for social or economic reasons that cannot be solved by broader selection of the training group.
During variable selection, confounding factors should be included, while making sure that variables favoring discrimination of a group have not been included. There are several examples illustrating the importance of this phase. During the COVID-19 pandemic, a lower risk of admissions due to virus-related pneumonias was observed in asthmatic individuals. This phenomenon may be due to risk underestimation in a specific subpopulation, namely, asthmatics developing pneumonia. This underestimation can be attributed to the lack of consideration of relevant factors, such as prior steroid use.52 Additionally, lower rates of heart failure-related admissions have been reported in exclusion risk populations, such as African American and Latino communities, which stresses the need for addressing disparities in risk assessment.53,54 In addition to defining the variables, we will have to select the error function that we want to optimize, i.e., the metric that will eventually evaluate our model. This selection is not trivial and can induce application biases.55 Therefore, it seems evident that it is essential to understand the effect of each evaluation metric on the problem being addressed.56
Various evaluation metrics, their drawbacks, clinical risks, transparency, and use cases are detailed in Table 3. Finally, it is advisable to define what it means for the developed model to be fair, understanding that individuals with similar characteristics are treated similarly.57,58 Several publications propose implementations considering the demographic parity and equality of opportunities; however, their use has not been standardized in the clinical field.59–61
Main metrics used in the evaluation of AI models.
| Metric | Description | Impact on clinical outcomes | Transparency | Examples |
|---|---|---|---|---|
| Accuracy | Percentage of data correctly classified. | Can lead to a higher rate of false negatives, which can delay or prevent appropriate treatment. | Easy to understand and interpret. | Prediction of the probability of death in COVID-19 patients |
| Precision | Percentage of positive data correctly classified. | Can lead to a higher rate of false positives, which can cause anxiety or stress in patients. | Can be difficult to interpret if the prevalence of the positive class is low. | Prediction of breast cancer presence in mammograms |
| F1-score | Weighted average of precision and recall. | Although it can be a good choice for balanced classification tasks, it is important to consider its potential impact on the clinical outcomes. | Can be difficult to interpret if the prevalence of the positive class is low. | Prediction of stroke probability in hypertensive patients |
| Specificity | Percentage of negative data correctly classified. | Can lead to a higher rate of false negatives, which can delay or prevent appropriate treatment. | Can be difficult to interpret if the prevalence of the positive class is low. | Biomarkers (used with precision) |
| ROC curve | Represents the relationship between true positive rate (TPR) and false positive rate (FPR). | Although it can be a good choice for comparing the performance of different models, it gives equal importance to both precision and specificity. | Can be difficult to interpret if the prevalence of the positive class is low. | Prediction of cancer survival probability |
| Area under ROC curve | Value of the ROC curve at points (0, 0) and (1, 1). | Although it can be a good choice to compare performance of different models, it gives equal importance to both precision and specificity. | Can be difficult to interpret if the prevalence of the positive class is low. | Prediction of mortality in ICU patients |
| Precision/Recall AUC | Represents the relationship between precision and recall. | Can be difficult to interpret if the prevalence of the positive class is low. | Can be a good choice for imbalanced classification tasks. | Prediction of acute hospitalizations in elderly receiving home care |
| Logarithmic loss | Sum of the logarithms of the probabilities of correct predictions. | Although it can be a good choice for comparing the performance of different models, it's sensitive to imbalanced data and lacks explainability. | It's a difficult metric to interpret, as it requires knowledge of logarithms. However, it's an objective metric that can be used to compare performance of different models. | Prediction of readmission 1 year after discharge |
| It does not take the severity of the error into account either | ||||
| Jaccard Index | Relationship between the number of elements correctly classified and the sum of the number of elements correctly classified and the number of elements misclassified. | Although it can be a good choice for comparing the performance of different models, it gives equal importance to both precision and specificity. Additionally, at an individual level (e.g., pixel in the case of images), it lacks gradation as it is a binary metric. | It's an easy metric to understand and interpret. However, it's less sensitive to false negatives than other metrics, such as accuracy or the F1-score. | Prediction of presence of brain damage on MRI images |
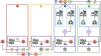
Having an AI generated securely with data from a development environment (DE) does not necessarily imply that it will function securely when implemented as a decision support tool in the routine clinca practice in real-time with data from an implementation environment (IE). It is important to clarify that, although these 2 environments can be different due to spatial dimension (2 different ICUs), they can also differ due to temporal dimension (same ICU, different time periods). Currently, there is no standardized protocol of the steps that should be followed to ensure its success. However, there are consensus guidelines among experts to help us during the process.62 Therefore, we propose a minimum of 4 necessary phases to safely transition an AI from a DE to a IE as a decision support tool (Fig. 2).
Map of the 4 phases of secure real-time AI implementation. In phase 1, the AI is tested to ensure it adapts to the reality of the data from the center where it is deployed. In phase 2, the data flow is constructed to evaluate its performance in real-time. In phase 3, the results of using AI vs not using it are compared in relation to patient benefit. Finally, in phase 4, the performance of the AI is continuously monitored, and necessary improvements are applied to ensure its evolution for the benefit of all.
AI requires a set of predictor variables (PV) to return the response variable (RV). An obvious first step is to ensure that PVs can be automatically obtained from the electronic health record (EHR) of the IE. The fewer PVs the AI requires and the less specific they are, the easier its implementation in different IE will be. Currently, AI models that have demonstrated high performance in literature are mostly not applicable to the routine clinical practice.63 If they can be automatically obtained, the AI's performance within the IE with retrospective data will be evaluated. If results are unsatisfactory, the decision should be made either to terminate the process or to open a new scenario for AI retraining to improve results in the IE, i.e., undergo a process of generalization.64 This latter step will depend greatly on the framework in which the integration process is being carried out, and all required ethical and legal guidelines must always be followed. It only makes sense to move on to phase 2 if good AI performance is achieved with retrospective data from the IE.
Phase 2: testing the AI with real-time data from the IE blindly for the clinicianTurning the extraction, transformation, and loading (ETL) process of PVs to run an AI in an ‘ad-hoc’ manner into a stable and scalable process or pipeline resistant to failures is a costly technological task. Without going into detail, once there is confirmation that everything works in real-time and that a protocol for handling system failures has been designed, the AI can be evaluated prospectively. This type of prospective evaluation is also necessary in cases where the DE and the IE are the same ICU, where what has changed is the temporal dimension. An AI that has shown good performance in its DE or in Phase 1 of the IE may be impaired by inherent time changes (new professionals, new habits, new drugs, pandemics, etc.). Therefore, ensuring sustained good performance in this Phase 2 is crucial, as it will indicate both that the IE has the necessary technological infrastructure to maintain the decision support tool and that the AI is robust enough to function stably over time.
Phase 3: clinical trial considering the use of AI as interventionIf Phase 2 is successfully passed, we know that we have a robust and stable AI capable of making accurate predictions in most cases. However, we do not know what impact its use by the clinical team could have had on the patient. This 3rd phase requires the design of a clinical trial capable of evaluating if there are significant differences between a control group without AI and an intervention group with AI.62 At this point, it can be crucial for the AI to be interpretable rather than a black box.65 An interpretable AI can give information to the clinician on the PVs that are impacting the RV, helping the clinician understand what should be changed to avoid that unwanted RV if they choose to do so. Conversely, if the AI is not interpretable, the task of finding out why the AI provides an unwanted RV will entirely fall on the clinician.
Phase 4: continuous monitoring and evolution of AIML-based AIs learn from a set of cases based on PVs and defining RVs. The temporal dimension inevitably turns any IE into a DE over time. New socio-economic contexts, new teams, new drugs, even new habits acquired from future human-AI synergies will render AIs obsolete if they do not evolve dynamically. For example, an AI trained to predict a certain adverse event in an environment where protocols did not use that same AI may stop working when the very AI is applied to prevent it, as new protocols adding the AI will have been generated, completely changing the context in which it was trained. In this final phase, a set of indicators of human actions motivated by the AI should be defined, whose monitoring will ensure that the coexistence of both intelligences (human-artificial) is beneficial for the patient. This set of indicators will depend on the type of AI and its objective. Finally, periodically and through constant clinical inputs, AIs should be retrained with new PVs to adapt to new IE and improve their performance.66
ConclusionsThe addition of artificial intelligence (AI) to the realm of security, while promising, faces key challenges. Predicting adverse events and aiding safe prescription represent significant opportunities. However, the lack of methodological quality in research and the need to address ethical concerns, such as trust and bias, are imperatives. Successful implementation requires not only technical robustness but also careful transition, ensuring understanding and acceptance by health care professionals. Continuous security and adaptability emerge as crucial foundations for effective collaboration between AI and health care, ensuring tangible benefits for patient safety.
During the preparation of this work, the authors used Chat-GPT 3.5 to request synonyms and improve the translation of technical expressions from English to Spanish. After using this service, the authors reviewed and edited the content as necessary, assuming full responsibility for the publication's content.
FundingNone declared.
Conflicts of interestJABM has worked for the artificial intelligence company Savana Médica. The remaining authors declared no conflicts of interest whatsoever.
Authors’ contributionJesús Abelardo Barea Mendoza: Conceptualization, drafting, editing, and final manuscript review. Josep Gómez Álvarez: Conceptualization, drafting, editing, and final manuscript review.
Alex Pardo Fernandez: Drafting and final manuscript review.
Marcos Valiente Fernandez: Drafting and final manuscript review.
None declared.