The RIFLE and AKIN criteria have definitely helped out to draw attention to the relationship between a deterioration of renal function that produces a small increase in serum creatinine and a worse outcome. However, the specific clinical utility of using these criteria remains to be well-defined. It is believed that the main use of these criteria is for the design of epidemiological studies and clinical trials to define inclusion criteria and objectives of an intervention.
AKI adopting term, re-summoning former ARF terminology, it is appropriate to describe the clinical condition characterized by damage to kidney, in the same way as the term is used to describe acute lung damage where the lung injury situation still has not increased to a situation of organ failure (dysfunction).
The serum and urine biomarkers (creatinine, urea, and diuresis) currently in use are not sensitive or specific for detecting kidney damage, limiting treatment options and potentially compromising the outcome. New biomarkers are being studied in order to diagnose an earlier and more specific AKI, with the potential to change the definition criteria of AKI with different stages, currently based in diuresis and serum creatinine.
Los criterios del RIFLE y del AKIN han ayudado definitivamente a llamar la atención sobre la relación entre un deterioro de la función renal que produce un pequeño incremente de la concentración sérica de creatinina y un peor pronóstico. Sin embargo, la utilidad clínica concreta del uso de estos criterios permanece por definir. Se cree que la principal utilidad de estos criterios reside en su uso en estudios epidemiológicos y en ensayos clínicos, para definir criterios de inclusión y objetivos de una intervención.
La adopción del término DRA, reemplazando a la antigua terminología de IRA, resulta apropiada para designar la condición clínica caracterizada por daño del órgano, de la misma forma que se utiliza el término daño pulmonar agudo para describir la situación de lesión pulmonar que todavía no ha progresado a una situación de insuficiencia del órgano (disfunción).
Los biomarcadores séricos y urinarios (creatinina, urea, diuresis) actualmente en uso no son sensibles ni específicos para la detección de daño renal, limitando las opciones terapéuticas y potencialmente comprometiendo el pronóstico. Nuevos biomarcadores se encuentran en estudio con el objeto de diagnosticar de una forma más precoz y específica el DRA, con el potencial de cambio de los criterios de definición y estadificación del DRA, actualmente basados en la diuresis y la concentración sérica de creatinina.
Acute renal failure (ARF) is common in the Intensive Care Unit (ICU). The incidence of the syndrome depends on the definition used. In a recent Spanish study,1 the incidence of ARF (defined as serum creatinine elevation ≥2mg/dl or diuresis <400ml/24h) was found to be 5.7%. The incidence in turn is 20–50% according to other studies (reviewed by Case et al.2) that use the more sensitive risk, injury, failure, loss, end-stage disease (RIFLE) criteria. ARF is an independent predictor of patient survival, and is associated with a mortality rate of 40–90%,2,3 or 43% according to the Spanish FRAMI study.1 Approximately 4–5% of all critical patients require renal replacement therapy.2 A multinational study reported the prevalence of renal replacement therapy to be 4%, or about two-thirds of all patients with ARF (defined in that study as diuresis <200ml/12h or blood urea nitrogen (BUN) >84mg/dl).4 The development of ARF, with the consequent risk of requiring renal replacement therapy, is associated to an increase in morbidity and costs.5
The aims of the present review are: (i) to review recent concepts referred to acute kidney injury (AKI) and the currently used diagnostic criteria; and (ii) to review the evidence on the usefulness of AKI biomarkers. The study in turn offers a series of comments on promising advances in the use of -omic scientific techniques for the identification of AKI biomarkers, as well as on the need to demonstrate improvement in some clinical outcome variable of interest in order to be able to recommend a concrete biomarker.
Serum creatinine and urea concentration, and diuresis, are renal dysfunction markers. Changes in these variables indicate that the kidney is no longer adequately performing its physiological functions. However, it is known that as a result of injury (ischemia, inflammation), the organ suffers damage (manifesting for example as changes in cell phenotype) that precedes dysfunction as such. The detection of such organ damage before dysfunction develops would allow the adoption of measures to correct the altered physiology before the situation progresses toward phases characterized by irreversibility, lesser treatment efficacy, and a poorer prognosis.
Concept of acute kidney injuryAcute renal failure is defined as an abrupt decrease in glomerular filtration, with the consequent increase in the concentration of nitrogenated products in blood, with or without associated oliguria.5 The last decades have been characterized by the coexistence of over 30 definitions of ARF, based on different serum creatinine concentration values.6,7
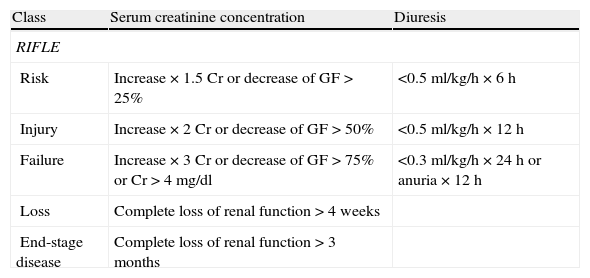
The Acute Dialysis Quality Initiative proposed the renal function classification known as RIFLE to classify patients with ARF.6 The RIFLE system contemplates three renal dysfunction categories (risk, injury and failure) and two clinical outcomes (loss of function and end-stage disease) (Table 1). Different clinical studies have demonstrated the correlation between the different categories and the prognosis of critical patients, and their independent association to mortality.5–8 The categories also represent evolutive stages, since 56% of the patients who are classified as being at risk progress toward another category, and 37% of those with injury progress toward the failure category.7
Comparison of the RIFLE and RIFLE modified by AKIN classification criteria.
| Class | Serum creatinine concentration | Diuresis |
| RIFLE | ||
| Risk | Increase×1.5Cr or decrease of GF>25% | <0.5ml/kg/h×6h |
| Injury | Increase×2Cr or decrease of GF>50% | <0.5ml/kg/h×12h |
| Failure | Increase×3Cr or decrease of GF>75% or Cr>4mg/dl | <0.3ml/kg/h×24h or anuria×12h |
| Loss | Complete loss of renal function>4 weeks | |
| End-stage disease | Complete loss of renal function>3 months | |
| Stage | Serum creatinine concentration | Diuresis |
| RIFLE modified by AKINb | ||
| 1 | Increase basal Cr≥0.3mg/dl or increase in basal value ≥150–200% in 48h | <0.5ml/kg/h×6h |
| 2 | Increase Cr>200–300% | <0.5ml/kg/h×12h |
| 3a | Increase basal Cr>300% of basal Cr≥4mg/dl with increase >0.5mg/dl | <0.3ml/kg/h×24h or anuria 12h |
AKIN: Acute Kidney Injury Network; Cr: serum creatinine concentration; AKI: acute kidney injury; GF: glomerular filtration; RIFLE: risk, injury, failure, loss, end-stage renal disease.
Patients requiring renal replacement therapy are included in stage 3, regardless of creatinine concentration or diuresis.
According to the Kidney Disease Improving Global Outcome (KDIGO), the time window needed to document an increase in serum creatinine concentration of 0.3mg/dl is maintained as 48h, while the time for an increase of 50% is 7 days, as initially recommended by the RIFLE criterion.
The KDIGO criteria only use changes in serum creatinine concentration and diuresis for AKI staging, and do not consider changes in glomerular filtration, except among patients under 18 years of age (see text).
According to the KDIGO criteria, AKI is classified as corresponding to stage 1, stage 2 or stage 3, following the AKIN criteria.
Posteriorly, the Acute Kidney Injury Network (AKIN), developed by the Acute Dialysis Quality Initiative and the European Society of Intensive Care Medicine, proposed replacing the term “failure” (which refers to the loss of renal filtration function) with the term “injury”.8 The AKIN system defined AKI as a structural or functional alteration, or evidence of renal damage, including any urine or blood test alteration, or imaging technique anomaly, with a duration of under three months.8–10 The current diagnostic criteria referred to ARF are described in Table 1.
The addition of the criterion corresponding to creatinine elevation ≥0.3mg/dl is based on epidemiological data which indicate an 80% increase in mortality associated to changes in creatinine concentration of 0.3–0.5mg/dl.9 This observation was subsequently reproduced in patients subjected to mechanical ventilation (MV).10,11
It is not clear whether the modification of the RIFLE criteria proposed by the AKIN system has substantially changed the classification of patients with AKI or improved the capacity to predict mortality.12
The ARF staging criteria of the AKIN allow the definition of three stages of increasing severity that correspond to the categories risk, injury and failure of the RIFLE classification-eliminating the categories loss of function and end-stage disease, which are defined as outcomes.
Lastly, the recently published Kidney Disease Improving Global Outcome (KDIGO) guidelines for the treatment of ARF have included a revised definition of AKI, maintaining the AKIN staging criteria13 (Table 1). The time window required for documenting an increase in serum creatinine concentration of 0.3mg/dl is maintained as 48h, while the time for an increase of 50% is taken to be 7days, as initially suggested according to the RIFLE criteria. For the staging of AKI, the KDIGO criteria only use changes in serum creatinine concentration and in diuresis, but not changes in glomerular filtration – with the exception of patients under 18 years of age, in which an estimated acute decrease in glomerular filtration to <35ml/min/1.73m2 body surface area is included as a criterion for stage 3. In the same way as with the RIFLE and AKIN criteria, patients must be classified according to the criteria associated to the most advanced stage of injury.
According to the KDIGO criteria, AKI is staged as follows:
- -
stage 1: a 1.5–1.9-fold increase in serum creatinine concentration, or an absolute increase of 0.3ml/dl, or diuresis <0.5ml/kg/h during 6–12h
- -
stage 2: a 2.0–2.9-fold increase in serum creatinine concentration, or diuresis <0.5ml/kg/h during ≥12h
- -
stage 3: a ≥3-fold increase in serum creatinine concentration, or concentration ≥4mg/dl, or diuresis <0.3ml/kg/h ≥24h, or anuria during ≥12h, or start of renal replacement therapy, or (in patients <18 years of age) reduction of estimated glomerular filtration <35ml/min/1.73m2
Although the new definitions and the proposal of the RIFLE and possibly AKIN criteria constitute clear advances in establishing the diagnosis and prognosis of AKI, they make use of variables (serum creatinine concentration and diuresis) that are subject to serious limitations (vide infra). This has led to the study of other biomarkers offering greater sensitivity and specificity in establishing the diagnosis and prognosis of AKI.
Serum creatinine concentration and urea: advantages and limitationsSerum creatinine concentration is useful as a marker of glomerular filtration, since creatinine is a solute that is freely filtered at glomerular level, with scant tubular processing. However, the use of serum creatinine concentration as an indicator of renal function has its limitations, particularly in critically ill patients. Firstly, serum creatinine concentration is not a sensitive or early marker of renal dysfunction, since a decrease in glomerular filtration of at least 50% is required in order to detect an increase in serum creatinine concentration.14 Secondly, in the absence of steady state conditions, the serum creatinine concentrations may be diminished while the glomerular filtration rate is very low, since there has not been enough time for the creatinine to accumulate. On the other hand, a decrease in glomerular filtration is accompanied by an increase in the proximal tubular secretion of creatinine, which initially makes it possible to maintain the serum creatinine values. In turn, serum creatinine concentration is not only dependent upon glomerular filtration but also on other variables such as: (a) muscle mass, which is usually diminished in critical patients; (b) liver function, which is responsible for metabolization of the molecule; and (c) the distribution volume, which is often increased under conditions of systemic inflammatory response. Serum creatinine concentration is therefore dependent upon a range of variables that also include, for example, patient age, sex, diet, muscle metabolism, medication and hydration.
In the same way as creatinine, serum urea concentration is not a specific marker of glomerular filtration. In this regard, it can increase under certain conditions in the presence of normal renal function, as in treatment with corticosteroids, gastrointestinal bleeding, or a hyperproteic diet.14
DiuresisDiuresis is used as an indicator of hemodynamic and renal function status. The presence of oligoanuria has strong positive predictive value in relation to renal failure and is a marker of poor prognosis in the critically ill patients. On the other hand, in critical patients oliguria develops before changes occur in serum creatinine concentration, and allows an early diagnosis of AKI. Likewise, oliguria is associated to increased mortality, independently of serum creatinine concentration.
However, the degree of oliguria is not correlated to the severity of kidney injury. A normal urine output does not rule out the presence of AKI, since many patients present a non-oliguric form of AKI. On the other hand, many interventions that are carried out in the ICU (e.g., the administration of diuretics, fluid resuscitation measures, the administration of dopamine) affect diuresis without necessarily modifying renal function.12,14
Markers of acute kidney injuryThe availability of an AKI biomarker may facilitate: (i) the early detection of kidney injury, thereby allowing the application of treatment potentially capable of preventing progression toward more advanced renal dysfunction categories; (ii) distinction among the different types of AKI (prerenal, renal, obstructive); (iii) risk stratification; (iv) monitoring of treatment response; and (v) prediction of treatment response. The ideal biomarker must be sensitive, specific, early, noninvasive, predictive, indicative of the site of injury, prognostic and economic.
Different molecules that can be detected in blood or urine have been investigated in recent years with these objectives in mind. The serum biomarkers include cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), and uric acid. The urinary biomarkers in turn are classified as follows:
- (1)
Enzymes released by damaged tubular cells (alkaline phosphatase, γ- glutamyl transpeptidase, alanine aminopeptidase, Ala-(Leu-Gly)-aminopeptidase, fructose-1–6-biphosphatase, glutathione-S-transferase α and π isoenzymes, N-acetyl-β-d-glucosaminidase)
- (2)
Low molecular weight molecules expressed in AKI (α-1-microglobulin, β-2-microglobulin, retinol binding protein, cystatin C, adenosine deaminase binding protein)
- (3)
Proteins specifically produced in the kidney under conditions of AKI (cysteine-rich protein 61, NGAL, KIM-1, cytokines and chemokines such as Gro-α or IL-18)
- (4)
Tubular structural and functional proteins (actin F, Na+/H+ exchanger isoform 3)
- (5)
glomerular filtration markers (pro-ANP, cystatin C)15
The present review examines the evidence on the possible clinical usefulness of the most widely studied AKI markers.
Each molecule offers different properties as a biomarker (e.g., increasing the sensitivity of the diagnosis, establishing a prognosis, etc.). On the other hand, the utilization of a panel of several biomarkers has been proposed as a way to improve upon the individual properties of each individual marker. As an example, a systematic review of the literature identified 25 studies with good methodological quality.15 Serum cystatin C, urinary IL-18 and urinary KIM-1 showed good behavior in application to the differential diagnosis of established AKI. Serum cystatin C and urinary NGAL, IL-18, glutathione-S-transferase-π and γ-glutathione-S-transferase showed good behavior in establishing an early diagnosis of AKI. NGAL in turn proved to be a good predictor of the need for renal replacement therapy.16 Urinary N-acetyl-β-d-glucosaminidase, KIM-1 and IL-18 were more useful in predicting mortality due to AKI.
Cystatin CCystatin C is a protein produced by all nucleated cells in the body, belonging to the cysteine-proteinase inhibitors superfamily. Under physiological conditions, cystatin C is freely filtered as a result of its low molecular weight, since it does not bind to proteins, and is reabsorbed in the proximal tubule, where it undergoes catabolism.15 Consequently, an increase in the urinary concentration of cystatin C is indicative of renal tubular damage.17 Under conditions of diminished glomerular filtration the serum concentration of cystatin also increases.18
However, the serum cystatin C levels are not specific of kidney injury, since they are also found to be elevated in elderly patients, in males, obese subjects, smokers, people with altered thyroid gland function, and in patients subjected to immunosuppressive therapy.19
The studies on the capacity of serum cystatin C concentration to predict the development of AKI have yielded discordant results. In critical patients at risk of developing AKI, an increase in serum cystatin C concentration was associated to the development of AKI, and preceded the rise in serum creatinine concentration by 1–2 days.20 In another study, serum cystatin C concentration predicted the development of AKI in a univariate analysis (area under the receiver operating characteristic [ROC] curve 0.80).21 In turn, among heart surgery patients, the serum cystatin C levels at the time of admission to the ICU predicted the development of AKI, the need for renal replacement therapy, and mortality.22
In a study of critically ill patients, the urinary concentration of cystatin C was associated to a diagnosis of sepsis or AKI, and to mortality.23 In a metaanalysis of 19 studies in heterogeneous patient populations (heart surgery, pediatrics, ICU), the serum cystatin C concentration predicted the development of AKI (odds ratio [OR] 23.5; 95% confidence interval [95%CI] 14.2–38.9). The urinary concentration was found to be less useful.24
Interleukin-18Interleukin-18 (IL-18) is a proinflammatory cytokine of the IL-1 family, produced by monocytes, macrophages and proximal tubular cells. Its urinary concentration in patients with acute lung injury (ALI) predicts the development of AKI in 24h, and a multivariate analysis has found it to be associated to mortality.25
In a recent study of 101 patients with AKI and a need for renal replacement therapy, the serum concentration of IL-18 was found to be associated to mortality after adjusting for the APACHE III score.26 In another study of 137 critical pediatric patients, the urinary concentration of IL-18 was significantly correlated to the severity of AKI.27 In non-septic patients, the urinary concentration of IL-18 increased two days before an increase was noted in serum creatinine concentration, was able to predict the development and duration of AKI on the first day of admission to the ICU, and was associated to mortality independently of the pediatric RIFLE score.27
A recent metaanalysis including 18 studies on the usefulness of the urinary concentration of IL-18 in diagnosing AKI of different causes (heart surgery, intravenous contrast injection, emergency care, or in the ICU setting)28 found the diagnostic odds ratio for AKI to be 4.22 (95%CI 2.90–6.14, with a sensitivity and specificity of 0.58 and 0.75, respectively). The diagnostic value of urinary IL-18 was greater in children than in adults.28
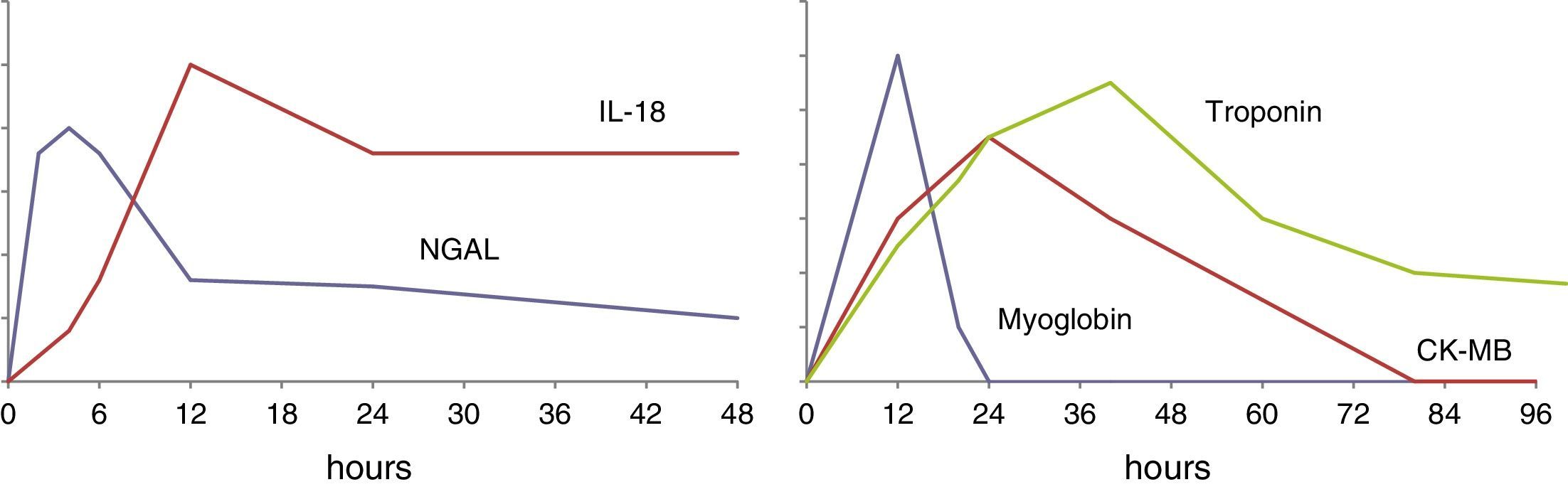
The urinary concentration of IL-18 exhibits a time course (Fig. 1) characterized by an initial increase after 4–6h, with a maximum concentration after 12h. The levels remain elevated for approximately 48h.26–29
Kidney injury molecule-1Kidney injury molecule-1 (KIM-1), also known as the cell receptor of the hepatitis A virus or T cell immunoglobulin and mucin-containing molecule (TIM-1), is a transmembrane glycoprotein exhibiting only minimal expression under normal conditions. However, KIM-1 expression is greatly increased following renal ischemia in the rat.30 KIM-1 participates in the regeneration process after epithelial damage, and in the elimination of dead cells in the tubular lumen.31 Loss of the tubular cell brush border in kidney injury includes the extracellular domain of KIM-1, with the consequent increase in its urine concentration. In this regard, the urinary concentration of KIM-1 has been proposed as a biomarker of proximal tubular damage.32
The urinary levels of KIM-1 are higher in cases of AKI due to ischemia than in AKI attributable to other causes.32 In critical patients, KIM-1 is related to the severity of the disease, the need for renal replacement therapy, and mortality.33 A number of studies have demonstrated the usefulness of the urinary concentration of KIM-1 in predicting the development of AKI in patients subjected to heart surgery.34
A recent systematic review35 has analyzed 8 articles comparing patients with AKI versus patients without AKI. The concentration of KIM-1 increased significantly 2h after heart surgery. It also increased in patients with established AKI, particularly in those with acute tubular necrosis. However, the correlation to the need for renal replacement therapy or mortality was weak.
Neutrophil gelatinase-associated lipocalinNeutrophil gelatinase-associated lipocalin (NGAL) is a small 178-amino acid protein belonging to the lipocalin superfamily of 20 proteins. These molecules share a beta-barrel-like structure that forms a goblet shaped element which binds to and transports a series of low molecular weight proteins that define the biological activity of each lipocalin. Examples are retinol binding protein, which transports vitamin A, or lipocalin α 1-microglobulin, which metabolizes the heme group. NGAL was initially identified as a protein bound to gelatinase in neutrophils, where it constitutes one of the proteins of the secondary granules (see Haase et al.36).
Bacteria produce siderophores which chelate or bind to extracellular iron. NGAL shows affinity for siderophores, and the resulting NGAL-siderophore complexes interact with specific membrane receptors and are internalized within the cell–thereby increasing the intracellular iron concentration. NGAL prevents the growth of bacteria that depend on siderophores to maintain their iron supply. Consequently, NGAL is a critical component of innate immunity.
NGAL is expressed at very low levels in different tissues such as the kidneys, trachea, lungs, stomach or colon, though its expression increases notoriously in the context of inflammation. NGAL is therefore a marker of systemic leukocyte activation, and is regarded as an acute phase reactant. Its specific function has not been fully established, though it reportedly exerts a renal protective effect, as demonstrated in animal models of ischemia-reperfusion AKI, as well as bacteriostatic action.37,38 There are different molecular forms of NGAL–the monomeric presentation being the form predominantly synthesized by the tubular cells, while the dimeric form is released by neutrophils. The different studies found in the literature focus on different molecular forms–a fact that could at least partially account for the diversity of the reported findings.39
NGAL is freely filtered and is reabsorbed at proximal tubular level through endocytosis. Damage to the proximal tubular epithelium alters its reabsorption. On the other hand, under conditions of kidney injury, the expression of NGAL in the distal tubular epithelium increases, particularly in the ascending arm of Henle's loop and in the collector tubules. The urinary concentration of NGAL increases under conditions of tubular damage as a result of both diminished reabsorption and increased release of the molecule into the tubular lumen–indicating both proximal and distal tubular damage.
NGAL is an early marker of kidney injury, since its serum concentration increases 2h after injury and precedes the rise in serum creatinine concentration by 24h. Its urinary and serum concentrations are also increased in other situations such as urinary tract infection and chronic renal disease.40
NGAL is one of the most intensively investigated AKI biomarkers, with the inclusion of several thousands of patients in different studies36–the most representative of which are reviewed here.
Heart surgeryThe urinary and plasma concentrations of NGAL are closely related to the development of AKI in patients subjected to heart surgery. In 16 of the 81 adults subjected to heart surgery and who developed AKI, the concentration of NGAL in urine after the operation predicted the development of AKI with a sensitivity of 81%, a specificity of 48%, and an accuracy of 58%.41 In a study involving 196 children subjected to heart surgery, of which 99 developed ARF (defined as an increase ≥50% of the basal creatinine concentration), the levels of NGAL in urine increased 15-fold 2h after the operation (area under the ROC curve 0.95, sensitivity 82% and specificity 90%), while the diagnosis based on the creatinine concentration criterion was delayed 2–3 days.42
In another study of 879 adults, of which 75 developed AKI (defined as an increase ≥50% of the basal creatinine concentration), plasma NGAL measured after the operation exhibited a sensitivity of 39% and a specificity of 81% in diagnosing AKI.43 Lastly, in 1219 adults, the plasma concentration of NGAL improved the predictive capacity of the clinical model (area under the ROC curve 0.69–0.75).44 The sensitivity of the plasma concentration of NGAL in diagnosing AKI was therefore more limited in these studies.44,45
Critical patientsIn general, since NGAL is released under conditions of systemic inflammation, its capacity to predict the development of AKI is poorer in critical patients in general, particularly in the presence of sepsis–with superior performance in the case of the urinary versus plasma measurements.
Referred to the general pediatric population, Zapitelli et al.,45 in a study of 140 critically ill children, showed the rise in urinary concentration of NGAL to precede the increase in serum creatinine concentration by two days among the individuals that developed AKI (area under the ROC curve 0.79), though no association to the severity of kidney injury was observed. The serum NGAL concentration in the first 24h following admission to the ICU differentiated among healthy children, children with a systemic inflammatory response, and those with sepsis and septic shock. In addition, the concentration was higher in the patients with AKI than in those without AKI.46
In a heterogeneous group of 451 critically ill adults, the urinary concentration of NGAL after 24 and 48h was found to be moderately correlated to the diagnosis of AKI: the median urinary concentration of NGAL was higher among the non-survivors, and was also higher in the patients requiring renal replacement therapy.47 In 307 adults admitted to a surgical ICU, the plasma concentration of NGAL at the time of admission to the Unit was found to be a good marker of the development of AKI over the subsequent 48h (area under the ROC curve 0.78) and of the need for renal replacement therapy (area under the ROC curve 0.82).48 Another study in critical patients did not find urinary or plasma NGAL concentration at the time of admission to the ICU to be superior to the calculated glomerular filtration based on the serum creatinine concentration in predicting the development of AKI. However, NGAL improved the properties of the predictive model which included clinical variables and glomerular filtration.49
The relationship between NGAL and inflammatory response and sepsis may worsen its performance as a biomarker of AKI - the main risk factor of which is sepsis. Bagshaw et al.50 found the urinary and plasma concentrations of NGAL to be greater in cases of AKI of septic origin than in cases of AKI of non-septic origin. However, another study showed that the plasma NGAL concentrations did not discriminate between cases of AKI and cases of septic shock, while the urinary concentration predicted the development of AKI in the following 12h in cases of septic shock.51 In a study of critical patients, Kümpers et al.52 found the serum NGAL concentration to be different in healthy individuals, patients with systemic inflammatory response syndrome (SIRS) and individuals with sepsis, and a correlation to both mortality and the severity of AKI was observed.
In a metaanalysis of 24 studies and 2538 patients, Haase et al.53 analyzed the properties of the urinary, serum or plasma concentrations of NGAL within the first 6h after injury or during the 24–48h preceding the conventional diagnosis of AKI. The odds ratio of NGAL in predicting the diagnosis of AKI was 18.6 (area under the ROC curve 0.81, with a sensitivity of 76% and a specificity of 85%). The results were slightly better in children than in adults. The performance of NGAL in cases of heart surgery was: OR 13.1, area under the ROC curve 0.77, sensitivity and specificity 75% and 75%, respectively. Urinary concentration presented a slightly higher predictive value than plasma concentration (area under the ROC curve 0.84 versus 0.77, respectively). NGAL was correlated to the need for renal replacement therapy (area under the ROC curve 0.78), but not to in-hospital mortality.53
Other situationsThe capacity of NGAL to predict the development of AKI has also been studied in patients in the Emergency Department. Nickolas et al.54 measured the urinary concentration of NGAL in 635 consecutive patients, recording a sensitivity of 90% and a specificity of 99% in predicting the development of AKI (for a serum creatinine concentration cutoff point of 1.3mg/dl). The urinary concentration of NGAL also discriminated between AKI and other causes of serum creatinine concentration elevation, such as chronic renal failure and pre-renal kidney failure.54
Recent studies have proposed NGAL as a predictor of nephropathy induced by contrast injection.55,56 The increase in serum NGAL concentration 24h after angiographic study is superior to the changes in serum creatinine concentration in predicting development of the early form of ARF induced by contrast injection.57 Likewise, a relationship has been demonstrated between the urinary concentration of NGAL measured on the day of kidney transplantation and the development of late graft dysfunction.58
Since NGAL is not filtered by continuous venous–venous hemofiltration, it has been proposed as a marker of renal functional recovery in patients subjected to renal replacement therapy.59
Hepatic form of fatty acid binding proteinFatty acid binding proteins (FABP) comprise a family of 9 intracellular long chain fatty acid transporting proteins named according to the tissues in which they were originally described. They limit the cell membrane oxidizing effects of toxic intermediate products.60 The hepatic or liver form (L-FABP) is expressed in the liver and, to a lesser extent, in the kidneys and small bowel. At kidney level, L-FABP is expressed in the proximal tubular cells. The urinary levels are undetectable under normal conditions, and the molecule is released in urine under conditions of hypoxia due to a decrease in peritubular blood flow.61
The relationship between the urinary concentration of L-FABP and the development of AKI has been demonstrated in patients subjected to heart surgery,62 patients with AKI induced by contrast injection,63 and in liver transplant patients.64
In patients subjected to heart surgery, the urinary concentrations of L-FABP and NGAL were found to be greater in 28 of the 77 patients who developed AKI, but the discriminatory capacity was modest (area under the ROC curve 0.72 and 0.75 for L-FABP and NGAL, respectively, measured after 4h, and 0.81 for the combination of both determinations).62 The measurement of urinary L-FABP showed great sensitivity, while the measurement of NGAL showed great specificity; a panel composed of both parameters was therefore proposed as potentially useful for diagnosing AKI.62 Indeed, the use of both biomarkers improved the diagnostic performance of each marker considered individually, and improved the prediction of AKI risk compared with a model based only on clinical variables.
In a group of 25 liver transplant patients, the urine concentration of L-FABP was found to be greater in the subjects who developed AKI, though the discriminative capacity of urinary NGAL was superior.64 On the other hand, the urinary concentration of NGAL increases earlier than that of L-FABP, as evidenced by a study of 220 pediatric patients subjected to heart surgery.65 However, in another study of 85 heart surgery patients,66 the biomarker with the greatest area under the ROC curve in diagnosing AKI was found to be urinary L-FABP. The urinary concentration of this biomarker before and during the first 6h after the operation was independently associated to the development of AKI.66 Lastly, in a group of 145 critically ill patients with septic shock complicated by AKI, the urinary concentration of L-FABP was shown to be greater among the non-survivors than in the survivors (area under the ROC curve 0.99).67
Discovery of new protein biomarkers in large patient cohorts: tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding proteinUsing rigorous methodology based on the search of biomarkers in a large population of critical patients with posterior validation in a different cohort of patients, it has been suggested that the measurement of certain molecules could be useful not for diagnostic purposes but with risk predicting intent.61 Kashani et al.68 determined 340 proteins in 522 adults from three different cohorts of surgical patients, subjects with sepsis, and trauma cases. They found tissue inhibitor of metalloproteinase (TIMP)-2 and insulin-like growth factor-binding protein (IGFBP)-7 to offer the best performance as predictors of moderate or severe AKI (KDIGO stages 2 or 3), as determined by the area under the ROC curve, in comparison with other biomarkers such as NGAL, KIM-1, IL-18, π-GST and L-FABP in urine, and cystatin C and NGAL in plasma.
A posterior validation study involving 744 critical patients without AKI at the time of inclusion (Sapphire)68 found the combination of both determinations (TIMP-2 and IGFBP-7) in samples obtained within the first 24h after admission to the ICU to predict the development of AKI in the 12h after sample collection, with an area under the ROC curve of 0.80.
TIMP-2 and IGFBP-7 are markers of G1 cell cycle progression, and are implicated in the response to different stimuli such as inflammation, ultraviolet radiation, drugs, toxins and cell damage.69,70 It is not known whether these biomarkers are also useful in predicting kidney injury progression and recovery.
Combined use of biomarkers: added benefitsMany studies have explored the added benefits of the combined use of more than one biomarker. As has been mentioned, the combined determination of TIMP-2 and IGFBP-7 predicts the development of AKI in the subsequent 12h.68 Likewise, it has been suggested that the combination of NGAL, KIM-1 and IL-18 could be useful in detecting damage at different timepoints, in view of their different time profiles.71
MetabolomicsMetabolomics consists of the evaluation of all the metabolites produced by the body in a sample of tissue or organic fluid. It provides complete information on the metabolic processes of the cells, in contrast to genomics, transcriptomics or proteomics, which do not offer information on the products of metabolic reactions. The identified metabolites include products of intermediate metabolism, hormones and other signaling molecules. This metabolic profile can be studied using magnetic resonance spectroscopy or mass spectrometry techniques. Based on multivariate analysis, the spectrum of all the metabolites obtained can generate a mathematical model for predicting a concrete diagnosis or outcome, allowing the discovery of diagnostic or prognostic biomarkers.72
This novel tool is currently under development for establishing the diagnosis and prognosis of conditions such as sepsis and acute lung injury.73,74 The role of magnetic resonance spectroscopy and mass spectrometry in diagnosing AKI is being investigated by a number of groups.75
Future perspectivesDespite advances in our understanding of the physiopathology of AKI, its morbidity–mortality rate remains high. The use of serum creatinine concentration and diuresis as biomarkers of AKI is hampered by numerous limitations related to the lack of earliness, specificity and sensitivity of these variables in diagnosing AKI. New biomarkers have been proposed that are characterized by earlier increases in concentration under conditions in which glomerular filtration decreases. However, these markers generally lack specificity. We need to identify biomarkers which in the same way as the markers of myocardial damage are able to offer satisfactory performance in terms of earliness and specificity. The role of other approaches such as metabolomics in establishing the diagnosis and prognosis of AKI appears promising but remains to be defined.
The recommendations on the routine clinical application (i.e., outside the research setting) of some of the examined biomarkers should be preceded by confirmation that a concrete determination is effectively able to improve some outcome variable of interest. It is not enough for a given marker to be increased in patients with AKI. Its use (and the associated costs) would be justified provided we are able to demonstrate that its measurement is able to improve patient treatment or modify some outcome variable of interest, such as earlier treatment, a lesser duration of AKI, or improved survival.
Conflicts on interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Seijas M, Baccino C, Nin N, Lorente J.A. Definición y biomarcadores de daño renal agudo: nuevas perspectivas. Med Intensiva. 2014;38:376–385.