The first cases of the European epidemic of Shiga toxin-producing Escherichia coli O104:H4 (STEC-O104:H4) infection were reported in Germany in April 2011.
ObjectivesTo characterize the 2011 STEC-O104:H4 outbreak and its management. A literature review is made to assess the state of the art in STEC–haemolytic–uraemic syndrome (HUS) epidemiology, pathogenesis, management and prognosis, focusing on critically ill adults.
MethodsReferences were obtained from the European Center for Disease Control and World Health Organization epidemiological updates, in addition to a PubMed search covering the period from 1980 to August 2011, including all published work on STEC-014:H4 and reviews on HUS management and prognosis.
ResultsThe epidemic originated from a bean and seed sprouts farm in Lower Saxony, and was caused by the O104:H4 strain – a highly antibiotic resistant, hybrid enteroaggregative – Shiga toxin producing E. coli strain (STEC). The infection was characterized by increased HUS (25%) and a higher mortality rate. STEC enteritis and HUS are associated with significant mortality and morbidity, especially amongst patients with severe renal and neurological disorders. Management should center on prompt kidney protection by maintaining adequate renal perfusion, in addition to avoiding diuretics and nephrotoxic agents.
ConclusionsThe published studies regarding antibiotic treatment lack good quality evidence. However, recent data suggest a potential modulating effect that explains the conflicting data but moreover suggests that azithromycin might be of use. Neutralizing monoclonal antibodies are a promising new therapy for STEC–HUS, with currently ongoing studies. Other treatments have not been shown to be superior to supportive therapy alone.
Los primeros casos de la epidemia europea de infección por E. coli productora de la toxina Shiga (STEC-O104:H4) se detectaron en Alemania en abril de 2011.
ObjetivosCaracterizar el brote de STEC-O104:H4 de 2011 y su gestión. Se realiza una revisión bibliográfica para evaluar el estado del arte en epidemiología, patogenia, tratamiento y pronósitico de la STEC-síndrome urémico-hemolítico (SHU), con especial hincapié en los adultos críticamente enfermos.
MétodosSe obtuvieron referencias de las actualizaciones epidemiológicas del Centro Europeo de Control de Enfermedades y de la Organización Mundial de la Salud, además de una búsqueda en PubMed relativa al periodo de 1980 a agosto de 2011, incluidas todas las publicaciones sobre la STEC-014:H4 y las revisiones sobre el tratamiento y el pronóstico del SHU.
ResultadosLa epidemia tuvo su origen en unos brotes germinados de una plantación de Baja Sajonia y estuvo causada por la cepa O104:H4, una cepa híbrida de E. coli enteroagregativa productora de la toxina Shiga (STEC) con una elevada resistencia a los antibióticos. La infección se caracterizó por un aumento del SHU (25%) y por una tasa de mortalidad más elevada. El SHU y la enteritis de la STEC se asocian a una morbimortalidad importante, especialmente en pacientes con trastornos neurológicos y renales graves. El tratamiento debe centrarse en una rápida protección renal, menteniendo una perfusión renal adecuada, además de evitar los diuréticos y los agentes nefrotóxicos.
ConclusionesLos estudios publicados en relación con el tratamiento antibiótico no están respaldados por datos de calidad. Sin embargo, los datos recientes sugieren un potencial efecto modulador que no solo explica la incoherencia en los datos sino que además sugiere que la azitromicina podría resultar de utilidad. Los anticuerpos monoclonales neutralizadores son un prometedor tratamiento nuevo para la STEC-SHU, y actualmente se están llevando a cabo estudios sobre ellos. Otros tratamientos no han resultado ser superiores a la administración de cuidados paliativos exclusivamente.
The objective of this review was the characterization of 2011 Shiga toxin-producing Escherichia coli (STEC) O104:H4 outbreak and its management. Also, a review of the literature to assess state of the art for STEC–haemolytic–uraemic syndrome (HUS) epidemiology, pathogenesis, management and prognosis, focusing on critically ill adults. References were obtained from European Center for Disease Control and World Health Organization's epidemiological updates until the outbreak was called to an end. In addition, a PubMed search from 1980 to August 2011, including all published work regarding STEC-014:H4 and reviews for HUS management and prognosis. The bias of this review included language (English, Spanish and Italian), publication (only published data are included) and poor reporting of an important proportion of included studies. In total, 34 publications were included.
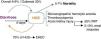
IntroductionOn April 2011 the first cases of Shiga toxin-producing E. coli (STEC) O104:H4 infection were reported in Germany and quickly rose to epidemic levels that was characterized by bloody diarrhoea and significantly increased association with haemolytic–uraemic syndrome (HUS) and mortality. ECDC outbreak case definition for STEC diarrhoea included “Acute onset of diarrhoea or bloody diarrhoea and at least one of the following laboratory criteria: isolation of an E. coli strain that produces Shiga-like toxin 2 (STX2) or harbours STX2 gene and/or direct detection of STX2 gene nucleic acid in faeces without strain isolation”. Whilst STEC–HUS was defined as “HUS defined as acute renal failure and at least one of the following clinical criteria: microangiopathic haemolytic anemia AND/OR thrombocytopenia”1 (Fig. 1).
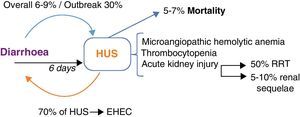
Enterohaemorragic E. coli infection and haemolytic–uraemic syndrome. HUS occurs at the 6th day after diarrhoea in EHEC enteritis; with an overall incidence of 6–9% and in STEC-O104:H4's outbreak of 30%. On the other hand, 70% of HUS cases occur in the context of EHEC enteritis. HUS triad comprises microangiopathic haemolytic anaemia, thrombocytopenia and AKI. Patients who develop AKI, 50% will require RRT and 5–10% will remain with renal sequelae. 5–7% of patients with HUS do not survive. EHEC: enterohaemorragic E. coli; HUS: haemolytic–uraemic syndrome; RRT: renal replacement therapies; STEC: Shiga-like toxin producing E. coli; AKI: acute kidney injury.
Till date, 941 STEC cases have been confirmed and 2.969 probable cases were reported (without STEC-O104 confirmation) in EU/EEA. Since May 30th cases reported markedly declined suggesting a change in consumption patterns and/or waning of the source of infection. The last confirmed case dates on July 7th and the Robert Koch Institute called the end of the outbreak on July 26th. Considered as Germany's outbreak, given that most cases were from the north of Germany or had history of travelling there (93.7% of HUS–STEC and 96.6% of non-HUS–STEC cases). Nonetheless, it also involved other EU countries such as Austria, Czech Republic, Denmark, France, Greece, Luxembourg, Netherlands, Norway, Poland, Spain, Sweden and the UK. Also in USA, three travel-related cases of EHEC–HUS (1 confirmed) were reported.2 Of the confirmed cases, 264 (28.1%) presented with haemolytic–uraemic syndrome.3 Mortality, in both confirmed and probable cases, totals 46 persons; of which 29 (63%) were HUS–STEC cases; reflecting a greater fatality rate than usual. Also a particular feature of this outbreak was the predominance of adults (88% were 20 years or older) with the highest attack rate in the group of 20–49 years. Women represented two-third of cases. At first, the source of the outbreak was attributed to cucumbers cultivated in Spain, afterwards it was found that bean and seed sprouts originated from a farm in Lower Saxony were the vehicles for the unusual E. coli bacterium.
Initially it was thought that E. coli 0157:H7 strain was responsible for the outbreak, but it was later recognized as due to the O104:H4 strain. A highly resistant, hybrid enteroaggregative – Shiga-toxin producing E. coli4 is thought to be an evolved combination of adhesion proteins that makes it particularly hard to remove from enterocytes and food, helping the bacteria to survive and persist in the environment. Another particular feature is its production of extended-spectrum beta-lactamase (ESBL),5,6 considering that E. coli enteritis usually are not treated with antibiotics. Both these characteristics could explain why the outbreak lasted so long (usually it lasts 2 weeks). Considered as the first outbreak caused by this strain, it is actually the second known outbreak. By 2009 in the Republic of Georgia, it was reported as a cluster of diarrhoeal illness in two patients caused by a STEC-O104:H4 strain that was similar to the current outbreak strain, but with different molecular fingerprint and less resistance to antibiotics.7
Epidemiology and microbiologyE. coli, a major facultative inhabitant of the large intestine, is a Gram-negative bacilli and facultatively anaerobic. Strains are classified according to the O (lipopolysaccharide) and H (flagellum) antigens. Enterohaemorragic (EHEC) strains are characterized by its genomic and plasmidic virulence factors. The first one translates into the production of one or two types of Shiga-like toxins (STX), STX-1 and STX-2. Whereas the latter endows it with genes encoding proteins key to its virulence and pathogenesis: such as catalase–peroxidase enzymes, haemolysins, proteases, secretion proteins and anticoagulants. Characterized by a low infectious dose (10–100 microorganisms), it causes haemorrhagic colitis that can be complicated with HUS. Strains associated with HUS usually harbour STX-2, curiously, it is less frequent amongst strains with both STX genes. The most common serotype of outbreaks and HUS is the STEC-O157:H7. Whilst enteroaggregative E. coli presents with persistent watery diarrhoea, usually in children. Infection with EHEC is commonly associated with contaminated beef; however, a retrospective review8 of 350 outbreaks revealed that even though gastrointestinal tract of cattle is the most important reservoir and food faecally contaminated by cattle (including raw milk) is the first mechanism of transmission (52% of cases), beef is a less frequent vehicle. Foodborne transmission is followed frequently by person-to-person (14%) and waterborne, including swimming (9%). Person-to-person transmission peaks during diarrhoea; post-symptomatic shedding can occur.
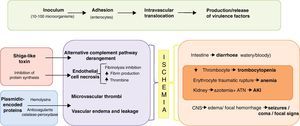
Pathogenesis and clinical presentationAfter its adherence to enterocytes, STEC produces attaching-effacing lesions, through its plasmidic encoded proteins. Providing an entry point into the intravascular space for toxins. Targeting endothelial cells, STX halt cell protein synthesis causing cell death. Which translates into vascular damage; predominantly locally (at the intestine) and at the kidneys. Producing variable prothrombotic states in addition to non-inflammatory bloody diarrhoea and kidney failure.9 Symptoms usually present after 72h of ingestion of the inoculum. Initially, watery diarrhoea is accompanied by abdominal pain, vomiting and usually without fever (although almost 50% of patients report fever during the prodromic phase). 48–72h later, haemorrhagic enterocolitis ensues in the 90% of cases. Improvement of diarrhoea occurs at the 7th day of symptoms with spontaneous resolution in the 85% of cases, whilst 15% develop HUS. Post-symptomatic shedding can be present from the 3rd to the 7th day of initiation of symptoms (Fig. 2).
Overall, HUS's incidence in STEC infections rounds 6–9% and in children 15%; whilst, in the STEC-O104:H4 outbreak it rose to 25%.5 Conversely, 70% of HUS are caused by EHEC; other causes include pneumococcal pneumonia and complement inherited-abnormalities. HUS is defined as microangiopathic haemolytic anaemia, thrombocytopenia and acute kidney injury (AKI). Despite its low mortality (5–7%), it is associated to significant morbidity: 50% of patients require extra-renal depurative techniques and 5–10% have renal sequelae.7 Initially considered as a part of the spectrum of the same thrombotic non-vasculitic disorder, manifesting either with thrombotic thrombocytopenic purpura (TTP) in adults and HUS in children. Actually a distinctive pathophysiology is known in each case; TTP is an auto-immune disorder caused by inhibition of Von Willebrand factor, whilst HUS is characterized by fibrinolysis inhibition, increased fibrin production and thrombin formation, entirely secondary to STX injury and almost never manifesting with bacteraemia. The core pathogenesis of HUS is the formation of microvascular thrombi, followed by endothelial oedema and vascular leakage. New findings suggest that STX also directly interferes with the alternative complement pathway and that it is a key pathogenic mechanism.10 Thrombocytopenia is the first abnormality in STEC infections, either the patient develops HUS or not, and lasts up to one week. It is secondary to thrombocyte consumption and somewhat to voluminous expansion. A reliable parameter of clinical resolution, platelet count with stable or ascending trends translates into improvement and in most cases presents the possibility of discharge. If renal failure has not developed up to this point, almost it unequivocally will not occur afterwards. Microangiopathic haemolytic anaemia is a result of the traumatic rupture due to endothelial thrombi and is the most persistent abnormality, usually preceding renal failure. Azotemia and acute tubular necrosis are responsible for AKI. Overload is due to oliguria and fluid resuscitation. Neurological complications of HUS are important determinant of morbidity and the most common cause of mortality.11 It encompasses a wide array of clinical findings, from minor changes in mental status to major neurologic deficits such as seizures, coma and hemiparesis.12 Proposed mechanisms include thrombotic occlusions (coagulative necrosis due to microthrombosis without haemorrhage),13 microangiopathic changes of small vessels (oedema and focal haemorrhage),14 direct injury of toxin, ischaemia–reperfusion injury, hypertension and metabolic disregulation (hypoglycaemia, dehydration, acid–base disturbances, changes in serum osmolarity or azothemia). Neurological damage occurs in 50–100% of children with HUS,15 the most frequent presentation is generalized or partial seizures, followed by coma, irritability and focal sings (hemiparesis or aphasia). Neurological sequelae follow in most cases. Diffuse encephalopathy and SWCP score are useful predictors of bad neurological outcome.16 Neuroimaging findings include ischaemia and are usually located at basal ganglia and thalamus.17
Both watery and bloody diarrhoea are probably due to intestinal ischaemia, rather than by invasive/inflammatory damage, and can evolve to necrosis and perforation. Elevations in serum amylase and lipase have been reported, without clinical implications. Heart failure is usually secondary to volume overload; however, elevation of cardiac markers such as troponins suggests myocardial infarction rather than reduced renal clearance.
WorkupSorbitol-MacConkey agar cultures allow STEC-O157:H7 serotype identification. The highest likelihood of positive culture occurs on days 1–6 from onset of diarrhoea; usually positive on the third day. Molecular diagnosis of STEC can be done in stool directly with the detection of STX with enzyme linked immunosorbent assay (ELISA) or indirectly (STX genes) with polymerase chain reaction (PCR). Specific serogroup identification can be achieved using ELISA and Western blot with specific antibodies detection, both in the acute and convalescent periods.
ManagementMost efforts are directed towards kidney protection. Maintaining an appropriate renal perfusion is key in preventing HUS. Studies show that adequate pre-HUS volume expansion is associated with lesser AKI in HUS.7 Therefore, therapies that could deplete the intravascular volume, such as diuretics and medication that could elevate the risk or worsen AKI (NSAIDs, ACE inhibitors and nephrotoxic agents) should be avoided. Renal replacement therapy should be implemented according to the usual indications in ARF. Careful fluid balance should be sought, taking into account the increased risk for third-space liquid accumulation secondary to vascular leakage and hypoalbuminaemia. Hypertension is preferred to be treated with vasodilators. Erythrocyte transfusions also should follow standard indications. Anti-motility agents and narcotics are not recommended, although is not clear whether they increase the risk of HUS and neurological complications or not.7
Ineffective interventions include those directed to equilibrate the prothrombotic state and to the inhibition of STX and auto-immune mechanisms.8 Anticoagulation, antiaggregation and fibrinolysis are not associated with better outcome, instead, they highly increase risk of bleeding (mainly intracranial bleeding); compared to supportive therapy, OR for bleeding is 25.89 (95% CI 3.67–182.83). Corticosteroids, plasmapheresis and plasma infusion are not superior to supportive therapy, which is logical considering the absence of immune-mediated pathways. Despite that SHU is mainly a consequence of direct action of the Shiga-toxin, binding agents also fail to contribute to its management, which can be explained because at the time of SHU onset, STX-endothelial damage has been already established.18
Currently much controversy revolves around antibiotic treatment of EHEC. Data from a retrospective study of 1993's USA E. coli-O157:H7 outbreak reported that antibiotic administration was not associated with a decreased risk for HUS development,8 setting a trend of not treating with antibiotics for these infections. Proposed mechanisms included that bacterial lysis caused liberation of STX and the induction of bacteriophages (where STX genes are located), which further increased STX levels. It also caused selection of resistant strains. Considering that the main pathogenic factor is direct toxin injury and that at the time of onset of diarrhoea, the STX is already in circulation; it suggested that little benefit would come from administration of antibiotics.7
Thereafter, on 2003, a meta-analysis19 that included 9 clinical studies, of which 6 retrospective, suggested that antibiotic treatment with fosfomycin might be a protective factor for HUS in E. coli O157:H7 enteritis, even though studies lacked adequate methodology to support the evidence. The pooled OR did not show a clear result to favour any trend and conclusions were based on a pooled-OR that excluded two studies that clearly associated antibiotic treatment with increased risk of HUS. Amongst the pro-antibiotic treatment studies, the Ikeda et al.,20 a prospective cohort, evaluated 36 cases of patients receiving fosfomycin vs. other antibiotic (not specified), OR was a result of a univariate analysis that yielded 0.12 (95% CI 0.02–0.74). Cimolai et al.21 published a retrospective case–control, including 27 cases reporting an OR: 0.63 (95% CI 0.27–1.48); however, neither the type of antibiotic nor the diarrhoea-antibiotic administration interval was stated. On the other hand, one of the excluded studies, Wong et al.,22 a prospective cohort that included 10 cases in children (<10 years) where antibiotics (trimethroprim–sulfamethoxazole, amoxicillin and cephalosporins) were administered within 3 days of diarrhoea onset (DO), yielding an adjusted OR for HUS of 17.9 with statistical significance (unadjusted OR: 14.25; 95% CI 3.62–56.1). The other excluded study was the Pavia et al.,23 a retrospective case–control and randomized trial of 8 cases which received antibiotics within 3 days of DO; with a OR for HUS of 80.6 with statistical significance (95% CI 3.38–1922).
Lynn et al.24 published a 4 years prospective surveillance of childhood HUS in United Kingdom and Ireland; including 413 cases of HUS. Statistical significance was found between neither mortality and antibiotic treatment, nor antibiotic treatment with ciprofloxacin vs. metronidazole/penicilline/2nd–3rd generation cephalosporin.
On 2006 a systematic review25 that included all cases published between years 1982 and 2005, analysed whether antibiotics are beneficial or detrimental for E. coli O157:H7 infection. In total, 63 in vitro and clinical studies were included, case-reports were excluded. Of the experimental data, they found that: (a) bacteria can recover and continue producing extracellular toxins after exposure to continuously changing sub-lethal antibiotics concentrations; (b) antibiotics might be detrimental, given that increased release of toxins was found in cultures exposed to sub-inhibitory and sub-lethal concentrations of polymixin B, trimethroprim–sulfamethoxazole, ciprofloxacin, cefixime and tetracycline; and (c) fewer STX is released when exposed to sub-minimum inhibitory concentrations of roxithromycin, rokitamycin and clindamycin. Concluding that sub-lethal concentrations of antibiotics might have a modulating effect on STX production. Within the clinical studies that advocate to the beneficial effect of antibiotic treatment that were not included in the 2003 meta-analysis were the Martin et al.,26 a retrospective cohort of 117 patients (<18 years), of which only 20 were treated with antibiotics during prodromal illness (without specification) showed a mild clinical course and good outcome; it also reported that antibiotic treatment was less common in the group of severe outcome. Higami et al.27 conducted a retrospective control including 216 children, comparing antibiotic regimens at the 3rd day of OD, less HUS incidence was found in the group treated with new quinolones in comparison to cephalosporins. Shiomi et al.,28 with a retrospective control study of 42 children, found that antibiotic treatment on 3rd day of OD with oral fluoroquinolones (FQ) prevents HUS when compared to a endovenous FQ plus cefotaxime regime. On the other hand, studies exposing a detrimental effect of antibiotics included the Slutsker et al.,29 a retrospective case–control comprising 230 patients of all ages, showed that sulfamethoxazole given at the 3rd day of OD was related with an OR for HUS 11.5%, in the subgroup of <13 years. Carter et al.,30 through a retrospective case–control of 73 patients also of all ages; analysed timing of initiation of antibiotics; when given before the onset of symptoms (OOS) increased secondary person to person infection was reported; whereas when given after OOS, elevated case-fatality rate was found, probably due to disease severity bias. Ostroff et al.,31 in a retrospective case–control of 80 patients (including all ages), compared TMP/SMX with gentamicin initiated at the 4th day; TMP/SMX showed a OR for HUS: 3.1 (even though the CI included 1); and gentamicin, OR: 9.1 (CI≈1). Finally, Dundas et al.,32 in a retrospective cohort of 120 patients of all ages, analysed those who received antibiotic 4 weeks before OOS and found association with increased incidence of HUS, except for ciprofloxacin that did not show any difference compared to controls; no association with mortality was found.
Amongst the latest evidence addressing this issue, despite its pure experimental nature, provides a coherent explanation that encompasses the evidence found out about antibiotics and STX modulation until now.33 It studied different E. coli O157:H7 strains, different plasmids and different antibiotics. Results showed that phages can infect harmless intestinal E. coli, recruiting them to produce STX. Grow-inhibitory levels of antibiotics decrease STX production. Even when O157:H7's viability remained high, azithromycin significantly reduced STX production. Nonetheless, sub-inhibitory levels that target DNA synthesis (ciprofloxacin/TMP-SMX) increase STX production; whilst ciprofloxacin's complete suppression of O157:H7 markedly increases levels of STX. Antibiotics targeting cell wall, transcription and translation do not have any effect on STX levels. Based on the evidence, during this outbreak Germany's infectious diseases society recommended to avoid the use of FQ, TMP-SMX, aminoglycosides and fosfomycin in EHEC patients.34Table 1 summarizes these studies according to the relationship between HUS and antibiotic treatment.
Summary of works studying the relationship between antibiotic treatment in STEC enteritis and development of HUS.
| Study (first author) | Type of study | Sample size (number of patients) | OR (95% CI) |
| Beneficialb | |||
| Safdar et al.19 | Meta-analysis | a | 0.83 (0.54–1.26)c |
| Ikeda et al.20 | Prospective cohort | 292 | 0.12 (0.02–0.75) |
| Cimolai et al.21 | Retrospective case–control | 128 | 0.63 (0.27–1.43) |
| Dundas et al.32 | Retrospective cohort | 120 | 1.23 (0.4–3.8) |
| Martin et al.26 | Retrospective cohort | 117 | 9.4 (1.2–199) |
| Higami et al.27 | Retrospective control | 216 | NSd |
| Shiomi et al.28 | Retrospective control | 42 | NSe |
| Detrimentalf | |||
| Safdar et al.19 | Meta-analysis | a | 1.15 (0.79–1.68)g |
| Wong et al.22 | Prospective cohort | 10 | 14.25 (3.62–56.1)h |
| Pavia et al.23 | Retrospective case–control | 23 | NSi |
| Slutsker et al.29 | Retrospective case–control | 93 | 11.5 (1.4–91.8)j |
| Ostroff et al.31 | Retrospective case–control | 80 | 3.1 (0.6–9.8)k9.1 (NS)l |
| Carter et al.30 | Retrospective case–control | 73 | NSm |
Abbreviations: OR: odds ratio, CI: confidence interval, FOM: fosfomycin, HUS: haemolytic–uraemic syndrome, IV: intravenous, NS: not stated, STEC: Shiga-like toxin producing E. coli, PO: oral, and TPM/SMX: trimethoprim–sulfamethoxazole.
Pooled sample size is not specified. 63 studies were included (prospective or retrospective cohort studies, case–control and placebo-controlled trials, in vitro and animal studies).
OR<1. Suggesting that antibiotic treatment is associated with decreased risk of HUS.
Pooled OR, excluding two statistically significant studies associating antibiotic treatment as a risk factor for HUS.
Lower incidence of HUS with previous treatment with new quinolone vs. cephalosporin (3.7% vs. 18.2%).
Lower incidence of HUS in patients treated with FOM PO+cefotaxime IV vs. FOM IV.
OR>1. Suggesting that antibiotic treatment is associated with increased risk of HUS.
Pooled OR including all studies.
Unadjusted OR. Adjusted OR 17.9, CI not stated.
HUS more frequent with previous TPM/SMX treatment (5/8 vs. 0/7), p=0.026.
Overall results did not yield any difference, OR obtained in the <13 years group.
OR for TPM/SMX.
OR for gentamicin. CI stated as “Low CI – 1.8”.
HUS more frequent amongst patients who received antibiotics. Relationship probably secondary to severity bias.
Neutralizing monoclonal antibody of STX is promising amongst new therapies in the development of Shiga toxin.10 Eculizumab is a recombinant, humanized, monoclonal immunoglobulin G antibody derived from murine myeloma cells, directed against protein C5 inhibiting activation of the terminal complement pathway. Authorized only for paroxysmal nocturnal haemoglobinuria; in this setting, reduces intravascular haemolysis, anaemia and thrombotic complications. However, since terminal complement is necessary to prevent and limit a N. meningitidis infections, all patients treated with it require vaccination against N. meningitidis. Recently, in a series of 3 paediatric patients with severe STEC–HUS treated with eculizumab, showed a rapid clinical response, suggesting that indeed STX directly activates complement.35 During this outbreak, in Germany experimental therapy with monoclonal antibodies was used. Studies regarding it are currently ongoing.
PrognosisUp to 10% of patients who suffered HUS, wind up with renal sequels. In a meta-analysis/meta-regression36 of 49 studies from 1950 to 2001 that included 3.476 patients aging from 1 month to 18 years from 18 countries, with a follow-up of 4.4 years, confirmed that death or end-stage renal disease (ERSD) occurs in approximately 12% with EHEC–HUS and that one quarter of survivors suffer long-term renal disease. Worse prognosis is associated with severity of acute illness, especially with early requirement of renal depurative techniques and CNS severe manifestations.
ConclusionsE. coli O104:H4 strain is characterized by a low infection dose, hybrid adhesion proteins and antibiotic resistance (ESBL). 2011's outbreak presented with increased HUS and greater fatality rate. Management should focus on promop kidney protection by mantaining adequate renal perfusion in addition to avoidance of diuretics and nephrotoxic agents. Antibiotics have potential modulator effect, of which azithromycin might be of use for STEC–HUS treatment. However, results are limited by the design of the selected studies: case–control, not randomized-controlled with high heterogeneity and lacking information of both sample and treatment. Well-designed studies are needed to establish the role of antibiotics in this setting. Neutralizing monoclonal antibodies are a promising new therapy for STEC–HUS, with currently ongoing studies. Platelet trend is a reliable parameter for management guidance. Other therapies have not proven to be superior to supportive therapy alone.
Conflicts of interestAuthors do not have any financial relationship with a commercial entity that has an interest in the subject of this manuscript.