To describe the incidence of cardiovascular adverse events in patients with sepsis in its various stages.
DesignA longitudinal, descriptive, observational study was carried out.
SettingIntensive care units (ICUs) of two university hospitals in Bogotá (Colombia).
PatientsA number of patients consecutively admitted to the adult ICU with a diagnosis of sepsis, and no evidence of previous ischemic myocardial injury.
InterventionsForty-eight hours of electrocardiographic record using Holter technology.
Main variablesIschemia, cardiac arrhythmia, heart rate variability (HRV).
ResultsA total of 100 patients were analyzed, 62% being staged as presenting septic shock. Three percent suffered ischemic events detected by Holter and unnoticed through conventional monitoring. Forty-six percent suffered an arrhythmic event detected by Holter, compared with only 6% as detected by conventional monitoring. Mortality was 40%. All patients showed loss of HRV.
ConclusionIn this study patients with sepsis showed a low incidence of cardiovascular ischemic events. In contrast, arrhythmic events showed a high incidence. Conventional monitoring failed to detect any of the ischemic events and most arrhythmic events. In this study, cardiovascular events generated by adrenergic discharge had no impact upon mortality.
Describir la incidencia de eventos cardiovasculares adversos en pacientes con diagnóstico de sepsis en sus diferentes estadios.
DiseñoEstudio observacional, descriptivo, longitudinal.
ÁmbitoUnidades de cuidados intensivos de tipo mixto de dos hospitales universitarios en la ciudad de Bogotá.
ParticipantesSe incluyeron una serie de pacientes mayores de edad, que ingresan a UCI con diagnostico de sepsis, sin evidencia de lesión miocárdica isquémica previa.
IntervencionesRegistros electrocardiográficos continuos de 12 derivaciones durante 48 horas con monitoría Holter.
Variables de interésIsquemia, arritmia cardiaca, variabilidad de la frecuencia cardiaca.
ResultadosSe analizaron 100 pacientes, 62% de los cuales se estadificó como choque séptico. El 3% presentó evento cardiovascular adverso de tipo isquémico detectado por Holter y desapercibido por monitoreo convencional. El 46% presentaron un evento de tipo arrítmico por Holter, comparado con solo un 6% detectado por monitoreo convencional. La mortalidad cruda fue de 40%. El 100% presentó pérdida de la variabilidad de la frecuencia cardiaca.
ConclusiónLa sepsis en esta experiencia mostró una baja incidencia de eventos cardiovasculares isquémicos. Los eventos arrítmicos, sin embargo, mostraron una incidencia alta. El monitoreo convencional falló en detectar la totalidad de los eventos isquémicos y en mayor proporción los eventos arrítmicos. En esta serie, los eventos cardiovasculares generados por descarga adrenérgica no impactan en la mortalidad.
The incidence of sepsis has increased in recent years due to different factors.1 It is the main cause of death in non-coronary critical patients, with a mortality rate of between 19.6% and 59%.2,3 Mortality in such cases is attributable to failure of different organs, including particularly the cardiovascular system.4
The pathogenesis of myocardial dysfunction due to sepsis is of a multifactorial nature, and includes ischemia.5,6 In this context, an increase in coronary flow is described, though some areas suffer oxygen deficiency–resulting in a phenomenon similar to that of myocardial hibernation.7 Alterations in capillary flow and in coagulation homeostasis are considered to be the bases of this process.8,9 Thus, myocardial dysfunction cannot be attributed to generalized myocardial ischemia.10,11 Other elements alter ventricular function in sepsis, including interleukins,12 tumor necrosis factor, and a specific circulating humoral factor probably of pancreatic origin.13
The initial clinical picture is characterized by a loss of vascular tone, a hyperdynamic phase with increased cardiac output, and subsequently myocardial depression with contractility alterations,14 relaxation disorders, tachycardia15 and dilatation of the heart cavities.16 There is no typical arrhythmogenic profile, though the systemic inflammatory response–independently of its etiology–is intrinsically arrhythmogenic.17 Alterations are observed in the equilibrium of the autonomous control of heart function, resulting in incompetent chronotropism that reduces HRV.18
Parasympathetic control is attributed with an antiinflammatory effect mediated by attenuation of the production of splanchnic tumor necrosis factor-alpha.18
Finally, the elevated heart rate increases myocardial stress and oxygen demand.19
In clinical practice, cardiovascular monitoring is the basis for the making of many decisions.20 To date, however, there are no technical elements that meet all the criteria of an ideal monitoring system.21 In the specific case of myocardial ischemia, basic monitorization does not meet the operative requirements for diagnosing an acute event.22
Considering a high probability of inadvertent myocardial ischemia in the septic patient, we contemplate close electrocardiographic monitorization of these patients.
Materials and methodsA longitudinal, descriptive observational study was designed, with information collected from 48-h Holter monitoring as the primary data source.
Study population and sampleThe study covered the period between July 2009 and October 2010, and included patients over 18 years of age with a diagnosis of sepsis and admitted to the ICU within the first 24h of symptoms onset, with the need for cardiovascular or ventilatory support. Exclusion criteria were clinical or electrocardiographic features complicating interpretation of the Holter recordings, and patients with coronary disease.
The Holter recordings were made using a Cardiovex MMC10L system with three electrocardiography channels. Recording was started in the first 6h of admission and was continued until completing 48h. The interpretation of the tracings was made by one of two cardiologists based on randomized assignment and following the current consensus parameters.23,24
Cardiovascular adverse events secondary to myocardial ischemia were regarded as arrhythmias and loss of variability of the heart rate. The detection of ischemia was based on ST-segment analysis.
For the evaluation of HRV, use was made of the statistical indices standard deviation of the RR intervals (SDNN) and percentage of intervals differing by more than 50ms from the preceding interval (PNN50), in relation to sympathetic and parasympathetic activity, respectively.25,26
These indices have been used for the prediction of mortality risk after infarction; SDNN<50ms and PNN50<3% identify those patients with severely reduced HRV26 (Table 1).
Conventional cardiac monitorization was carried out with the visioscope, obtaining registries of one to two channels.
Statistical analysisThe STATA SE version 10.1 package was used for the statistical analysis. A descriptive study of the data was made based on absolute frequencies and percentages in the case of categorical variables, and central tendency and dispersion measures in the case of quantitative variables. Multiple correspondence analysis was performed with SPAD 7.0. The illustrating variable was sepsis stage, while the cardiovascular adverse events represented the active variables.
Ethical issuesThe study was approved by the Ethics Committees of the participating institutions, being regarded as entailing only minimum risk according to current legislation.27 Informed consent was obtained from all patients.
ResultsA total of 130 eligible adults were admitted between July 2009 and October 2010 with a diagnosis of sepsis and requiring cardiovascular and/or ventilatory support, and who met the screening criteria. Thirty subjects were excluded due to poor data quality.
The general population and the patients grouped by sepsis stage were characterized.
Ten percent of the patients were admitted with a diagnosis of sepsis, 28% with a diagnosis of severe sepsis, and 62% with septic shock–the latter category being defined not only by the use of vasopressor drugs but also by the presence of clinical manifestations of tissue hypoperfusion. The global median age was 55±26 years (range 18–88 years). The male:female ratio was 1.38:1.
In the general population, admission due to clinical causes was slightly predominant (53%). This tendency was also seen in the groups of patients with sepsis and severe sepsis, though surgical patients were seen to predominate in the septic shock group (56.5%). Regarding the cardiovascular risk factors, arterial hypertension predominated (33%), followed by patient age over 65 years (24%), obesity (18%) and diabetes mellitus (12%).
The median APACHE severity score was 16.5±10 among the global patients, versus 18±9 in those with septic shock.
Globally, the most frequent septic focus was the lungs (38%), while abdominal cavity infections were seen to predominate in the septic shock group (41.9%) (Table 2).
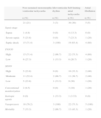
Epidemiological characteristics of the studied patients.
| Characteristics | Sepsis | Severe sepsis | Septic shock | General |
| 10 (10%) | 28 (28%) | 62 (62%) | 100 (100%) | |
| Median age±IQRa[minimum, maximum] | 50±31 [32.81] | 54±29.5 [20.85] | 56±23 [18.88] | 55±26 [18.88] |
| Gender, n (%) | ||||
| Female | 2 (20) | 16 (57.1) | 40 (64.5) | 58 (58) |
| Male | 8 (80) | 12 (42.9) | 22 (35.5) | 42 (42) |
| Admission, n (%) | ||||
| Clinical | 7 (70) | 19 (67.9) | 27 (43.5) | 53 (53) |
| Surgical | 3 (30) | 9 (32.1) | 35 (56.5) | 48 (48) |
| Risk factors, n (%) | ||||
| Hypertension | 1 (10) | 10 (35.7) | 22 (35.5) | 33 (33) |
| Obesity | 1 (10) | 4 (14.3) | 13 (21) | 18 (18) |
| Age>65 years | 1 (10) | 8 (28.6) | 15 (24.2) | 24 (24) |
| Diabetes | 1 (10) | 2 (7.1) | 9 (14.5) | 12 (12) |
| Hypothyroidism | 0 (0) | 0 (0) | 7 (11.3) | 7 (7) |
| Dyslipidemia | 0 (0) | 1 (3.6) | 5 (8.06) | 6 (6) |
| APACHE median±IQRa[minimum, maximum] | 15.5±13 [6.29] | 14.5±11.5 [6.30] | 18±9 [6.30] | 16.5±10 [6.30] |
| Focus, n (%) | ||||
| Lung | 4 (40) | 13 (46.4) | 21 (33.9) | 38 (38) |
| Abdomen | 2 (20) | 6 (21.4) | 26 (41.9) | 34 (34) |
| Urinary | 1 (10) | 2 (7.1) | 3 (4.8) | 6 (6) |
| Bacteremia | 0 (0) | 1 (3.6) | 2 (3.2) | 3 (3) |
| Soft tissues | 0 (0) | 2 (7.1) | 0 (0) | 2 (2) |
| Others | 3 (30) | 4 (14.3) | 10 (16.1) | 17 (17) |
| Monitorization | 1 (10) | 5 (17.9) | 39 (62.9) | 45 (45) |
| Inotropic agents | 0 (0) | 0 (0) | 7 (11.3) | 7 (7) |
| Vasopressors | 4 (40) | 7 (25) | 61 (98.4) | 72 (72) |
| Arrhythmic event, n (%) | 4 (40) | 11 (39.3) | 31 (50) | 46 (46) |
| Ischemic event, n (%) | 0 (0) | 2 (7.1) | 1 (1.6) | 3 (3) |
| Mortality | 1 (10) | 9 (32.1) | 30 (48.4) | 40 (40) |
Inter-quartile range.
A total of 72% of the patients required vasopressor drugs, 7% were prescribed inotropic agents, and 45% required monitorization of cardiac output. This was done using Vigileo® technology; the recorded hemodynamic profile indicated a mean systolic volume index (SVI) of 32ml/beat and a mean systolic volume variability (SVV) of 10%.
Conventional monitorization detected no ischemic event, while arrhythmias were identified in 6%. Holter analysis determined the presence of ischemia in three patients (Table 3).
Cardiovascular adverse events.
| General | Arrhythmia | Ischemia | |
| Detection by conventional monitorization, n (%) | 6 (6) | 0 (0) | |
| Detection by Holter | 46 (100) | 3 (100) | |
| Mortality, n (%) | 40 (40) | 18 (40.1) | 1 (33) |
| Days of stay Mea [range] | 10 [1–71] | 9.5 [1–47] | 18 [8–6] |
Median.
Arrhythmic events were detected by Holter recording in 46% of the population, their origin being classified as follows: monomorphic ventricular tachycardia (21%), idioventricular rhythm (3%), atrial tachycardia (30%) and atrial fibrillation (5%). A higher frequency of arrhythmias was observed in the patients with septic shock and in patients with an increased loss of HRV (Table 4).
Description of arrhythmic events.
| Non-sustained monomorphic ventricular tachycardia | Idioventricular rhythm | Self-limiting atrial tachycardia | Atrial fibrillation | |
| n (%) | n (%) | n (%) | n (%) | |
| 21 (21) | 3 (3) | 30 (30) | 5 (5) | |
| Sepsis stage | ||||
| Sepsis | 1 (4.8) | 0 (0) | 4 (13.3) | 0 (0) |
| Severe sepsis | 5 (23.8) | 0 (0) | 7 (23.3) | 1 (20) |
| Septic shock | 15 (71.4) | 3 (100) | 19 (63.4) | 4 (80) |
| PNN50 | ||||
| High | 15 (71.4) | 2 (66.7) | 22 (73.3) | 4 (80) |
| Low | 6 (27.3) | 1 (33.3) | 8 (26.7) | 1 (20) |
| SDNN | ||||
| High | 5 (23.8) | 0 (0) | 10 (33.3) | 2 (40) |
| Moderate | 11 (52.4) | 2 (66.7) | 11 (36.7) | 2 (40) |
| Low | 5 (23.8) | 1 (33.3) | 9 (30) | 1 (20) |
| Conventional monitorization | 2 (9.5) | 0 (0) | 3 (10) | 1 (20) |
| Inotropic agents | 0 (0) | 1 (33.3) | 1 (3.33) | 0 (0) |
| Vasopressors | 16 (76.2) | 3 (100) | 22 (73.3) | 5 (100) |
| Mortality | 7 (33.3) | 2 (66.7) | 13 (43.3) | 1 (20) |
The global mortality rate was 40%. One of the three patients with a diagnosis of ischemia died, while in the patients with arrhythmic events the mortality rate was 40.1%.
The loss of HRV was less pronounced in the sepsis category, with median values for SDNN and PNN50 of 81.5ms and 1.5%, respectively, versus 68ms and 0% in the septic shock category (Table 5).
Heart rate variability indices.
| SDNN | PNN50 | |||||||
| Median | IQR | Minimum | Maximum | Median | IQR | Minimum | Maximum | |
| 93 | 47 | 14 | 221 | 0 | 2 | 0 | 30 | |
| Sepsis | 81.5 | 38 | 16 | 161 | 1.5 | 6 | 0 | 7 |
| Severe sepsis | 62 | 47.5 | 14 | 161 | 0 | 1.5 | 0 | 7 |
| Septic shock | 68 | 45 | 24 | 221 | 0 | 3 | 0 | 30 |
| With vasopressors | 68 | 46.5 | 17 | 221 | 0 | 2 | 0 | 71 |
| Without vasopressors | 73 | 45 | 14 | 163 | 0 | 3.5 | 0 | 9 |
| With inotropic drugs | 61 | 87 | 33 | 163 | 0 | 3 | 0 | 9 |
| Without inotropic drugs | 68 | 45 | 14 | 221 | 0 | 2 | 0 | 71 |
| Alive | 72.5 | 42 | 17 | 219 | 1 | 4 | 0 | 30 |
| Deceased | 61 | 45 | 14 | 221 | 0 | 1 | 0 | 9 |
In the patients administered vasopressor drugs, the median SDNN was 68±46.5, versus 61±87 in those administered inotropic agents. In turn, the median SDNN in the patients administered with neither vasopressors nor inotropic agents was greater.
Among the survivors, the median SDNN was 72.5±42, compared with 61±45 among those who died.
Application was made to this population of the mortality risk categorization used in coronary patients–a PNN50 proportion of 76% being found in the high risk category, where the mortality rate was 44.7%. In turn, according to SDNN, 78% of the population corresponded to the moderate and high risk groups, with a 51.9% mortality rate in the latter group (Table 6).
Classification of cardiovascular risk according to heart rate variability indices.
| Alive | Deceased | General | |
| n (%) | n (%) | n (%) | |
| PNN50 | |||
| High | 42 (55.3) | 34 (44.7) | 76 (76) |
| Low | 18 (75) | 6 (25) | 24 (24) |
| SDNN | |||
| High | 13 (48.1) | 14 (51.9) | 27 (27) |
| Moderate | 33 (64.7) | 18 (35.3) | 51 (51) |
| Low | 14 (63.6) | 8 (36.4) | 22 (22) |
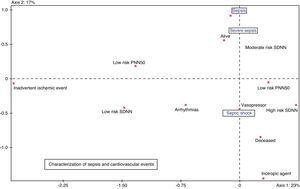
In the multiple correspondence analysis, and taking as illustrating variable the sepsis stage, with arrhythmic events, HRV, mortality and vasoactive drug use as active variables, septic shock was seen to be related to an increased HRV, more arrhythmias and greater mortality. Ischemic events were not related to sepsis stage (Fig. 1).
DiscussionAn evaluation was made of the incidence of ischemic events based on 4800h of Holter monitorization. The findings were scantly significant, and ischemia does not appear to be the primary cardiovascular event induced by sepsis.28
Activation of the systemic inflammatory response is the cause of the main manifestations of sepsis.29,30 The available evidence suggests that uncontrolled inflammation can trigger myocardial ischemia in previously diseased coronary vessels,31–33 and can depress ventricular function from the start of sepsis.34
In the present study ischemia was not shown to be the main physiopathological event related to ventricular dysfunction, though the presence of arrhythmias and the loss of HRV were seen to be important elements in that they constituted inadvertent cardiovascular events related to the imbalance between sympathetic and parasympathetic activity.35
An inconvenience of this study is its descriptive nature, given the scant theoretical basis upon which to support an analytical study.
The patients who suffered ischemic events were not detected by conventional monitorization, and the diagnosis was established by Holter monitorization. The events were transient, and none of them required stratification to invasive management.
This low incidence of ischemic events coincides with the observations of Cunnion and Schaer,10 who described normal or increased coronary flow in four of 7 septic patients with myocardial depression, and with the data published by Dhainaut and Huyghebaert, who identified myocardial ischemia in 6 of 40 septic patients on the basis of myocardial lactate production–though they also underscored that lactate is a poor indicator of myocardial ischemia.11
Arrhythmias were present in close to 50% of the patients, with a larger number of cases in the shock staging groups–almost all being diagnosed during Holter monitorization and characterized as atrial tachycardia, atrial fibrillation, ventricular tachycardia and idioventricular rhythm. None of these events was of a sustained nature.
HRV reflects the equilibrium of autonomous nervous system control of heart function,18 and has been reported to offer many applications.26 Sepsis has been found to involve a loss of HRV–this in turn being related to negative patient outcomes.36
This is the first series involving 100 patients to demonstrate a loss of HRV, as evaluated by the statistical indices SDNN and PNN50, which are associated to sympathetic hyperactivity and to a loss of parasympathetic control, respectively–this phenomenon being observed in septic myocardiopathy.35
No validation has been made of the use of the post-infarction risk stratification scale with HRV in septic patients. It was applied with the aim of grouping the data referred to these indices in the population. In this context, an increased distribution in the high risk categories was observed.
The higher median values of SDNN and PNN50 in the sepsis category suggest a lesser loss of HRV. The deceased patients showed a greater loss of variability–this finding coinciding with the observations of Chen and Kuo,37 who demonstrated the predictive capacity of HRV in relation to in-hospital mortality among patients with sepsis.37
The use of inotropic agents and vasopressors was concordant with the current resuscitation protocols,38 and the patients who received such therapy showed a greater loss of HRV. The utilization of inotropic agents was low, even while acknowledging that advanced stage septic myocardiopathy is a myocardial depression state. With this consideration in mind, an analysis was made of the hemodynamic profile of 26 patients subjected to cardiac output monitorization in parallel to Holter recording. The technique used was based on analysis of the pulse wave profile, using the Vigileo® system. We accumulated 100h of monitorization, with mean values for SVI and SVV of 32 and 10, respectively, which is consistent with myocardial depression, as described and confirmed by Parrillo.39
In our group of patients, cardiovascular behavior related to sepsis was no different from that found in previous descriptions, discarding the possibility that myocardial ischemia is significant in septic myocardiopathy–which makes more feasible the intervention of humoral myocardial depressor factors.
On the other hand, the incidence of arrhythmic events and the loss of HRV deserve close attention–representing the true inadvertent cardiovascular events in sepsis, and opening a new field of research for establishing the potential benefits of different treatments, with a view to modifying sympathetic activity. New studies with control groups are needed to compare the phenomenon of HRV which we have found in septic patients versus other disease conditions commonly observed in the ICU.
Improvements are required in continuous monitorization techniques, with the consideration of modifications in conventional electrocardiography or more closer monitoring of contractility in patients at risk, giving the limitations of conventional cardiac monitorization in diagnosing cardiovascular events in septic patients.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Gomez Duque M, et al. Estudio ECAIS: eventos cardiovasculares adversos inadvertidos en sepsis. Med Intensiva. 2012;36:343–50.