Brain Trauma incidence is about 235/100000 inhabitants/year in Europe, frequently co-existing with thoracic trauma (35%).1 Avoiding hypoxic brain damage is crucial but difficult to achieve in case of respiratory failure, given the potential harmful effect of ventilatory strategies (prone positioning, alveolar recruitment) on intracranial hypertension (ICH).
ECMO is a lifesaving technique, assisting the failing heart or lungs, but circuit anticoagulation is required, which could increase the risk of bleeding. Consequently, trauma bleeding, and especially brain trauma are still formal contra-indications for ECMO.2
We present the case of a severe BTI with intracranial bleeding requiring surgical drainage and VV ECMO for severe respiratory failure.
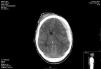
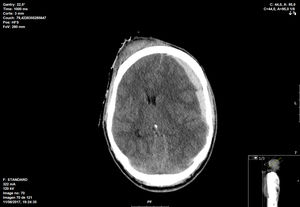
26 years old male suffered a motorbike crash accident. Initially presenting with GCS 4 on the scene, bilateral nonreactive pupils; BP 110/60mmHg. Injury severity score (ISS) 25. He was intubated and transferred to our hospital; initial CT scan showed temporal bone fracture and hemispheric subdural hematoma with middle line shift (12mm) and signs of uncal herniation (Fig. 1).
Multiple rib fractures and right lung consolidation (lung contusion vs. aspiration pneumonitis).
He was immediately transferred to the operating theater; left frontoparietal craniotomy and hematoma drainage was performed. Direct visualization detected loss of pulsatility in frontal and parietal lobes. Parenchymal intracranial pressure (ICP) monitoring (Integra Neurosciences, San Diego) after drainage showed ICP of 25mmHg, needing deep sedation, neuromuscular blocking, and osmotherapy. Postoperative CT scan showed correct drainage with diffuse brain edema.
The patient develops severe ARDS on day 2. Despite lung protective ventilation, bilateral pneumothorax occurs, requiring chest tube placement.
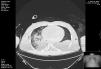
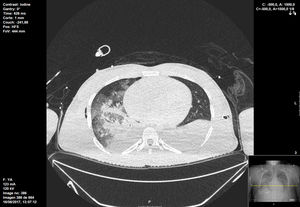
Open lung strategies such as recruitment maneuvers were not implemented due to the presence of pneumothorax, and prone positioning was tried but unfeasible due to rise in ICP (Fig. 2).
Given the refractory hypoxemia and its impact on secondary brain damage, we decided to put the patient on ECMO (Cardiohelp-Maquet-Getinge group, Rastatt, Germany).
Pre-ECMO respiratory setting: FiO2 1; PEEP 10 cmH2O; P/F ratio 90; compliance 20mlcm H2O-1 Plateau pressure 33cm H2O; dP 14. Murray score 3.
We utilized 23 Fr femoral drainage and 19 Fr jugular return Maquet HLS cannulae. A bolus of UFH (70IU/kg) was administered during cannulation. We decided no anticoagulation thereafter.
Initial ECMO settings: 4.2lpm, gas flow 5lpm, FiO2 1. Ventilator settings after ECMO: VCV FiO2 0.4; tidal volume 3cc/kg; RR 10bpm PEEP 10cmH2O.
Given the still uncontrolled ICH, cannulation was performed under deep sedation, paralysis, and osmotherapy.
Percutaneous tracheostomy was performed on day fifteen, and the last chest tube removed on day twenty.
ECMO system was closely monitored according to our protocol. Oxygenator was replaced on day seven due to an increase in LDH and reticulocyte count and a decrease in haptoglobin levels.
A decision to stop ECMO was made after ECMO time of fifteen days given respiratory improvement, correct weaning parameters, and labs showing incipient coagulopathy (slight increase in PT and aPTT ratio, and slightly decreasing platelets and fibrinogen). Having started decannulation procedure, the patient experience sudden airway bleeding. ECMO was immediately stopped and the patient decannulated, after which bleeding was controlled. 1g fibrinogen and 600IU of Human Prothrombin Complex were administered. Bronchoscopy was performed immediately afterward for airway clearance.
On day thirty the patient is awake, obeying commands, no motor deficit but with severe ICU acquired weakness. He was discharged after 50 days to the neurosurgery ward. Discharged home after four months with full neurological and functional recovery.
Multiple trauma is still a formal contra-indication for ECMO, given the increased risk of bleeding.2
ECMO circuit needs anticoagulation, which could be avoided in particular circumstances. But ECMO also exposes the blood to a foreign surface, triggering inflammation and coagulation, which can lead to a DIC-like consumptive coagulopathy.3 Although biocompatible coatings may decrease activation of coagulation, it has not solved the issue. Therefore, running ECMO without anticoagulation does not prevent the occurrence of bleeding diathesis.
These factors, in the context of co-existing severe BTI, could lead to catastrophic intracranial bleeding. Therefore, evidence of ECMO in this scenario is anecdotic.
Biscotti4 described two patients with severe BTI and intracranial bleeding who needed ECMO for ARDS. The circuit was anticoagulated with UFH with an aPTT target of 40–60s. However, no patient experienced ICH or underwent surgery.
Muellenbach5 published three trauma patients suffering BTI, none of them requiring surgery. The circuit was not anticoagulated, but ECMO time was short (maximum five days).
Friesenecker6 described the first case of severe BTI requiring craniotomy who underwent ECMO for ARF for 17 days. Initially presenting with CT scan showing brain edema, ECMO was started due to severe ARDS under anticoagulation with UFH targeted to ACT of 150s, but CT scan revealed large brain hematoma on day two, potentially influenced by anticoagulation. There are no further similar cases published to our knowledge.
In our case, ECMO was run for 15 days without anticoagulation. It was started shortly after surgery in a patient with severe intracranial bleeding and ICH aggravated by severe ARDS, with a good outcome.
Thorough system monitoring allowed safe management of a non-anticoagulated circuit, and early detection and control of complications. On the other hand, no ICP deterioration during ECMO time was detected.
This case suggests that ECMO can be implemented safely without anticoagulation, and should not be withheld from the therapeutic armamentarium in case of a severe brain trauma bleeding. Appropriate protocol implementation and close monitoring are paramount in this scenario.