To evaluate the impact of the infusion of sodium lactate 500ml upon different biochemical variables and intracranial pressure in patients admitted to the intensive care unit.
DesignA prospective experimental single cohort study was carried out.
ScopePolyvalent intensive care unit of a university hospital.
PatientsCritical patients with shock and intracranial hypertension.
ProcedureA 500ml sodium lactate bolus was infused in 15min. Plasma levels of sodium, potassium, magnesium, calcium, chloride, lactate, bicarbonate, PaCO2, pH, phosphate and albumin were recorded at 3 timepoints: T0 pre-infusion; T1 at 30min, and T2 at 60min post-infusion. Mean arterial pressure and intracranial pressure were measured at T0 and T2.
ResultsForty-one patients received sodium lactate: 19 as an osmotically active agent and 22 as a volume expander. Metabolic alkalosis was observed: T0 vs. T1 (p=0.007); T1 vs. T2 (p=0.003). Sodium increased at the 3 timepoints (T0 vs. T1, p<0.0001; T1 vs. T2, p=0.0001). In addition, sodium lactate decreased intracranial pressure (T0: 24.83±5.4 vs. T2: 15.06±5.8; p<0.001). Likewise, plasma lactate showed a biphasic effect, with a rapid decrease at T2 (p<0.0001), including in those with previous hyperlactatemia (p=0.002).
ConclusionsThe infusion of sodium lactate is associated to metabolic alkalosis, hypernatremia, reduced chloremia, and a biphasic change in plasma lactate levels. Moreover, a decrease in intracranial pressure was observed in patients with acute brain injury.
Evaluar el impacto de la infusión de lactato de sodio 0,5M sobre variables del medio interno y sobre la presión intracraneana en pacientes críticos.
DiseñoEstudio prospectivo experimental de cohorte única.
ÁmbitoUnidad de cuidados intensivos de un hospital universitario.
PacientesPacientes con shock y neurocríticos con hipertensión intracraneana.
IntervencionesSe infundió una carga de 500 cc de infusión de lactato de sodio 0,5M en 15min y se midió el nivel plasmático de sodio, potasio, magnesio, calcio, cloro, lactato, bicarbonato, PaCO2 arterial, pH, fosfato y albúmina en 3 tiempos: T0 preinfusión; T1 a los 30min y T2 a los 60min postinfusión. Se midieron la presión arterial media y presión intracraneana en T0 y T2.
ResultadosRecibieron el fluido N=41: n=19 como osmoagente y 22 como expansor. Se constató alcalosis metabólica: T0 vs. T1 (p=0,007); T1 vs. T2 (p=0,003). La natremia aumentó en los 3 tiempos (T0 vs. T1; p<0,0001; T1 vs. T2; p=0,0001). Se demostró un descenso de la presión intracraneana (T0: 24,83±5,4 vs. T2: 15,06±5,8; p<0,001). El lactato aumentó inicialmente (T1) con un rápido descenso (T2) (p<0,0001), incluso en aquellos pacientes con hiperlactatemia basal (p=0,002).
ConclusionesLa infusión de lactato de sodio 0,5M genera alcalosis metabólica, hipernatremia, disminución de la cloremia y un cambio bifásico del lactato, y muestra eficacia en el descenso de la presión intracraneana en pacientes con daño encefálico agudo.
Fluid therapy is a key element in the management of the critically ill. Its main objectives are the optimization of tissue perfusion, the replacement of fluid losses, and the maintenance of homeostasis1,2. The administration of a fluid acts upon the volume and content of the different body compartments, modifying water-electrolyte composition, tonicity and the acid–base balance. Fluid therapy should be conceived in the same way as the administration of any other drug, considering not only the therapeutic effects obtained but also the side effects and potential adverse events3.
In recent years there has been a growing body of evidence of the deleterious effects of fluid therapy, particularly in relation to the administration of unbalanced solutions (those with an in vivo difference in strong ions of zero) that give rise to the development of hyperchloremia4. While this condition has been observed with the administration of large and repeated amounts of normal saline solution (physiological saline), it manifests more often, earlier and with greater severity when hypertonic (3%, 7.5%, 10%, etc.) saline solutions are used. Such solutions are currently administered on a routine basis for the initial control of intracranial hypertension, and also for the correction of hyponatremia associated to salt wasting syndromes5.
Experimental models in animals have demonstrated that the presence of hyperchloremia secondary to fluid therapy generates a proinflammatory effect6,7, as well as alterations in intrarenal hemodynamics, with vasoconstriction and a decrease in glomerular filtration8. On the other hand, different observational clinical studies have established an association between hyperchloremia and the development of kidney damage9, as well as a significant increase in mortality among different groups of critical patients10–12. Such alterations in renal function generated by hyperchloremia would constitute the basis of water-saline retention, positive water balance13 and expansion of the extracellular space. This circumstance is the norm among patients admitted to the intensive care unit (ICU), and has also been described as a prognostic factor in different observational studies14,15.
Due to these problems, new water-electrolyte solutions have been investigated with the purpose of minimizing the development of hyperchloremia, and of limiting the positive fluid balance in the critically ill. It is in this setting where hypertonic sodium lactate solution (HLS) has been evaluated in different experimental models16–19 and in clinical trials involving heterogeneous populations of critical patients – including subjects in the postoperative period of heart surgery20–22, with septic shock, acute heart failure23 and major burns24. A special application of HSL is referred to the neurocritical patient, due to its osmotic activity25,26 and its capacity to be used as an energy substrate by the damaged brain tissues27–29.
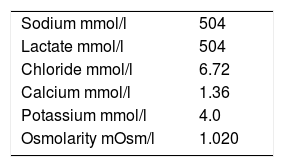
However, due to its water-electrolyte composition and osmolarity, half-molar HSL (HSL 0.5M) (Table 1) is far from being a balanced solution, and can significantly modify a number of variables of the internal body environment.
The primary objective of the present study was to evaluate the impact of the infusion of a 500-ml dose of HSL 0.5M upon different biochemical variables in a heterogeneous population of critical patients. As secondary objectives, we examined the impact of the administration of HSL upon intracranial pressure in a subpopulation of patients with neural damage and intracranial hypertension.
Patients and methodsA prospective, single-center experimental study was carried out in the intensive care unit of the Clinic Hospital of the Faculty of Medicine of the University of the Republic of Uruguay between the months of March 2017 and September 2018. This polyvalent adult ICU with 11 beds belongs to the Clinic Hospital, which is a tertiary level center in Montevideo.
The inclusion criteria were adults (over 18 years of age) requiring fluid provision with two indications: Group 1: volume expansion in the presence of arterial hypotension (mean blood pressure [MBP]<65mmHg), with clinical manifestations suggestive of hypovolemia and hypoperfusion, and with predictors of response to fluid loading and Group 2: osmotic action in the presence of acute brain damage of traumatic, vascular or postoperative origin with intracranial hypertension (intracranial pressure [ICP]>20mmHg or >15mmHg in subjects with temporal parenchymal damage, reduced basal cisterns or prior decompressive craniectomy), following the adoption of nonspecific measures for the control of intracranial hypertension and with an indication of osmotherapy.
Two necessary conditions associated to the presence of arterial hypotension were established for fluid administration in group 1: (a) the existence of elements predictive of a favorable response to fluids using dynamic variables (pulse pressure variation >13%; systolic volume variation >10%; distensibility index of the inferior vena cava >15%) and (b) the presence of elements indicative of hypoperfusion (lactate >2mmol/l or central venous O2 saturation <70% or diuresis <0.5ml/kg per hour or capillary filling time >3s).
The protocolized management of intracranial hypertension in our Unit is based on the adoption of nonspecific measures (mechanical ventilation, sedation and analgesia, correct patient positioning, treatment of hyperthermia, prevention of epileptic disorders, control of glycemia, hemodynamic stabilization) and specific measures at three levels: (a) cerebrospinal fluid drainage, use of neuromuscular blockers, osmotherapy with HSL 0.5M (in this case) and moderate hyperventilation (PaCO2 30mmHg); (b) infusion of indomethacin, point transient and intensive hyperventilation for reducing increased ICP (PaCO2 25mmHg); and (c) anesthetic coma (propofol), moderate controlled hypothermia 32–33°C, and decompressive craniectomy or lumbar drainage. All these measures are adopted progressively and stepwise until reaching ICP <20mmHg (or 15mmHg in case of decompressive craniectomy or non-evacuated focal temporal damage).
The exclusion criteria were: (a) age under 18 years; (b) pregnancy; (c) acute kidney injury according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria30; (d) liver failure; (e) hyponatremia (Na<135mmol/l); and (f) hypernatremia (Na>155mmol/l).
Study protocolThe eligible patients received a 500-ml loading dose of HSL 0.5M (Laboratorio Fármaco Uruguayo, Uruguay) as an intravenous infusion in 15min through a central venous catheter. The biochemical characteristics of the crystalloid are shown in Table 1.
Immediately before infusion of the crystalloid (T0), we extracted a 5-ml blood sample from the arterial line for use in the study of blood gases (PaCO2, PaO2, pH, base excess) and for the measurement of plasma osmolality, ions (lactate, Cl−Na+, ionic Ca2+, phosphate [PO42−], magnesium [Mg2+] and bicarbonate [HCO3−]) and albumin. The same measurements were repeated at 30min (T1) and at 60min (T2) postinfusion. Before the infusion of HSL 0.5M (T0) and 60min after infusion (T2), we recorded MBP, as well as ICP and brain perfusion pressure (BPP) in all those patients in which HSL 0.5M was used as osmotic agent. Before inclusion in the protocol, the patients received 0.9% sodium chloride and potassium chloride as maintenance fluid adjusted according to the water balance and ionogram established on a daily basis.
Analysis of samplesIons in blood processing was performed immediately, using the selective electrode method for Na+, K+, Ca2+ and Cl− on an ABL Flex 835 analyzer (Radiometer). From the same sample we also recorded the values corresponding to pO2, pCO2, pH, HCO3−, base excess and lactate.
Samples were collected in lithium heparin tubes for the analysis of albumin, PO42− and Mg2+. The tubes were immediately sent to the core laboratory and centrifuged within four hours after extraction of the samples to obtain the heparinized plasma. The samples were processed using a Cobas C 311 analyzer (Roche Diagnostics).
The heparinized plasma samples were used to measure osmolality with an Osmometer 3250 analyzer (Advanced Instruments), based on the lowering of freezing point method.
The quantitative analysis of the results was based on the physicochemical approach of Stewart modified by Figge31, with consideration of the effects of the plasma proteins. The following formulas were used for the different calculations:
Apparent strong ions difference (aSID)
=(Na++K++Mg2++Ca2+)
=(Cl−+lactate) all concentrations in mmol/l
Effective strong ions difference (eSID)
=2.46×10−8×pCO2/10−pH
+[albumin] g/dl×(0.123×pH−0.631)
+[PO42−] mmol/l×(0.309×pH−0.469)
Total weak acids (Atot)
=[albumin] g/dl×(0.123×pH−0.631)
+[PO42−] mmol/l×(0.309×pH−0.469)
Anion gap (AG)=Na++K+−(Cl−+HCO3−)
Strong ion gap (SIG)=aSID−eSID
The study was carried out following the ethical principles of the Declaration of Helsinki on research in human subjects. All the records were filed by the principal investigator of the study. The study protocol was approved by the Ethics Committee of the Clinic Hospital of the Faculty of Medicine of the University of the Republic of Uruguay, and informed consent was obtained from the relatives before patient inclusion in the study.
Statistical analysisQualitative variables were reported as frequencies and percentages, and quantitative variables were reported as the mean±standard deviation (SD) or as the median and interquartile range (IQR), as applicable.
Quantitative variables with a normal distribution were compared using the Student t-test for dependent samples in the case of two measurements, while analysis of variance (ANOVA) was used for the comparison of multiple variables with more than two measurements. Nonparametric tests were used for the comparison of variables exhibiting a non-normal distribution: the Wilcoxon test for two samples and the Friedman test for more than two samples. The level of confidence was 95%, with an alpha-error of 5%. Statistical significance was considered for p<0.05.
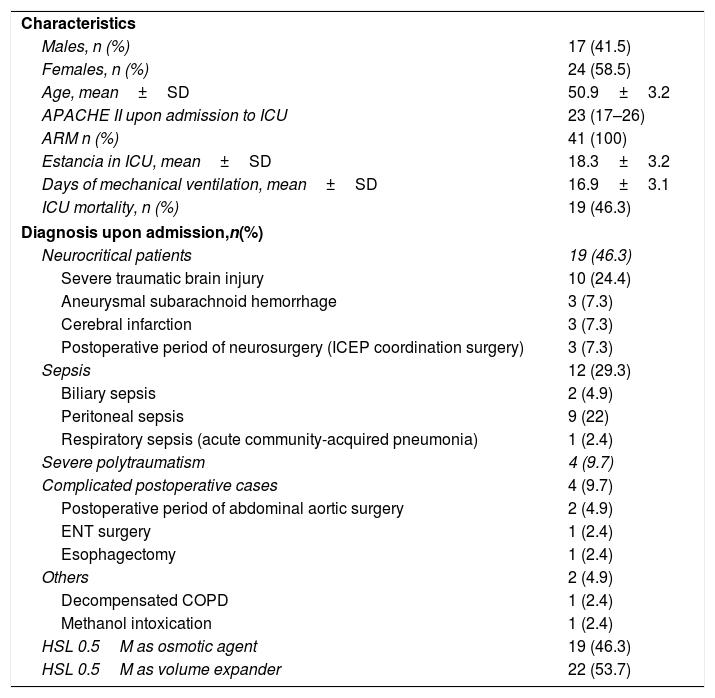
ResultsA total of 41 critical patients were enrolled in the study, of which 19 (46.3%) received HSL 0.5M as osmotic agent and 22 (53.7%) as volume expansion measure. There were 24 males (58.5%). The mean patient age was 50.9±3.1 years. The median APACHE II score upon admission to the ICU was 23 (IQR 17–26), and the in-ICU mortality rate was 46.3%. The mean duration of stay in the ICU was 18.3±3.2 days, while the duration of mechanical ventilation was 16.9±3.1 days. Of the 19 patients that received HSL because of cranial hypertension, 10 were admitted due to severe traumatic brain injury (Glasgow Coma Score [GCS]<8), and of these three required decompressive craniectomy, with a tomographic classification (Traumatic Coma Data Bank) of II (4 patients), III (2 patients) and IV (4 patients). A total of 12 patients were admitted due to sepsis, and four patients were admitted due to severe polytraumatisms, with an Injury Severity Score (ISS) of 27.75±3.9. The main demographic and baseline characteristics of the patients included in the study are shown in Table 2. None of the patients received hypertonic crystalloids or colloids before the study. Those patients that received HSL 0.5M as osmotic agent did so after 22.8±13.2h of admission to the Unit, while those receiving HSL as volume expander did so after 14.5±8.2hof admission.
Demographic and baseline characteristics of the patients included in the study.
| Characteristics | |
| Males, n (%) | 17 (41.5) |
| Females, n (%) | 24 (58.5) |
| Age, mean±SD | 50.9±3.2 |
| APACHE II upon admission to ICU | 23 (17–26) |
| ARM n (%) | 41 (100) |
| Estancia in ICU, mean±SD | 18.3±3.2 |
| Days of mechanical ventilation, mean±SD | 16.9±3.1 |
| ICU mortality, n (%) | 19 (46.3) |
| Diagnosis upon admission,n(%) | |
| Neurocritical patients | 19 (46.3) |
| Severe traumatic brain injury | 10 (24.4) |
| Aneurysmal subarachnoid hemorrhage | 3 (7.3) |
| Cerebral infarction | 3 (7.3) |
| Postoperative period of neurosurgery (ICEP coordination surgery) | 3 (7.3) |
| Sepsis | 12 (29.3) |
| Biliary sepsis | 2 (4.9) |
| Peritoneal sepsis | 9 (22) |
| Respiratory sepsis (acute community-acquired pneumonia) | 1 (2.4) |
| Severe polytraumatism | 4 (9.7) |
| Complicated postoperative cases | 4 (9.7) |
| Postoperative period of abdominal aortic surgery | 2 (4.9) |
| ENT surgery | 1 (2.4) |
| Esophagectomy | 1 (2.4) |
| Others | 2 (4.9) |
| Decompensated COPD | 1 (2.4) |
| Methanol intoxication | 1 (2.4) |
| HSL 0.5M as osmotic agent | 19 (46.3) |
| HSL 0.5M as volume expander | 22 (53.7) |
Values reported as frequencies (n) and percentage (%).
Mean±standard deviation and median with interquartile range.
ENT: ear, nose and throat surgery; ICEP: intracranial expansive process; ICU: intensive care unit.
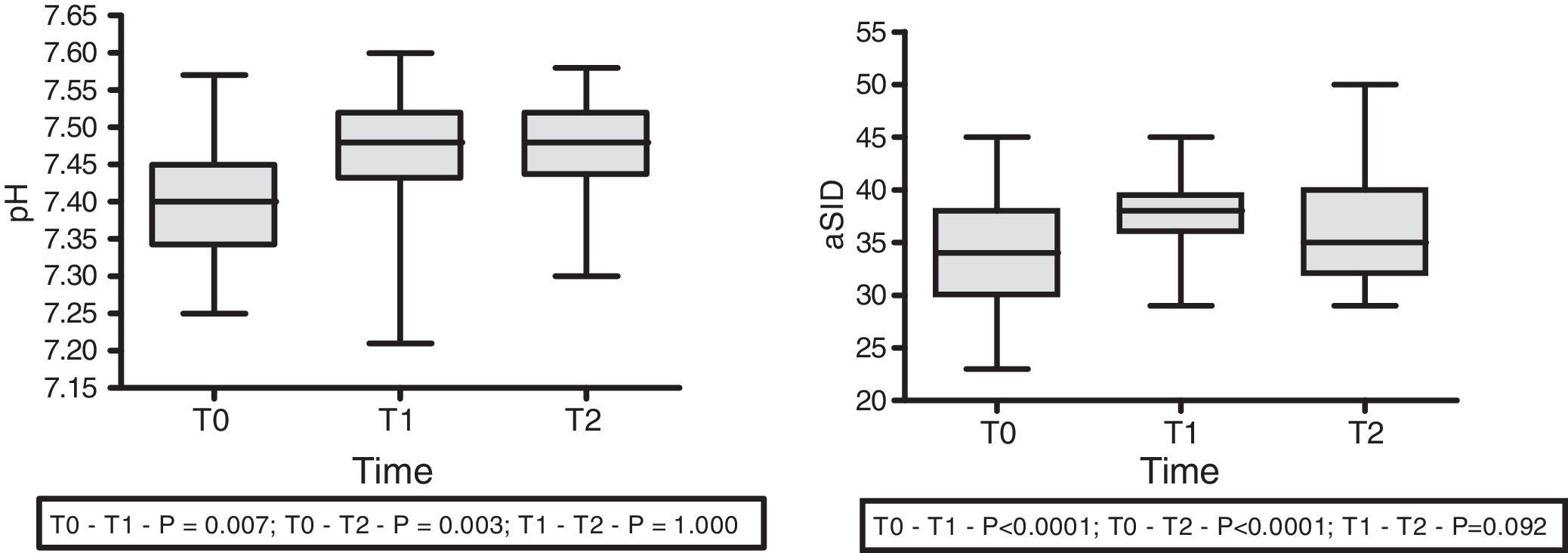
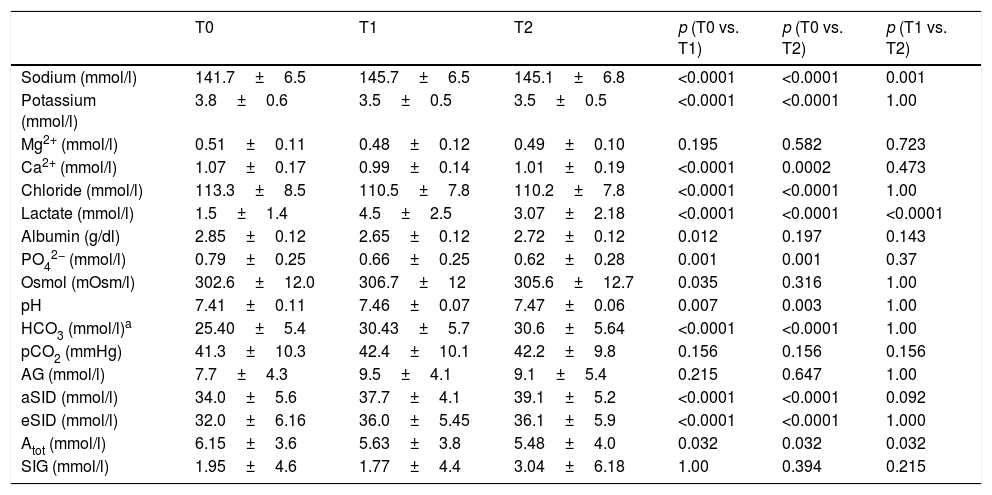
With regard to the effect of the infusion of HSL 0.5M upon acid–base status, moderate immediate metabolic alkalosis was recorded, with a rise in pH (7.41±0.11 at T0 vs. 7.46±0.07 at T1; p=0.007 vs. 7.47±0.06 at T2; p=0.003) and aSID (34.00±5.61 at T0 vs. 37.72±4.08 at T1; p<0.0001 vs. 39.11±5.20 at T2; p<0.0001) (Fig. 1). In turn, we recorded a decrease in Atot at T0 (6.15±3.58 vs. 5.628±3.85; p=0.032) that persisted at T1 (5.479±3.98; p=0.032), with no significant difference between T1 and T2. No changes were observed in the arterial blood CO2 levels.
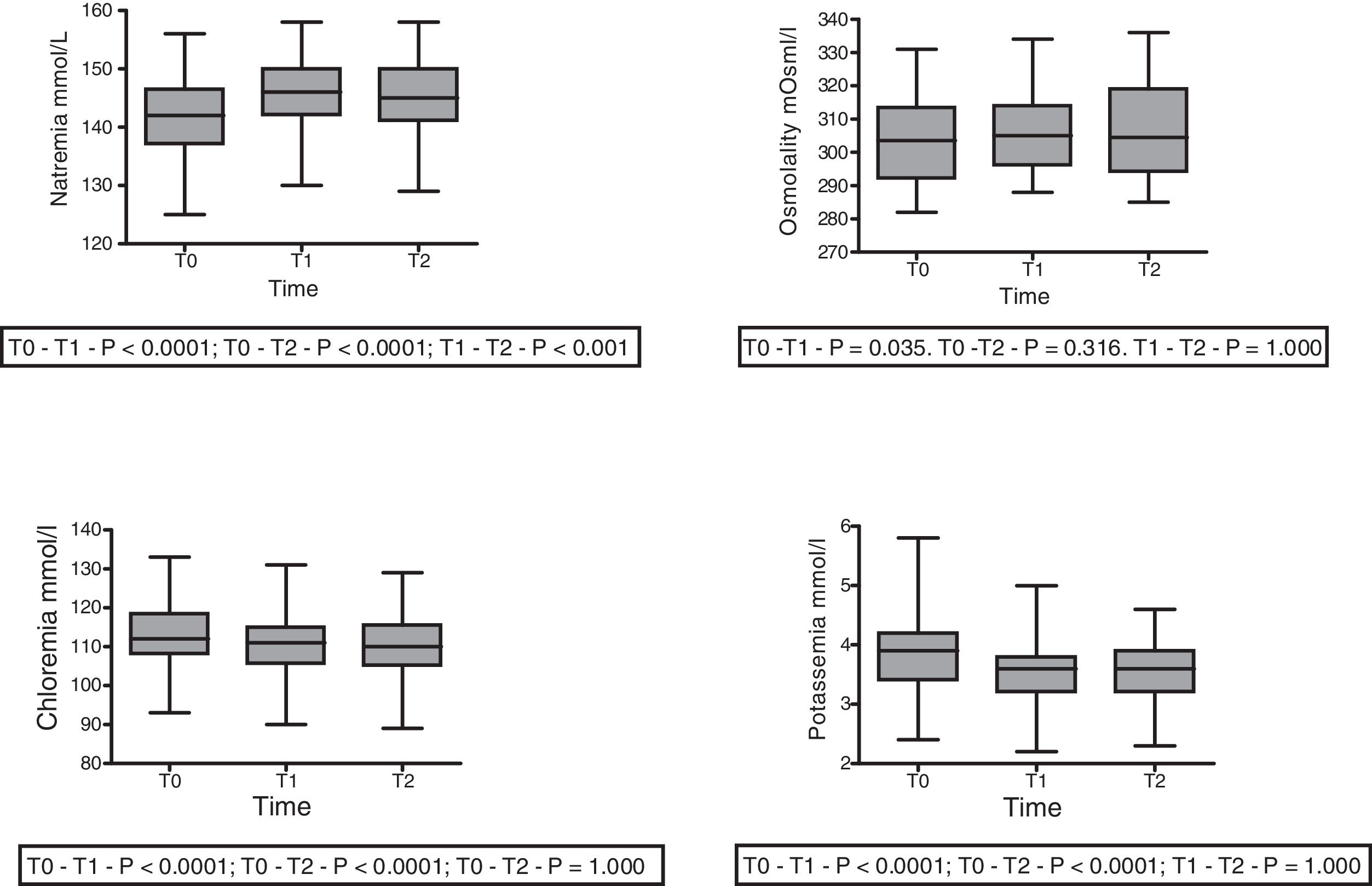
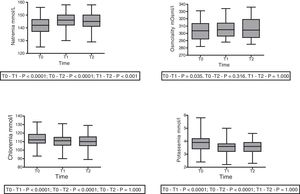
With regard to the impact upon the blood ion profile, variations in natremia were recorded at all three timepoints: 141.7±6.6 (T0) vs. 145.8±6.5mmol/l (T1) with p<0.0001; 141.7±6.6mmol/l (T0) vs. 145.1±6.9mmol/l (T2) with p<0.0001; and 145.8±6.5mmol/l (T1) vs. 145.1±6.9mmol/l (T2) with p=0.001. In turn, osmolality was seen to increase after 30min (302.6±12.0 vs. 306.7; p=0.035), with no significant differences between the measurements at 30 and 60min (306.7±12 vs. 305.6±12.7; p=0.31) (Fig. 2).
A slight but statistically significant decrease was observed in the concentrations of Cl− (113.3±8.5 vs. 110.5±7.8 vs. 110.225±7.859mmol/l) and K+ (3.84±0.6 vs. 3.54±0.5 vs. 3.54±0.5mmol/l) at T1 that remained without changes at T2 (Fig. 2). The variations of the biochemical variables are summarized in Table 3.
Time course of the different biochemical parameters during the study.
| T0 | T1 | T2 | p (T0 vs. T1) | p (T0 vs. T2) | p (T1 vs. T2) | |
|---|---|---|---|---|---|---|
| Sodium (mmol/l) | 141.7±6.5 | 145.7±6.5 | 145.1±6.8 | <0.0001 | <0.0001 | 0.001 |
| Potassium (mmol/l) | 3.8±0.6 | 3.5±0.5 | 3.5±0.5 | <0.0001 | <0.0001 | 1.00 |
| Mg2+ (mmol/l) | 0.51±0.11 | 0.48±0.12 | 0.49±0.10 | 0.195 | 0.582 | 0.723 |
| Ca2+ (mmol/l) | 1.07±0.17 | 0.99±0.14 | 1.01±0.19 | <0.0001 | 0.0002 | 0.473 |
| Chloride (mmol/l) | 113.3±8.5 | 110.5±7.8 | 110.2±7.8 | <0.0001 | <0.0001 | 1.00 |
| Lactate (mmol/l) | 1.5±1.4 | 4.5±2.5 | 3.07±2.18 | <0.0001 | <0.0001 | <0.0001 |
| Albumin (g/dl) | 2.85±0.12 | 2.65±0.12 | 2.72±0.12 | 0.012 | 0.197 | 0.143 |
| PO42− (mmol/l) | 0.79±0.25 | 0.66±0.25 | 0.62±0.28 | 0.001 | 0.001 | 0.37 |
| Osmol (mOsm/l) | 302.6±12.0 | 306.7±12 | 305.6±12.7 | 0.035 | 0.316 | 1.00 |
| pH | 7.41±0.11 | 7.46±0.07 | 7.47±0.06 | 0.007 | 0.003 | 1.00 |
| HCO3 (mmol/l)a | 25.40±5.4 | 30.43±5.7 | 30.6±5.64 | <0.0001 | <0.0001 | 1.00 |
| pCO2 (mmHg) | 41.3±10.3 | 42.4±10.1 | 42.2±9.8 | 0.156 | 0.156 | 0.156 |
| AG (mmol/l) | 7.7±4.3 | 9.5±4.1 | 9.1±5.4 | 0.215 | 0.647 | 1.00 |
| aSID (mmol/l) | 34.0±5.6 | 37.7±4.1 | 39.1±5.2 | <0.0001 | <0.0001 | 0.092 |
| eSID (mmol/l) | 32.0±6.16 | 36.0±5.45 | 36.1±5.9 | <0.0001 | <0.0001 | 1.000 |
| Atot (mmol/l) | 6.15±3.6 | 5.63±3.8 | 5.48±4.0 | 0.032 | 0.032 | 0.032 |
| SIG (mmol/l) | 1.95±4.6 | 1.77±4.4 | 3.04±6.18 | 1.00 | 0.394 | 0.215 |
Data reported as the mean±SD. Repeated measures ANOVA at preinfusion (T0); 30min after infusion (T1), 60min after infusion (T2) for all variables except lactate, PCO2 and Atot (Friedman test).
Atot: weak total acids; AG: anion gap; SIG: strong ion gap; aSID: apparent strong ion difference; eSID: effective strong ion difference.
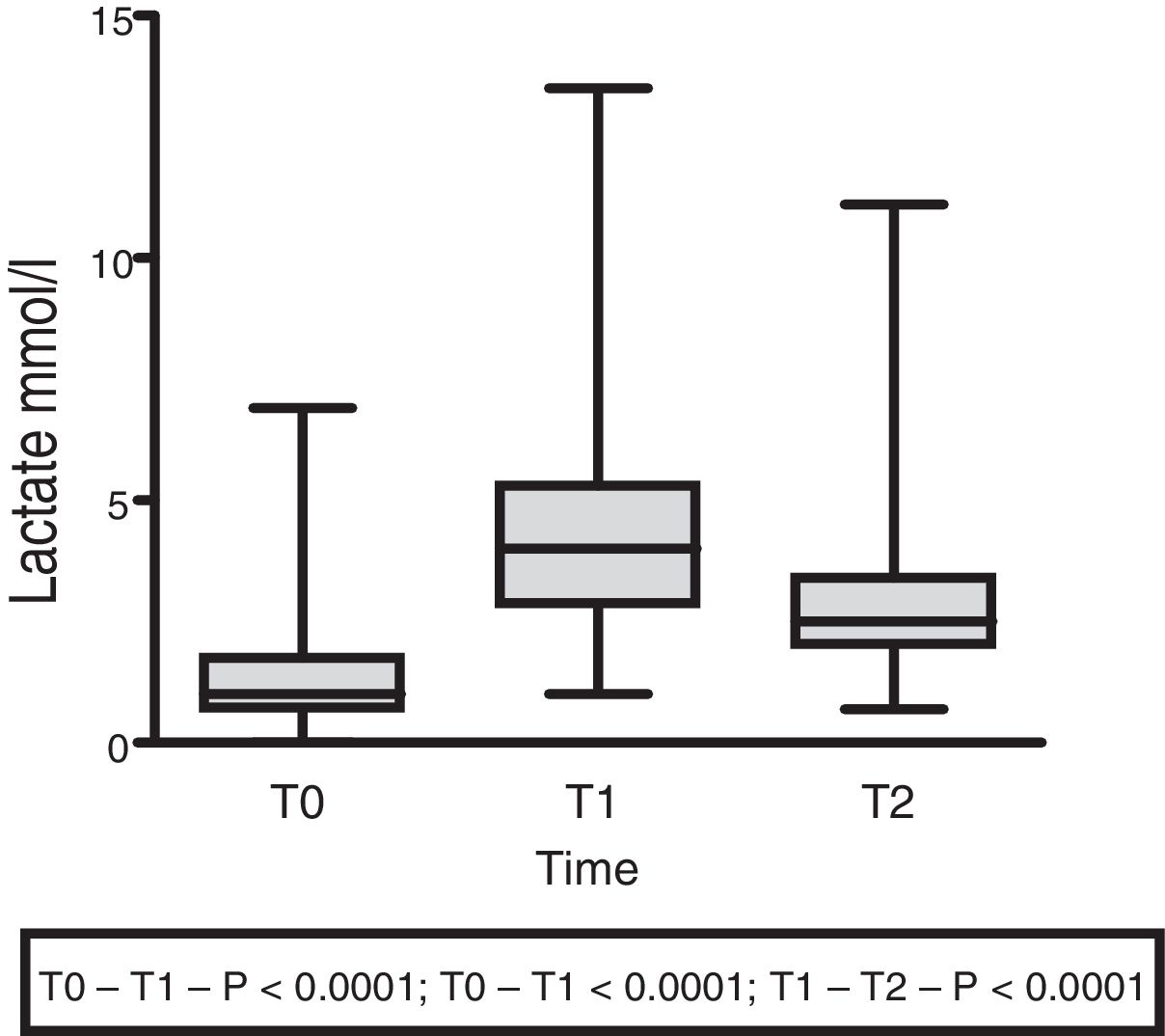
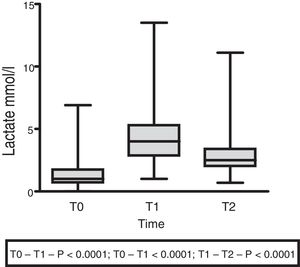
The lactate levels with respect to external loading initially exhibited a rising trend (1.5±1.4 at T0 vs. 4.5±2.5 at T1; p<0.001), followed by a decrease at 60min (3.07±2.18; p<0.0001) (Fig. 3).
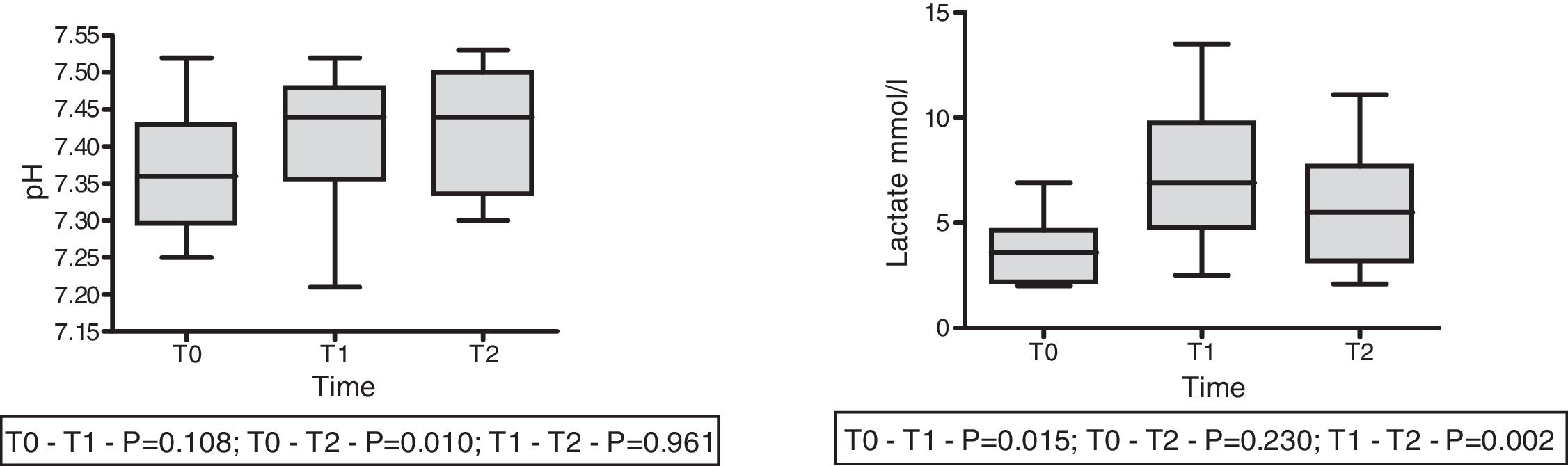
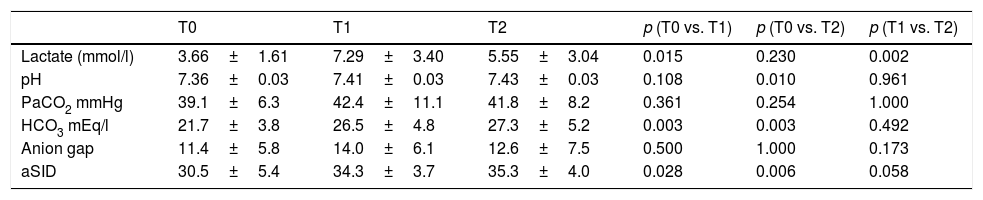
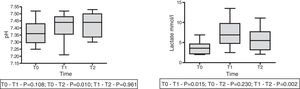
On the other hand, in 9 patients with lactatemia >2mmol/l prior to the infusion of HSL 0.5M, we observed an increase in plasma lactate of 3.67±0.53 (T0) vs. 7.23±1.13mmol/l (T1) (p<0.015), with a decrease after 60min of 5.54±1.01mmol/l (T2) (p=0.002). In this same group we recorded a rise in arterial pH: 7.36±0.03 (T0) vs. 7.41±0.032 (T1) with p=0.108, and 7.43±0.028 (T2) with p=0.01. The behavior of lactate and pH in this special patient subgroup is shown in Fig. 4. The data referred to pH, HCO3, anion gap, PCO2, lactate and aSID of this subgroup are summarized in Table 4.
Time course of the acid–base balance parameters in patients with hyperlactatemia (lactate >2mmol/l) prior to the infusion of HSL 0.5M.
| T0 | T1 | T2 | p (T0 vs. T1) | p (T0 vs. T2) | p (T1 vs. T2) | |
|---|---|---|---|---|---|---|
| Lactate (mmol/l) | 3.66±1.61 | 7.29±3.40 | 5.55±3.04 | 0.015 | 0.230 | 0.002 |
| pH | 7.36±0.03 | 7.41±0.03 | 7.43±0.03 | 0.108 | 0.010 | 0.961 |
| PaCO2 mmHg | 39.1±6.3 | 42.4±11.1 | 41.8±8.2 | 0.361 | 0.254 | 1.000 |
| HCO3 mEq/l | 21.7±3.8 | 26.5±4.8 | 27.3±5.2 | 0.003 | 0.003 | 0.492 |
| Anion gap | 11.4±5.8 | 14.0±6.1 | 12.6±7.5 | 0.500 | 1.000 | 0.173 |
| aSID | 30.5±5.4 | 34.3±3.7 | 35.3±4.0 | 0.028 | 0.006 | 0.058 |
Data reported as the mean±standard deviation.
Repeated measures ANOVA at different timepoints.
aSID: apparent strong ion difference; T0: preinfusion value; T1: value 30min after infusion; T2: value 60min after infusion.
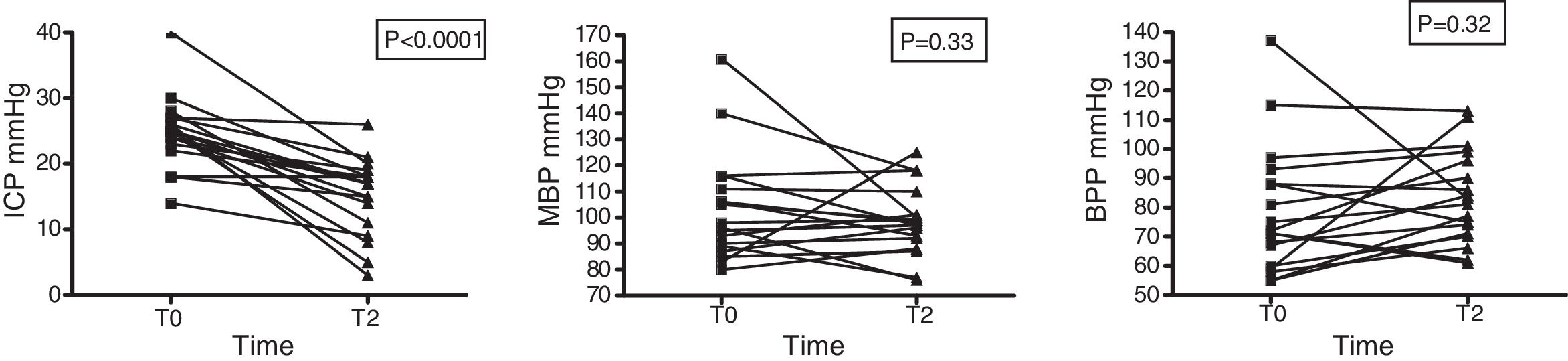
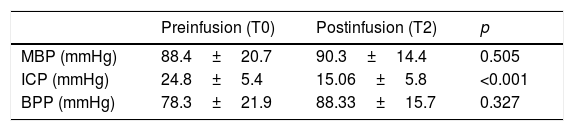
Intracranial pressure was seen to decrease significantly: 24.83±5.4 (T0) vs. 15.06±5.8 (T2) with p<0.001. In contrast, no changes in systemic hemodynamics were observed on the basis of the variations in MBP (88.4±20.7 at T0 vs. 90.3±14.4mmHg at T2; p=0.506) in the total sample of patients or in those individuals that received HSL as an osmotic agent (103.2±20.7 vs. 98.4±13.0mmHg; p=0.33) (Fig. 5). The data referred to ICP, MBP and BPP of the 19 patients administered HSL as osmotic agent are reported in Table 5.
Time course of MBP, ICP and BPP in 19 patients administered HSL 0.5M as osmotic agent.
| Preinfusion (T0) | Postinfusion (T2) | p | |
|---|---|---|---|
| MBP (mmHg) | 88.4±20.7 | 90.3±14.4 | 0.505 |
| ICP (mmHg) | 24.8±5.4 | 15.06±5.8 | <0.001 |
| BPP (mmHg) | 78.3±21.9 | 88.33±15.7 | 0.327 |
Data reported as the mean±standard deviation. Student t-test for the comparison of means.
MBP: mean blood pressure; ICP: intracranial pressure; BPP: brain perfusion pressure; T0: preinfusion value; T2: value at 60min postinfusion.
The present study has shown that the infusion of HSL 0.5M is able to modify a series of biochemical variables, particularly acid–base balance and the blood ion profile. With regard to acid–base status, the development of immediate metabolic alkalosis was the characteristic alteration recorded. This may be explained on the basis of the physicochemical theory of Stewart, fundamented upon the principles of electron neutrality and mass conservation32. According to this theory, pH is regulated by three independent variables: the apparent strong ion difference (aSID), the concentration of total weak acids (Atot), and arterial blood PaCO231,32. Following the infusion of HSL 0.5M, the latter dissociates into its two main strong ions, namely sodium (cation) and lactate (anion). Lactate is quickly taken up by the cells for use as an energy substrate, while the sodium load remains within the extracellular compartment, determining an increase in aSID. This fact, together with the dilutional effect upon Atot observed in our study, determines a decrease in the dissociation of plasma water (primary proton source). This decrease in the concentration of hydrogen ions (H+) generates initial and transient metabolic alkalosis. The metabolic alkalosis observed secondary to the infusion of HSL 0.5M could have beneficial effects, especially upon myocardial contractility and, indirectly, upon systolic (stroke) volume and cardiac output. In this regard, Nalos et al.23 reported improved cardiac function in patients with heart failure and systolic dysfunction – a condition attributed at least in part to the effects of metabolic alkalosis upon the production of endogenous catecholamines23. However, such metabolic alkalosis could have physiologically negative effects as well, such as for example a left-shift of the hemoglobin dissociation curve – thereby altering oxygen release at tissue level.
On the other hand, the administration of HSL 0.5M generated hypernatremia and hypopotassemia, in concordance with the observations of previous studies20,21,33. The increase in natremia is secondary to the administered sodium load, which results in a significant increase in plasma osmolality. This finding is explained by the fact that sodium is the osmotic backbone of the extracellular compartment, and therefore the main determinant of plasma osmolality.
In our series, 19 patients with neural damage and intracranial hypertension received HSL 0.5M, with a significant decrease in ICP after 60min. It is important to underscore that the sample consisted of patients with neural damage of diverse origins. On the other hand, a small proportion (n=3) presented ICP <20mmHg before the administration of HSL. This therapeutic action may be regarded as controversial. Of note in our series is the finding that 5 of the 19 patients showed a decrease in MBP, and although a concomitant drop in ICP was also recorded, the latter was of lesser magnitude – thus determining an ultimate decrease in BPP. These changes – some of which are of large magnitude – clearly imply a loss of statistical significance of the relationship between the administration of HSL and the eventual increase in BPP. This fact could be related to a Cushing response deactivation phenomenon (hyperadrenergic response secondary to ICP levels >30 and 40mmHg), referred to by some authors as Cushing in reversa, and would be associated to rapid and substantial reductions in ICP. Our results, together with those published by Ichai et al.25, demonstrate the effectiveness of this crystalloid as an osmotic agent and establish the bases for the development of future larger scale clinical trials.
In the present study, lactate showed a two-phase behavior characterized by a significant increase 30min after infusion and a subsequent decrease to preinfusion levels after 60min. This behavior could be explained by the deviation of lactate toward the intracellular compartment, where it is used as an energy substrate. This was also observed in those patients with hyperlactatemia (>2mmol/l) prior to infusion. It is interesting to note that although lactatemia increased even more in this group, metabolic acidosis decreased. The phenomenon could be regarded as paradoxical under the classical acid–base metabolic conception. However, the exogenous administration of lactate in the form of sodium lactate, its rapid cellular uptake, and the consequent increase in aSID explain the metabolic alkalosis and afford support of the Stewart approach to acid–base metabolism.
With regard to the impact upon plasma chloride, the administration of HSL 0.5M produced hypochloremia in our study. Chloremia was initially elevated (reflecting previous replacements with high-chloride content solutions), but decreased slightly (almost 3%) after 30min and remained lowered after one hour, with a chloremia-reducing effect of the infusion. Similar results have been obtained in studies in which HSL was administered in the form of bolus doses25. However, when HSL was administered in prolonged infusions (24–48h), it appeared to exhibit a two-phase behavior with respect to plasma chloride concentration – with an initial decrease and subsequent gradual increase until reaching baseline levels. However, in the comparison with other fluids used in the mentioned studies (sodium chloride, Ringer lactate), the chloride levels were significantly lower23,26,33. This normalization of chloremia following sustained infusion practically without chloride supply supports the hypothesis of an endogenous chloride source conditioned by emergence of the anion from the intracellular space, seeking to maintain electron neutrality after lactate penetration into the cells34. Based on this important finding, an additional mechanism has been contemplated in which HSL acts as an osmotic agent in the control of ICP, as a consequence of increased plasma tonicity secondary to an increase in natremia and chloride transcellular efflux – generating a “drag effect” with a decrease in water and intracellular volume, and thus the control of ICP25,26.
The absence of development of hyperchloremia during the infusion of HSL could be clinically advantageous, considering that hyperchloremia has been found to be deleterious, particularly in neurocritical patients in which the use of hypertonic saline solution has become the cornerstone of the management of intracranial hypertension35. However, due to the high chloride content, hyperchloremia rapidly develops with repeated use. In this regard, a growing number of observational studies have evidenced the effect of hyperchloremia upon the neurocritical patient mortality rate, including the recent study published by Riha et al.36. In turn, Huang et al. have described the association between hyperchloremia and mortality risk after 30 days in patients with ischemic stroke and intracranial hemorrhage37. Likewise, Sadan et al. reported an association between the use of hypertonic saline solution and hyperchloremia and acute renal failure risk in patients with subarachnoid hemorrhage38.
The present pilot study analyzes a series of cases, and thus has limitations. It is a single-center study with a limited sample consisting of two heterogeneous groups of critical patients, and with no prolonged duration of follow-up that could contribute to improve understanding and definition of the pharmacokinetics HSL. Likewise, the long-term variations of chloremia and acid–base disorders were not evaluated. Lastly, there was no monitoring of patient hemodynamics or brain metabolism, which could have produced information about the effects of HSL 0.5M upon cardiac output and brain metabolism.
ConclusionsIn patients with neural damage and shock, the infusion of HSL 0.5M was associated to the development of metabolic alkalosis reflected by the increase in aSID and decrease in Atot. This effect was seen to be transient and was associated to the appearance of hypernatremia, hyperosmolarity and hypochloremia. The significant increase in plasma osmolality generated a decrease in ICP; the fluid therefore acted as an effective osmotic agent. However, this decrease was not associated to an increase in BPP. The lactate concentrations showed a two-phase behavior characterized by an initial increase and a subsequent decrease. Such behavior was also observed in those patients with hyperlactatemia prior to the infusion.
We consider that future well designed, randomized phase III clinical trials of sufficient statistical power are needed, involving the use of HSL 0.5M in different groups of critical patients, and that comparative evaluations versus hypertonic sodium chloride are of particular interest.
Financial supportThe present study received no financial support.
Authorship/collaborationDr. Ignacio Aramendi: study conception and design, data analysis and interpretation, drafting of the manuscript and final approval of the article.
Dr. Alejandra Stolovas: data acquisition, data analysis and interpretation, drafting of the manuscript and final approval of the article.
Dr. Sebastián Mendaña: data acquisition, data analysis and interpretation, drafting of the manuscript and final approval of the article.
Dr. Anna Barindelli: study conception and design, critical review of the intellectual content and final approval of the article.
Dr. William Manzanares: study conception and design, data analysis and interpretation, drafting of the manuscript and final approval of the article.
Dr. Alberto Biestro: study conception and design, data analysis and interpretation, drafting of the manuscript and final approval of the article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Aramendi I, Stolovas A, Mendaña S, Barindelli A, Manzanares W, Biestro A. Efecto de la infusión de lactato de sodio 0,5 molar sobre el medio interno de pacientes críticos. Med Intensiva. 2021;45:421–430.