To assess the effectiveness and identify predictors of failure of noninvasive ventilation.
DesignA retrospective, longitudinal descriptive study was made.
SettingAdult patients with acute respiratory failure.
PatientsA total of 410 consecutive patients with noninvasive ventilation treated in an Intensive Care Unit of a tertiary university hospital from 2006 to 2011.
ProceduresNoninvasive ventilation.
Main variables of interestDemographic variables and clinical and laboratory test parameters at the start and 2h after the start of noninvasive ventilation. Evolution during admission to the Unit and until hospital discharge.
ResultsThe failure rate was 50%, with an overall mortality rate of 33%. A total of 156 patients had hypoxemic respiratory failure, 87 postextubation respiratory failure, 78 exacerbation of chronic obstructive pulmonary disease, 61 hypercapnic respiratory failure without chronic obstructive pulmonary disease, and 28 had acute pulmonary edema. The failure rates were 74%, 54%, 27%, 31% and 21%, respectively. The etiology of respiratory failure, serum bilirubin at the start, APACHE II score, radiological findings, the need for sedation to tolerate noninvasive ventilation, changes in level of consciousness, PaO2/FIO2 ratio, respiratory rate and heart rate from the start and 2h after the start of noninvasive ventilation were independently associated to failure.
ConclusionsThe effectiveness of noninvasive ventilation varies according to the etiology of respiratory failure. Its use in hypoxemic respiratory failure and postextubation respiratory failure should be assessed individually. Predictors of failure could be useful to prevent delayed intubation.
Evaluar la efectividad e identificar predictores de fracaso de la ventilación mecánica no invasiva en la insuficiencia respiratoria aguda.
DiseñoEstudio retrospectivo, longitudinal y descriptivo.
ÁmbitoPacientes adultos con insuficiencia respiratoria aguda.
PacientesUn total de 410 pacientes (muestra consecutiva) tratados mediante ventilación mecánica no invasiva en una unidad de cuidados intensivos de un hospital universitario terciario entre 2006 y 2011.
IntervencionesVentilación mecánica no invasiva.
Variables principales de interésVariables demográficas, clínicas y analíticas desde el inicio de la ventilación mecánica no invasiva y 2h después. Variables evolutivas durante el ingreso en la unidad y hasta el alta hospitalaria.
ResultadosEl fracaso fue del 50%, y la mortalidad global del 33%. Un total de 156 pacientes presentaban insuficiencia respiratoria aguda hipoxémica, 87 insuficiencia respiratoria postextubación, 78 reagudización de enfermedad pulmonar obstructiva crónica, 61 insuficiencia respiratoria hipercápnica sin enfermedad pulmonar obstructiva crónica y 28 edema pulmonar agudo cardiogénico. El fracaso fue del 74, del 54, del 27, del 31 y del 21%, respectivamente. El tipo de insuficiencia respiratoria, la bilirrubina sérica al inicio, APACHE II, la existencia de hallazgos radiológicos, la necesidad de sedación para tolerarla y los cambios en el nivel de consciencia, ratio PaO2/FiO2, frecuencia respiratoria y frecuencia cardiaca entre el inicio y 2h después se asociaron con el fracaso.
ConclusionesLa efectividad de la técnica varió dependiendo del tipo de insuficiencia respiratoria. Su uso en la insuficiencia respiratoria aguda hipoxémica y la insuficiencia respiratoria postextubación debería valorarse individualmente. Los predictores de fracaso podrían ser útiles para prevenir el retraso en la intubación orotraqueal.
In recent years, noninvasive mechanical ventilation (NIMV) has become an alternative to orotracheal intubation and invasive mechanical ventilation for the management of respiratory failure (RF), since it can reduce the complications and shorten patient stay in the Intensive Care Unit (ICU). The first acute disorders treated with NIMV were exacerbations of chronic obstructive pulmonary disease (COPD)1 or acute lung edema (ALE).2,3 Since these first studies, the cumulative supporting evidence has been very consistent in reference to patients of this kind,4 and over the last 20 years the use of NIMV has been extended to patients with other kinds of diseases,5 such as hypoxemic RF or postextubation RF. The usefulness of NIMV in patients of this kind is much less certain, however. The latest clinical guides6 suggest that NIMV should be the first management option in patients with exacerbated COPD or with ALE, and that NIMV may be considered in patients with postoperative RF or in immune depressed individuals. In turn, the use of NIMV should also be considered for allowing the early extubation of patients with COPD.7 These guides offer no recommendations on the use of NIMV in patients with asthma exacerbations, acute lung injury (ALI), severe community-acquired pneumonia or thoracic trauma. Nevertheless, NIMV is often used in patients of this kind.
Considering the above, it is important to know the use of NIMV outside protocolized studies, in patients with different types of RF.
The present study has two main objectives:
- -
To know how NIMV is being used in clinical practice and its effectiveness.
- -
To identify early predictors of NIMV failure that can be used for improved indication of the technique in future patients.
A retrospective observational study was made of all the patients receiving treatment with NIMV in the ICU of a third-level university hospital between the years 2006 and 2011.
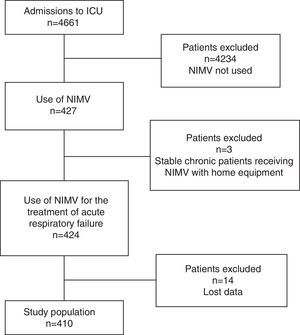
The inclusion criteria were: patients diagnosed with acute RF (ARF) of any origin and subjected to NIMV between the years 2006 and 2011. Stable chronic patients receiving NIMV as continuation of their home treatment were excluded (Fig. 1).
The hospital Ethics Committee approved publication of the results of the study.
Application of NIMV and definition of failureNoninvasive mechanical ventilation was applied using face masks with two types of respirators: BiPAP Vision (Respironics, Murrysville, PA, USA) and Carina (Dräger Medical, Lübeck, Germany).
Failure of NIMV was defined as the need for intubation and mechanical ventilation in the 72h following its suspension.
Regarding the causes of NIMV failure, labored breathing was defined as the persistence of tachypnea and use of the accessory muscles.
Data collectionThe patient case histories were consulted to compile demographic data, personal history, admission and discharge dates (referred to both the hospital and ICU), in-hospital mortality, duration of NIMV, NIMV parameters (inspiratory positive airway pressure [IPAP], expiratory positive airway pressure [EPAP] and fraction of inspired oxygen [FiO2]), the appearance of complications (NIMV associated pneumonia, hemodynamic alterations, pneumothorax), radiological findings, APACHE II score, and different physiological variables (systolic blood pressure, diastolic blood pressure, respiratory frequency, heart rate, Glasgow Coma Score [GCS] and temperature), and laboratory test parameters (pH, PaCO2, PaO2, bicarbonate, lactate, hematocrit, leukocytes, urea, creatinine, albumin, sodium, potassium and bilirubin). The physiological and blood gas parameters were recorded at the start and 2h after the start of NIMV, while the biochemical and hematological parameters were recorded only at the start.
Respiratory failure was classified into 5 groups8 based on the underlying etiology (as determined from the diagnoses, radiological and laboratory test findings, and personal history):
- -
Exacerbation of COPD.
- -
Acute hypercapnic RF or exacerbation of chronic hypercapnic RF without COPD (PaCO2>50mmHg).
- -
Hypoxemic ARF (PaO2/FiO2 ratio<300 and PaCO2<50mmHg).
- -
ALE in patients diagnosed with acute or chronic heart failure.
- -
Postextubation RF (defined as that manifesting in the 48h following extubation).
The patients with PaO2/FiO2 ratio<300 and PaCO2>50mmHg (except those included in the postextubation RF group, according to definition) were classified within the exacerbation of COPD group or acute hypercapnic RF group or exacerbation of chronic hypercapnic RF without COPD group, depending on whether COPD was diagnosed or not.
The chest X-ray findings at the start of NIMV were classified as follows:
- -
Clear parenchyma.
- -
Infiltration/consolidation/unilateral effusion.
- -
Infiltration/consolidation/bilateral effusion.
Quantitative variables were described as the mean and standard deviation, while qualitative variables were reported as percentages.
The Student t-test for independent and dependent data was used for the comparison of means between two groups. The Welch test was applied in the case of heteroscedasticity. Analysis of variance (ANOVA) was used in the case of more than two groups. The corresponding nonparametric versions of the abovementioned tests were used in the case of data exhibiting a non-normal distribution: the Mann–Whitney U-test for independent data and the Wilcoxon test in the case of paired data. The analysis of qualitative variables was based on the chi-squared test with contingency tables. The normality and the heteroscedasticity of the variables were contrasted with the Kolmogorov-Smirnov test and Levene test, respectively.
A logistic regression model was developed to explore the possible prognostic factors determining the presence or not of NIMV failure (response variable). We started with the variables considered to be important as predictors from the clinical perspective. A total of 281 patients presented full information referred to these variables. These subjects were randomized to two groups: a group of 250 patients for development of the model, and a group of 31 patients for validation of the model. Regarding the variables for which data were available corresponding to the start of ventilation and 2h after the start of ventilation, we decided to use the difference between them. Furthermore, in order to facilitate the analysis, the types of RF were reclassified into two groups: (a) patients with hypercapnic RF (exacerbations of COPD and hypercapnic RF without COPD), serving as reference variable; and (b) the remaining three types (hypoxemic ARF, ALE and postextubation RF). The variable chest X-ray was also recoded, pooling the patients with condensation/infiltration/effusion (unilateral or bilateral) to form two groups: clear X-ray (reference) versus the rest. For the selection of variables we used the procedure described by Collett9 to obtain the model that best explains and fits the NIMV failure response variable. The test used for the comparison of models was the likelihood ratio. Goodness of fit was assessed using the Nagelkerke R2 and tables of correctly classified values. The sensitivity and specificity of the model were calculated, with determination of the positive and negative predictive values from the validation sample.
Statistical significance was considered for p<0.05. The SPSS version 21 statistical package (IBM Corp. Armonk, NY, USA) was used throughout.
ResultsA total of 410 cases of NIMV were recorded, with a mean patient age of 66.7±13.4 years and a mean APACHE II score of 20.8±7.4. Males predominated (60.2%).
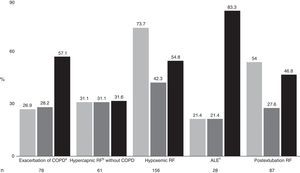
The total percentage failure rate was 50.7%, with a global mortality rate of 33.4%. Fig. 2 shows the distribution according to etiology, together with percentage failure, mortality, and mortality among the patients in which NIMV failed. The most frequent indication of the technique was acute hypoxemic RF (38%), followed at a distance by postextubation RF (21.2%) and the exacerbation of COPD (19%). The least numerous groups were hypercapnic RF without COPD (14.9%) and ALE (6.8%). On analyzing failure according to the type of RF involved, the poorest results corresponded to acute hypoxemic RF (73.7%), while the best results corresponded to ALE (21.4%), the exacerbation of COPD (26.9%) and hypercapnic RF without COPD (31.1%). The percentage failure rate in postextubation RF was 54%. The highest mortality corresponded to patients with hypoxemic RF. In the patients with NIMV failure, the mortality rate was very high (83.3%) among the individuals with ALE, though the small number of patients in this group means that the data must be viewed with caution. In contrast, the lowest mortality in patients with NIMV failure corresponded to the group of individuals with hypercapnic RF without COPD (31.6%).
The patients in which NIMV proved successful presented lesser mortality (14.4 vs 51.9%, p<0.001), with a shorter stay in the ICU (4.7±5.0 vs 16.3±19.2 days, p<0.01) and in hospital (20.8±17 vs 31.7±26.7 days, p<0.01) compared with the patients in which NIMV failed.
Regarding the differences in stay according to the type of RF, we observed significant differences in stay in the ICU among the patients with exacerbation of COPD (2.7±1.6 vs 9.9±16.7 days, p<0.005), hypercapnic RF without COPD (4.1±5.6 vs 14.4±12.4 days, p<0.001), acute hypoxemic RF (6±7.4 vs 16.5±21.4 days, p<0.001) and postextubation RF (7.6±4 vs 20.8±16.7 days, p<0.001)—the difference in the ALE group being nonsignificant (3.4±2.6 vs 5.2±3.4 days, p=0.13). Regarding the stay in hospital, we observed significant differences in the postextubation RF group (23.9±11.7 vs 37.1±25 days, p<0.05)—the rest of the differences being nonsignificant: exacerbation of COPD (14.5±8.6 vs 18.2±19.2 days, p=0.94); hypercapnic RF without COPD (19.9±12.9 vs 25.9±17.8 days, p=0.11); acute hypoxemic RF (29±28.9 vs 33.6±29.1 days, p=0.19) and ALE (18.1±11.7 vs 20.3±24.1 days, p=0.53).
We also observed differences in mortality in all the groups except in the hypercapnic RF without COPD group (31% vs 31.6%, p=0.96): exacerbation of COPD (17.5% vs 57.1%, p<0.001); acute hypoxemic RF (7.3% vs 54.8%, p<0.005); ALE (4.5% vs 83.3% p<0.005); postextubation RF (5% vs 46.8% p<0.005).
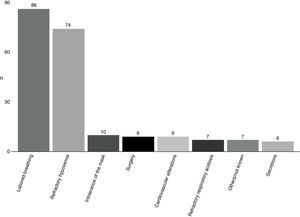
The most frequent cause of NIMV failure (Fig. 3) was excessively labored breathing (41.3%), followed by refractory hypoxemia (35.6%).
There were few complications associated to the technique. In effect, only 18 hemodynamic alterations (4.4%) and a single case of NIMV-associated pneumonia (0.2%) were documented.
Table 1 shows the differences in general characteristics of the patients in which NIMV proved successful and those in which NIMV failed. There were significant differences in the APACHE II score, the use of NIMV before admission to the ICU, the use of inotropic/vasopressor support, home oxygen therapy, the need for sedation in order to tolerate NIMV, and the presence of some type of immunosuppression (according to the definition of the APACHE II scale).
General characteristics of the patients according to the result of NIMV.
| NIMV success (n=202) | NIMV failure (n=208) | p | |
|---|---|---|---|
| Age (years) | 68.3±11.9 | 65.1±14.5 | 0.066 |
| APACHE II score | 19.3±6.7 | 22.3±7.9 | <0.001 |
| Male gender | 119 (58.9%) | 128 (61.5%) | 0.587 |
| Vasoactive/inotropic support | 49 (24.3%) | 72 (34.6%) | <0.05 |
| Pre-ICU NIMV | 43 (21.3%) | 24 (11.5%) | 0.008 |
| Home O2 | 34 (16.8%) | 18 (8.7%) | 0.013 |
| Immunosuppression | 18 (8.9%) | 53 (25.5%) | 0.000 |
| Previous liver disease | 9 (4.5%) | 7 (3.4%) | 0.587 |
| Need for sedation | 15 (7.4%) | 45 (21.6%) | 0.000 |
APACHE II, Acute Physiology and Chronic Health Evaluation II scale; ICU, Intensive Care Unit; NIMV, noninvasive mechanical ventilation.
Table 2 in turn shows the clinical and laboratory test findings at the start and 2h after the start of NIMV. At the start, the patients in which NIMV failed showed lower values corresponding to pH, respiratory frequency, GCS, serum lactate and serum bilirubin, and greater PaCO2, PaO2/FiO2, bicarbonate and systolic blood pressure values.
Clinical and laboratory test findings (expressed as mean±standard deviation) before the start and 2h after the start of NIMV, according to the result obtained.
| Before the start of NIMV | 2h after the start of NIMV | |||||
|---|---|---|---|---|---|---|
| Success | Failure | p | Success | Failure | p | |
| pH | 7.29±0.13 | 7.34±0.12 | <0.001 | 7.35±0.10 | 7.36±0.11 | 0.73 |
| PaCO2 (mmHg) | 61.9±28.7 | 45.6±21 | <0.001 | 51.9±20.4 | 44.1±17.8 | <0.001 |
| PaO2/FiO2 ratio | 148.6±62.1 | 118.5±56.4 | <0.001 | 194.5±76.3 | 145.1±65.6 | <0.001 |
| Bicarbonate (mEq/l) | 28.4±10 | 23.6±7.8 | <0.001 | 28.7±9.8 | 24.2±7.8 | <0.001 |
| SBP (mmHg) | 138.3±30.9 | 129±30.3 | 0.002 | 129.8±19.1 | 125.7±24.7 | 0.07 |
| HR (bpm) | 104.5±22.4 | 106.4±22.3 | 0.39 | 92.6±17.6 | 101.4±19.9 | <0.001 |
| RFr (rpm) | 28.2±7.9 | 31.4±8.4 | <0.001 | 23.3±5.1 | 29±8 | <0.001 |
| IPAP (cmH2O) | 16.3±4.2 | 15.5±3.4 | 0.06 | |||
| EPAP (cmH2O) | 6.6±1.8 | 6.9±1.7 | 0.11 | |||
| GCS | 13.8±2.1 | 14.3±1.7 | 0.007 | 14.7±0.8 | 14.5±1.2 | 0.21 |
| Hematocrit (%) | 36.0±8.5 | 32.1±7.1 | <0.001 | |||
| Creatinine (mg/dl) | 1.5±1.4 | 1.5±1.2 | 0.88 | |||
| Albumin (g/dl) | 3.1±0.8 | 2.5±0.7 | <0.001 | |||
| Lactate (mmol/l) | 1.9±2.2 | 2.2±1.9 | 0.16 | 1.6±1.8 | 1.9±1.6 | 0.08 |
| Bilirubin (mg/dl) | 0.7±0.6 | 1.5±2.4 | <0.001 | |||
EPAP, expiratory positive airway pressure; HR, heart rate; RFr, respiratory frequency; GCS, Glasgow Coma Scale; IPAP, inspiratory positive airway pressure; SBP, systolic blood pressure; NIMV, noninvasive mechanical ventilation.
Table 3 shows the variables affording the best fit in the logistic regression model. The goodness of fit of the model was 0.48. The percentage of correct classifications was 77%, with a sensitivity of 70% and a specificity of 84%. With the validation sample, the correct classifications rate was 74%, with a positive predictive value of 66% and a negative predictive value of 72%.
Results of the logistic regression analysis identifying risk factors for NIMV failure.
| p | OR | 95%CI OR | |
|---|---|---|---|
| APACHE II score | 0.001 | 1.09 | (1.03–1.14) |
| Etiology of the RF | 0.017 | 2.66 | (1.19–5.91) |
| Need for sedation | 0.001 | 7.13 | (2.26–22.54) |
| Serum bilirubin | 0.007 | 2.16 | (1.24–3.78) |
| Change in GCS score | 0.016 | 1.51 | (1.08–2.12) |
| Change in PaO2/FiO2 ratio start-2h | 0.028 | 1.01 | (1.001–1.02) |
| Change in heart rate start-2h | 0.077 | 0.98 | (0.96–1.01) |
| Change in respiratory frequency start-2h | 0.025 | 0.94 | (0.89–0.99) |
| Existence of radiological findings | 0.072 | 2.06 | (0.94–4.52) |
| Constant | 0 | 0.04 |
APACHE II, Acute Physiology and Chronic Health Evaluation II scale; GCS, Glasgow Coma Scale; CI, confidence interval; RF, respiratory failure; OR, odds-ratio calculated by the coefficient of the model (eβˆ); NIMV, noninvasive mechanical ventilation.
Although the change in heart rate and the existence of radiological findings were not significant, they were retained in the model due to their clinical importance and because their presence in the construct afforded better correct classification rates. The need for sedation, the type of RF (hypercapnic versus non-hypercapnic), serum bilirubin at the start, and the existence of radiological findings were the risk factors with the greatest magnitude of effect, while the change in heart rate and respiratory frequency were found to be protective factors, though with a small magnitude of effect. It is necessary to clarify the interpretation of the variables defining the change from the start to 2h after the start: in the case of the change in GCS in patients who are improving, negative values are obtained (as a result of change from a lower to a higher GCS). A more negative result reflects better evolution than a less negative result; consequently, an increase in this scale (i.e., a less negative result) identifies lesser improvement and therefore points to a poorer prognosis.
DiscussionThe NIMV failure rate in our series was 50.7%. On examining the results of previous studies,10,11 the failure rate was found to be between 24% and 58%—the latter being the rate observed in a randomized study involving different types of RF.12 There is also evidence consistent with our own results in favor of a decrease in stay both in the ICU13,14 and in hospital14 among those patients in which NIMV proves successful.
However, the broad range in percentage failure observed in the literature is notorious. The fundamental reason for such variability is probably the different proportions of types of RF in the different studies. It therefore might be more useful to compare results according to type of RF.
In this regard, the success rate was high in exacerbations of COPD, in ALE and in hypercapnic RF without COPD, ranging between 70 and 78%. In the first two groups, the scientific evidence is overwhelmingly in favor of the use of NIMV.1,15,16 In these patients, our results are similar to those of previous studies as regards the success rates15,16 and decreases in mortality and stay in the UCI16 when NIMV proves successful.
Likewise, in the patients with ALE the observed failure rate was 21.4%, which is similar to the data published elsewhere,17 and lesser mortality was also recorded in the patients in which the technique did not fail. Despite these observations, however, the small number of patients involved in this group means that the data must be viewed with caution.
Regarding the last group, i.e., hypercapnic RF without COPD, the existing evidence is much less clear, with few studies and moreover involving patients with highly diverse disease conditions. The reported success rate in this case ranges from 89%18 to 38%.11 In our study, and in contrast to the previous evidence, we recorded no increased mortality or greater NIMV failure among the patients without COPD versus those with COPD.
In our experience, NIMV can be used with a high probability of success in these three types of patients (exacerbation of COPD, ALE and hypercapnic RF without COPD).
In contrast, the failure rate in patients with hypoxemic ARF was very high (73.7%). In this type of disease there is much less available evidence in support of the use of the technique. The great variability of causes of hypoxemic ARF complicates the comparison of results, with failure rates of between 12 and 60%.11,19,20
In our study, the patients in which NIMV proved successful presented lesser mortality and a shorter stay in the ICU. In the literature, although there are studies reporting a decrease in the number of serious complications, a lesser need for mechanical ventilation, and a shorter stay in the ICU,19–21 the metaanalysis conducted by Keenan et al.22 concluded that the great variability of results in the different studies does not allow recommendation of the generalized use of NIMV in patients with hypoxemic ARF.
The data of our study should cause reconsideration of the use of NIMV in patients of this kind.
In the postextubation RF subgroup, success was only moderate, with failure being observed in 54% of the cases. While the use of NIMV in the prevention of postextubation RF during the weaning process is warranted by the scientific evidence,23 its effectiveness in postextubation RF is much more controversial. In the same way as in hypoxemic ARF, the great variety of causes of RF and the diversity of disease conditions for which the patient required orotracheal intubation in the first place all complicate the evaluation of NIMV in these patients. Two randomized studies24,25 found no differences in reintubation, and one of them24 demonstrated greater mortality in the NIMV group.
The complications of NIMV were found to be infrequent (one case of NIMV-associated pneumonia and 18 hemodynamic alterations), though the retrospective nature of the study made it impossible to adequately evidence the minor complications such as the appearance of skin lesions secondary to application of the interface. There were 6 cases of cardiorespiratory arrest (1.5%)—all in asystole or electrical activity without a pulse. The incidence of cardiorespiratory arrest in earlier studies varied between 0%15 and 7%.12 We identified no myocardial ischemia episode (defined as the presence of acute changes in the ECG tracing and/or the elevation of cardiac enzymes), in contrast to the study of Mehta et al.,17 who found an increased incidence of myocardial infarction in the group subjected to bilevel positive airway pressure (BPAP) ventilation. The 6 cases of cardiorespiratory arrest occurred in patients with multiple previous disease conditions justifying conservative management (in fact, three of them were patients with instructions against intubation).
The results of the logistic regression analysis are concordant with the previous evidence which identified the following predictors of failure: a diagnosis of pneumonia,12 high APACHE II score,12 respiratory frequency >35rpm,12 high SAPS II score,8,11,26 poor tolerance of NIMV,26 high IPAP,27 low pH,27,28 GCS before NIMV,11 PaO2/FiO2 before NIMV11 or PaO2/FiO2 after 1h of NIMV.28 The exacerbation of COPD has been identified as a predictor of success.29
In general, our data suggest that the more seriously ill patients, as well as those with a poorer initial response to NIMV, are more likely to suffer NIMV failure.
The identification of serum bilirubin before the start of NIMV as a predictor of failure of the technique has not been reported elsewhere, and could help to identify patients with multiorgan dysfunction (since there were no significant differences regarding the existence of previous liver disease between the NIMV success and failure groups). Previous evidence30 showed that patients with greater organ failure (as measured by the SOFA score) had poorer results.
The main limitation of our study is the fact that it is not a randomized controlled trial but a retrospective study. On the other hand, its single-center nature means that the results might not be extrapolatable. Furthermore, its long duration (6 years) might imply the existence of changes in technology and in the management of patients that must be taken into account in interpreting the data obtained.
Since one of the key factors in deciding the use of NIMV is the probability of failure, which can worsen the prognosis of patients with RF, our results could contribute to the decision making process, since we have identified several predictors of failure that might be useful. With these predictors it may be possible to decide not to start NIMV, or to suspend it early, if the probability of failure is seen to be high.
Noninvasive mechanical ventilation is useful for managing patients with RF, and can avoid the need for orotracheal intubation in approximately one-half of the cases. Its effectiveness is greater in patients with exacerbation of COPD, with ALE or with hypercapnic RF without COPD. The use of NIMV in patients with hypoxemic RF or postextubation RF should be assessed on an individualized basis.
Financial supportThere has been no financial support for the conduction of this study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martín-González F, González-Robledo J, Sánchez-Hernández F, Moreno-García MN, Barreda-Mellado I. Efectividad y predictores de fracaso de la ventilación mecánica no invasiva en la insuficiencia respiratoria aguda. Med Intensiva. 2016;40:9–17.
Study carried out in the Intensive Care Unit of Salamanca University Hospital.