The availability of continuous plasma filtration adsorption (CPFA) to treat patients with septic shock created a lot of expectations at
the time. However, after several randomized clinical trials, there is tremendous uncertainty on the efficacy and safety profile of CPFA in these patients. The objective of this letter is to review the information available on this issue and establish evidence-based recommendations regarding its clinical application.
Therefore, we started by analyzing the systematic review conducted by Poole and D’Arrigo1 updated until October 2021. Only randomized clinical studies of patients with septic shock and short/mid-term mortality data were included (primary clinical endpoint). The synthesis of evidence was conducted using a conventional meta-analysis (random effects model) and sequential analysis (Trial Sequential Analysis [TSA]).2 The risk of study bias was assessed using the Cochrane risk-of-bias tool for randomized trials (RoB2).3 The quality of the body of evidence and the formulation of recommendations was performed using GRADE approach.4 Details on the methodology, results, and references can be found in the Electronic Supplementary Data (ESD).
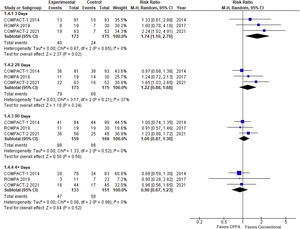
The meta-analysis included 3 randomized clinical trials5–7 with a total of 341 patients diagnosed as septic shock. The 3 trials were stopped before they could ever reach the predetermined sample size on grounds of futility,5 increased early mortality in the experimental branch6 or indirectly through the COMPACT-2 results.7
In the conventional meta-analysis (Table 1, Fig. 1) a non-significant trend can be seen towards an increased mortality rate in the experimental arm at 28 and 90 days followed by significant excess mortality at 3 days. The information available on non-critical outcomes (like shock duration, ICU stay, hemodynamic instability) was scarce and did not allow an overall synthesis.
Results of meta-analysis.
| RR (95%CI) | I2 | D2 | |||
|---|---|---|---|---|---|
| Fixed effects | DerSimonian | TSA (DS) | |||
| 28-day mortality rate* | 1.19 (0.93−1.53) | 1.22 (0.88−1.68) | 1.22 (0.81−1.82) | 0.37 (0−0.69) | 0.41 |
| 90-day mortality rate | 1.06 (0.86−1.30) | 1.06 (0.87−1.30) | 1.06 (0.84−1.35) | 0 (0−0.60) | 0 |
| 3-day mortality rate | 1.75 (1.10−2.77) | 1.74 (1.10−2.76) | 1.74 (0.84−3.62) | 0 (0−0.60) | 0 |
Abbreviations: 95%CI, 95% confidence interval; DS, DerSimonian-Laird; RR, relative risk; TSA, Trial Sequential Analysis.
The level of certainty of evidence available was considered moderate due to inaccuracy,5 which did not allow us to discard a small protective effect. Sequential analysis (TSA) allowed us to discard, however, a significant reduction (>25%) of the risk of death at 28 days.
The increased mortality rate reported within the first 3 days deserves some reflection. In the first place, this estimate refers to a clinical outcome based on data (not predefined) and, therefore, is hindered by a high risk of bias. Also, at 90 days, excess mortality dropped nominally down to 6% (RR, 1.06; 95%CI between 0.87 and 1.30; non-significant P value). Excess early mortality did not reach statistical significance in the sequential analysis (95% confidence interval between 0.84 and 3.62), which is suggestive that it was a random maximum.
Despite this loss of statistical significance, the possible increased early mortality rate reported is worrying and deserves careful attention. The hypothetical early deaths described could be associated with the fact that extracorporeal blood purification therapies are being received. The pathophysiological nexus here could be the hemodynamic instability associated with the application of the technique,8 which has demonstrated to increase mortality in the critically ill patient setting.9 We should mention that, in the protocol of the 3 clinical trials, the early administration of this technique was being sought after (not beyond the first 12 h after diagnosis), and that the early application of renal replacement techniques (RRT) in critically ill patients is associated with more hemodynamic complications and no survival benefits.10 As a matter of fact, investigators from the COMPACT-2 trial suggested that patients who do not need RRT to treat acute kidney injury (AKI) seem to be more susceptible to the deleterious effects of this technique.6
In conclusion, although statistically and formally a small protective effect cannot be ruled out, the 3 trials available suggest that CPFA is associated with excess mortality in the mid-term (moderate level of certainty) without evidence of improvement in other clinical outcomes. Also, if we take into consideration the cost associated with the intervention (some US$ 3000 per patient plus the work overload of the healthcare personnel), we will see that the risk-benefit ratio is truly unbalanced towards risk. Therefore, a strong recommendation should be made against the generalized use of CPFA in patients with septic shock.
Although the risk-benefit ratio is clearly unfavorable for the entire group of patients with septic shock, the existence of certain subgroups who may benefit from treatment cannot be discarded like patients who need RRT due to AKI. Conducting a meta-analysis of individual data could be a useful tool in the identification process of these different subgroups.