To evaluate the efficacy of the Start to move protocol compared to conventional treatment in subjects over 15 years of age hospitalized in the ICU on an improvement in functionality, decrease in ICU-acquired weakness (DAUCI), incidence of delirium, days of mechanical ventilation (MV), length of stay in ICU and mortality at 28 days.
Designrandomized controlled clinical trial.
SettingIntensive Care Unit.
ParticipantsIncludes adults older than 15 years with invasive mechanical ventilation more than 48h, randomized allocation.
InterventionsStart to move protocol and conventional treatment.
Main variables of interestFunctionality, incidence of ICU-acquired weakness, incidence of delirium, days on mechanical ventilation, ICU stay and mortality-28 days, ClinicalTrials.gov number, NCT05053724.
Results69 subjects were admitted to the study, 33 to the Start to move group and 36 to conventional treatment, clinically and sociodemographic comparable. In the “Start to move” group, the incidence of IUCD at ICU discharge was 35.7% vs. 80.7% in the “conventional treatment” group (p=0.001). Functionality (FSS-ICU) at ICU discharge corresponds to 26 vs. 17 points in favor of the “Start to move” group (p=0.001). The difference in Barthel at ICU discharge was 20% in favor of the “Start to move” group (p=0.006). There were no significant differences in the incidence of delirium, days of mechanical ventilation, ICU stay and 28-day mortality. The study did not report adverse events or protocol suspension.
ConclusionsThe application of the “Start to move” protocol in ICU showed a reduction in the incidence of IUAD, an increase in functionality and a smaller decrease in Barthel score at discharge.
Evaluar la eficacia del protocoloStart to move comparado con tratamiento convencional en sujetos mayores de 15 años hospitalizados en UCI sobre una mejoría en funcionalidad, disminución de debilidad adquirida en UCI (DAUCI), incidencia de delirio, días de ventilación mecánica (VM), estadía en UCI y mortalidad a los 28 días.
DiseñoEnsayo clínico controlado aleatorizado.
ÁmbitoUnidad de paciente crítico.
ParticipantesIncluye adultos mayores a 15 años con VMI mayor a 48 horas, asignación aleatoria.
IntervencionesProtocolo “Start to move” y tratamiento convencional.
Variables de interés principalesSe analizó funcionalidad, incidencia DAUCI, incidencia delirio, días VM, estadía UCI y mortalidad-28 días, ClinicalTrials.gov número, NCT05053724.
Resultados69 sujetos fueron ingresados al estudio, 33 al grupoStart to move y 36 a tratamiento convencional, comparables clínico y sociodemograficamente. En grupo Start to move la incidencia DAUCI al egreso de UCI fue de 35,7% vs 80,7% grupo tratamiento convencional (p=0,001). La funcionalidad (FSS-ICU) al egreso UCI corresponde a 26 vs 17 puntos a favor del grupo Start to move (p=0,001). La diferencia en Barthel al egreso UCI fue de 20% a favor del grupo Start to move (p=0,006). No hubo diferencias significativas en incidencia de delirio, días de VM, estadía UCI y mortalidad-28 días. El estudio no reportó eventos adversos ni suspensión de protocolo.
ConclusionesLa aplicación del protocol Start to move en UCI se asoció reducción en la incidencia DAUCI, aumento en funcionalidad y menor caída en puntaje Barthel al egreso.
Patients admitted to the Intensive Care Unit (ICU) are exposed to prolonged periods in bed and to different factors that directly or indirectly affect the muscle and organ structures, which may result in ICU-acquired weakness (ICU-AW)1,2 and functional limitation.3
The aforementioned factors are of a metabolic, pharmacological and organic nature, including particularly sustained hyperglycemia, corticosteroid use, sedoanalgesia, neuromuscular block and multiorgan failure associated with sepsis or septic shock.4 This situation in turn results in direct loss of muscle mass (specifically type II fibers), which is explained by increased myosin protein degradation, decreased protein synthesis and increased proinflammatory cell activity, which favor weakness in the critically ill patient.4
According to Brower et al.,5 the effects of prolonged resting periods include muscle atrophy and deconditioning. After 14 days of immobilization, young individuals and adults suffer a quadriceps muscle loss of 9%, with a resulting loss of muscle strength of up to 27%.6,7 In individuals subjected to invasive mechanical ventilation (IMV), it has been seen that the cross-sectional area of the quadriceps muscle can decrease by up to 12.5% in the first week of ICU stay. This figure, in turn, can reach 15.7% in the presence of multiorgan failure, versus 3% in the presence of single-organ failure.8
Both ICU-AW and functional loss are also directly related to prolonged sedoanalgesia, neuromuscular block and an increased incidence of delirium in the ICU.9 The presence of delirium is associated with low participation in physical therapy due to poor cooperation and/or psychomotor agitation; as a result, it directly impacts upon muscle condition and posterior functional recovery.9
Brummel et al.10 reported that delirium is common in the ICU, affecting 60–80% of all patients subjected to IMV, and 20–50% of those subjected to noninvasive mechanical ventilation (NIMV). It increases the risk of tearing out invasive devices, accidental extubation, and the need for physical restraints that can cause delays in the start of functional recovery.27,28
In order to assess the consequences of prolonged resting periods and quantify ICU-AW, use is made of the Medical Research Council (MRC) score. This validated tool bilaterally measures the strength of 6 muscle groups with a score of 30 points per side of the body, yielding a total score of 60 points. A score of ≤ 48 points indicates the presence of ICU-AW.11,29
On the other hand, in order to assess functionality in the critically ill, use is commonly made of the validated Functional Status Scale – Intensive Care Unit (FSS-ICU), which scores functional performance from 0 to 35 points.12
The present study was carried out to compare the efficacy of the “Start to move” protocol versus conventional treatment in subjects over 15 years of age admitted to the ICU in terms of improvement of functionality, decreased ICU-AW, incidence of delirium, days of mechanical ventilation (MV), length of ICU stay, and mortality at 28 days.
Material and methodsMethodological designA single-blind, randomized controlled clinical trial was carried out. Patient randomization to the intervention was carried out using sealed envelopes (proportion 1:1) with a non-probabilistic consecutive sampling of patients admitted to the ICU (Clínica Ensenada, Santiago de Chile, Chile) between January 2018 and July 2019 and who met the study inclusion criteria. The study is registered with ClinicalTrials.gov (number NCT05053724) and protocol No. 010/2018 (Servicio de Salud Metropolitano Occidente).
ParticipantsWe recruited all medium complexity cases admitted to the ICU, stabilized in another emergency service and transferred to Clínica Ensenada, since the latter has no emergency service. The patients were required to be over 15 years of age, with a need for IMV for more than 48h, and with informed consent to participation in the study signed by the legal representative. It should be noted that medium complexity cases with an APACHE II score of close to 20 points are admitted to Clínica Ensenada, since prior patient stabilization is required for transfer to the clinic.
We excluded patients with neuromuscular disease, a history of psychiatric disorders (suicide attempts, schizophrenia, senile dementia, etc.) capable of biasing functional treatment and assessment, patients with limb amputations, pregnant women, subjects presenting cardiac arrest with severe cerebral hypoxia-ischemia, and patients with total dependency before hospitalization, as reflected by a Barthel Index of < 20 points.
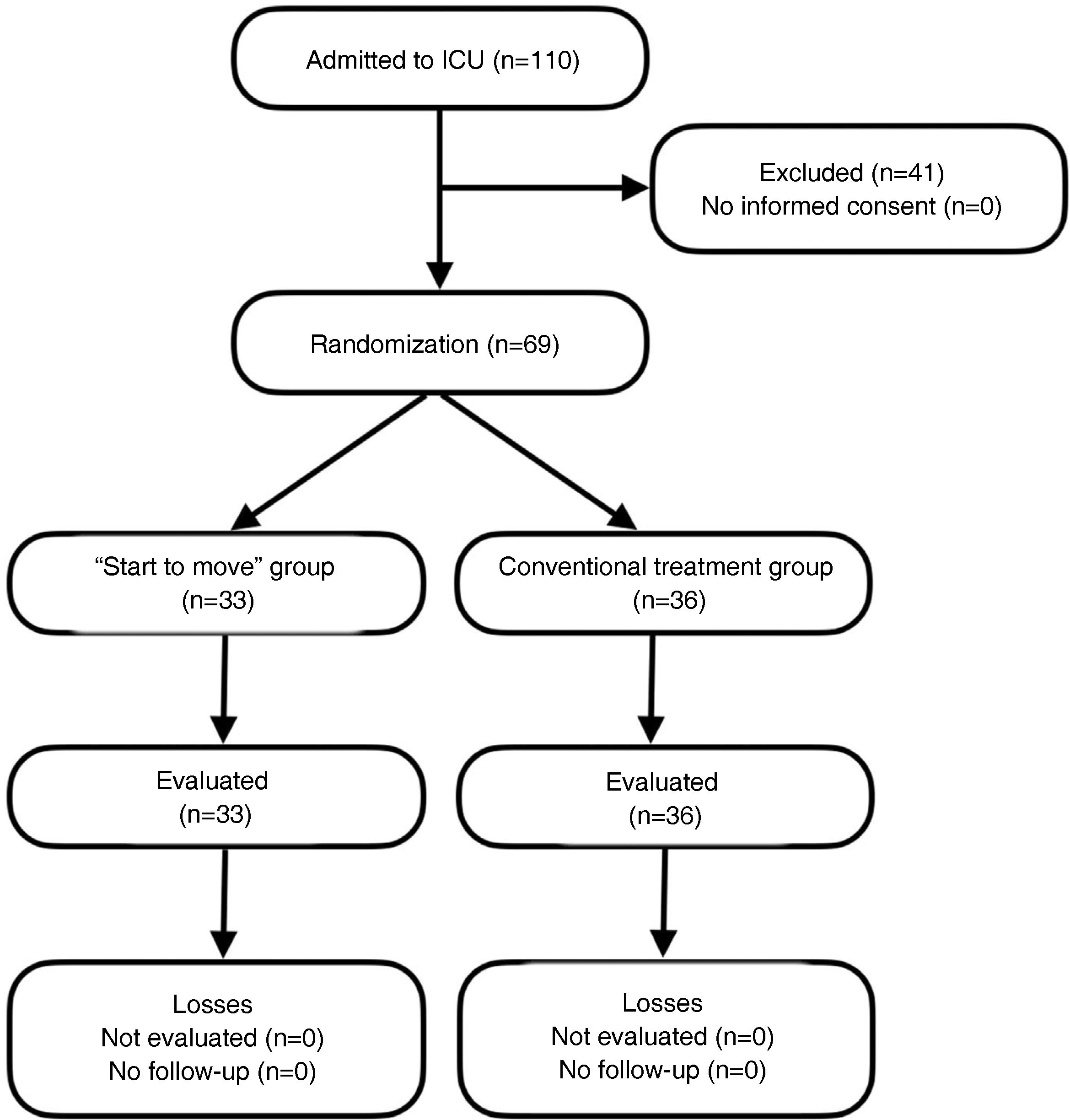
Recruitment and randomizationThe study was carried out in a single center with 12 ICU beds during the period 2018−2019. In those patients that met the inclusion criteria, informed consent was obtained from the legal representative, followed by 1:1 randomization to the “Start to move” group or to conventional treatment. Randomization was carried out using sequentially numbered sealed envelopes only accessible to research members not participating in the clinical trial (Fig. 1).
ProcedureIn both intervention groups the kinesiologist evaluated the patient's waking status and cooperation with the De Jonghe scale,3,13 in which the subject is required to follow at least three of 5 instructions proposed by the scale. In addition, psychomotor agitation was scored using the Riker sedation-agitation scale (between 3–5 points), assigning the patient to one of the 6 kinesic therapy levels in both the “Start to move” group according to Gosselink3 and in the conventional treatment group (Annexes 1 and 2).
In both groups, the intervention started within the first 48h of ICU stay and continued until discharge from the Unit. The conventional treatment group included passive and active-assisted mobilization, together with resistance exercises, and the facilitation of functional positions (sitting, standing and walking) according to the conventional treatment protocol. The total duration of therapy was 45min per session (Annex 1).
The “Start to move” group included kinesic therapy according to the protocol of Gosselink,3 which establishes 6 levels of attention divided according to systems stability, cognitive status associated with sedation, muscle weakness and the proposed functional objectives. At level 0 kinesic therapy is not applied, due to systemic lability. At levels 1–5 progression is made from passive mobilization, the use of electrical muscle stimulation (EMS), active mobilization and resistance exercises and the application of conventional cycle ergometry, to assisted walking if possible for the patient. The total duration of therapy was 45min per session (Annex 2).
Neuromuscular stimulation (EMS) was performed using four surface electrodes positioned bilaterally on the quadriceps, and four electrodes on the tibialis anterior, as muscles associated with walking function.26 Muscle stimulation was applied at an intensity sufficient to generate visible or palpable muscle contractions. The electrostimulation device was set to an intensity of up to 140mA, with a pulse duration of 300−400μs (rise time 0.8–2s, start 2–15s, pause 0.7–1s, and stop 4–10s,)26 a frequency of 30−50Hz, with a duration of 60min per day.14,15 Cycle ergometry in turn was set to a rhythm of 30–45 rpm.15
Once the subject awakened with a standardized 5 questions (S5Q) score of 3 out of 5 points13, we applied the scales referred to the evaluation of muscle strength (Medical Research Council [MRC]),11 functionality (FSS-ICU),12 delirium (Confusion Assessment Method for the Intensive Care Unit [CAM-ICU])9 and functionality before hospital admission (Barthel Index). Those patients unable to awaken during the follow-up period were excluded from the study.
Initial sample assessment: We recorded and analyzed sociodemographic parameters such as patient age and gender, reason for admission, severity of disease (APACHE II score), comorbidities (Charlson Index)16 and body mass index (BMI). Clinical characteristics were also recorded, including multiorgan failure (MOF), corticosteroid use, the administration of vasoactive drugs, neuromuscular blockers, sedoanalgesia, hyperglycemia requiring insulin, and acute and chronic renal replacement therapy. Further clinical data such as the arterial partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) ratio and oxygenation index, extubation failure and need for tracheostomy were likewise documented. With regard to the primary outcomes, we analyzed functionality using the Barthel Index, FSS-ICU, ICU-AW based on the MRC scale, and delirium using the CAM-ICU score. The secondary outcomes in turn comprised days of IMV, days of ICU stay, and mortality at 28 days.
Safety protocols for suspending treatment were established in both groups (Annex 3).
Sample: The sample size was calculated based on functional independence at hospital discharge (59% in the treatment group versus 35% in the control group; p=0.02).17 The effect size was 24% in favor of the intervention group versus the control group. For an alpha error of 5% and a statistical power of 80%, a total of 67 subjects per group were needed (total n=134). However, the present study was discontinued early due to internal reasons of the center (staff replacements); post hoc calculation of power with an alpha error of 5% was therefore carried out, yielding 95.8% for the variable ICU-AW and 90.5% for the variable FSS-ICU at discharge from the Unit compared with awakening.
Statistical analysisA descriptive analysis was made of the baseline characteristics of the subjects according to the intervention group. Normal data distribution was assessed using the Shapiro-Wilks test (p<0.05). The comparison of groups was carried out with the Fisher exact test, Mann-Whitney U test, Wilcoxon test or Student t-test, as applicable.
Functionality was evaluated based on the variation in FSS-ICU score, using crude and adjusted ordinal regression analysis, in the same way as the days of MV and the Barthel Index. In turn, ICU-AW, delirium and mortality at 28 days were assessed based on crude and adjusted logistic regression analysis. An internal analysis of safety variables was performed, in which each reported event implied a review of the causes to suspend the study protocol.
Statistical significance was considered for p<0.05 in two-tailed analyses, and the regression models reported the odds ratio (OR) with the corresponding 95% confidence interval (95%CI) for the ordinal and logistic models.
Analysis was performed on an intention-to-treat (ITT) basis. The data were entered using Epidata, and statistical processing was made using the Stata 14.2 SE package (StataCorp LLC, College Station, TX, USA.).
Ethical particularsThe present study, the informed consent and the protocol were approved by the Ethics Committee of Servicio de Salud Metropolitano Occidente (No. 010/2018).
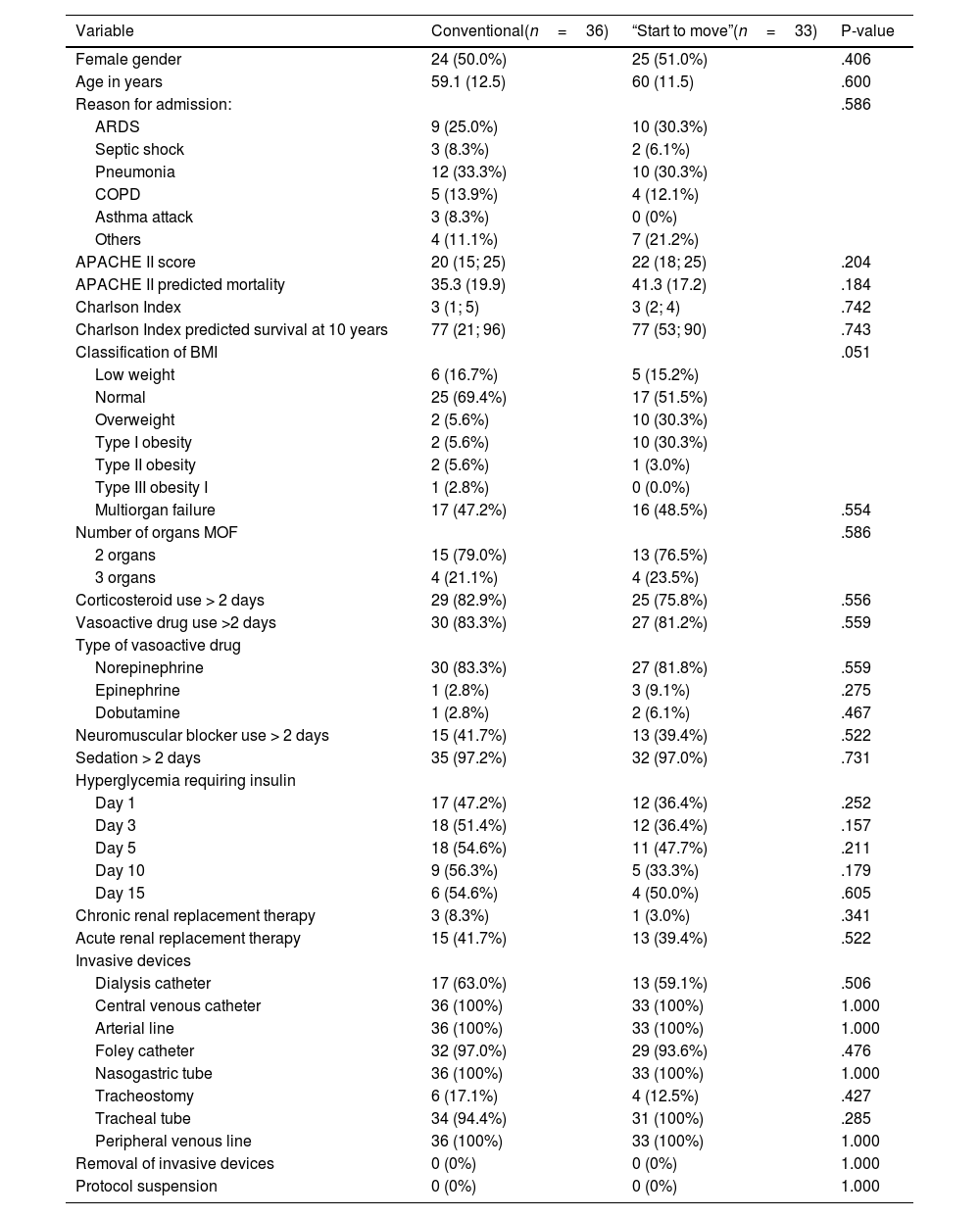
ResultsThe study included 69 subjects out of a calculated sample of 134 individuals. None of the patients abandoned or rejected participation in the study, and none were eliminated due to failure to meet the established criteria. Thirty-three subjects were allotted to the “Start to move” group (47%) and 36 to the conventional treatment group (52%). There were no significant differences in the sociodemographic or clinical characteristics between the two groups. The mean age of the “Start to move” group was 60 years, with an APACHE II score of 22 points (41% mortality) and a Charlson Index of 3 points (77% survival at 10 years (Table 1).
Comparison of sociodemographic characteristics between the conventional treatment group and the “Start to move” group.
| Variable | Conventional(n=36) | “Start to move”(n=33) | P-value |
|---|---|---|---|
| Female gender | 24 (50.0%) | 25 (51.0%) | .406 |
| Age in years | 59.1 (12.5) | 60 (11.5) | .600 |
| Reason for admission: | .586 | ||
| ARDS | 9 (25.0%) | 10 (30.3%) | |
| Septic shock | 3 (8.3%) | 2 (6.1%) | |
| Pneumonia | 12 (33.3%) | 10 (30.3%) | |
| COPD | 5 (13.9%) | 4 (12.1%) | |
| Asthma attack | 3 (8.3%) | 0 (0%) | |
| Others | 4 (11.1%) | 7 (21.2%) | |
| APACHE II score | 20 (15; 25) | 22 (18; 25) | .204 |
| APACHE II predicted mortality | 35.3 (19.9) | 41.3 (17.2) | .184 |
| Charlson Index | 3 (1; 5) | 3 (2; 4) | .742 |
| Charlson Index predicted survival at 10 years | 77 (21; 96) | 77 (53; 90) | .743 |
| Classification of BMI | .051 | ||
| Low weight | 6 (16.7%) | 5 (15.2%) | |
| Normal | 25 (69.4%) | 17 (51.5%) | |
| Overweight | 2 (5.6%) | 10 (30.3%) | |
| Type I obesity | 2 (5.6%) | 10 (30.3%) | |
| Type II obesity | 2 (5.6%) | 1 (3.0%) | |
| Type III obesity I | 1 (2.8%) | 0 (0.0%) | |
| Multiorgan failure | 17 (47.2%) | 16 (48.5%) | .554 |
| Number of organs MOF | .586 | ||
| 2 organs | 15 (79.0%) | 13 (76.5%) | |
| 3 organs | 4 (21.1%) | 4 (23.5%) | |
| Corticosteroid use > 2 days | 29 (82.9%) | 25 (75.8%) | .556 |
| Vasoactive drug use >2 days | 30 (83.3%) | 27 (81.2%) | .559 |
| Type of vasoactive drug | |||
| Norepinephrine | 30 (83.3%) | 27 (81.8%) | .559 |
| Epinephrine | 1 (2.8%) | 3 (9.1%) | .275 |
| Dobutamine | 1 (2.8%) | 2 (6.1%) | .467 |
| Neuromuscular blocker use > 2 days | 15 (41.7%) | 13 (39.4%) | .522 |
| Sedation > 2 days | 35 (97.2%) | 32 (97.0%) | .731 |
| Hyperglycemia requiring insulin | |||
| Day 1 | 17 (47.2%) | 12 (36.4%) | .252 |
| Day 3 | 18 (51.4%) | 12 (36.4%) | .157 |
| Day 5 | 18 (54.6%) | 11 (47.7%) | .211 |
| Day 10 | 9 (56.3%) | 5 (33.3%) | .179 |
| Day 15 | 6 (54.6%) | 4 (50.0%) | .605 |
| Chronic renal replacement therapy | 3 (8.3%) | 1 (3.0%) | .341 |
| Acute renal replacement therapy | 15 (41.7%) | 13 (39.4%) | .522 |
| Invasive devices | |||
| Dialysis catheter | 17 (63.0%) | 13 (59.1%) | .506 |
| Central venous catheter | 36 (100%) | 33 (100%) | 1.000 |
| Arterial line | 36 (100%) | 33 (100%) | 1.000 |
| Foley catheter | 32 (97.0%) | 29 (93.6%) | .476 |
| Nasogastric tube | 36 (100%) | 33 (100%) | 1.000 |
| Tracheostomy | 6 (17.1%) | 4 (12.5%) | .427 |
| Tracheal tube | 34 (94.4%) | 31 (100%) | .285 |
| Peripheral venous line | 36 (100%) | 33 (100%) | 1.000 |
| Removal of invasive devices | 0 (0%) | 0 (0%) | 1.000 |
| Protocol suspension | 0 (0%) | 0 (0%) | 1.000 |
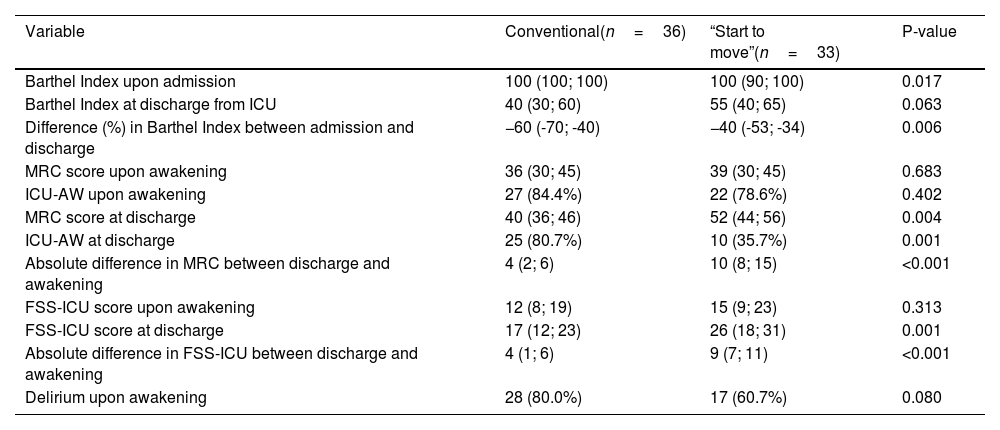
The incidence of ICU-AW at discharge in the conventional treatment group was 80.7% (95%CI 62.5–92.6) versus 35.7% in the “Start to move” group (95%CI 18.6–55.9) (p=0.001), with OR=0.44 (95%CI 0.26−0.75) (p<0.001). The difference in the MRC score at awakening versus the MRC score at discharge was 4 points (range 2–6) in the conventional treatment group and 10 points (range 8–15) in the “Start to move” group (p<0.001).
The functionality score as assessed with the FSS-ICU at discharge from the ICU was 17 points in the conventional treatment group (95%CI 12–23) versus 26 points in the “Start to move” group (95%CI 18–31; p=0.001). The absolute difference in FSS-ICU score between awakening and discharge was 4 points in the conventional treatment group (95%CI 1–6) and 9 points in the “Start to move” group (95%CI 7–11) (p<0.001).
Functional independence as evidenced by the percentage difference in the Barthel Index was 60% in the conventional treatment group (range −70 to −40) versus 40% in the “Start to move” group (−52.5 to −34.2) (p=0.006)(Table 2).
Before and in hospital comparison of functionality, muscle strength and delirium between conventional treatment and the “Start to move” protocol.
| Variable | Conventional(n=36) | “Start to move”(n=33) | P-value |
|---|---|---|---|
| Barthel Index upon admission | 100 (100; 100) | 100 (90; 100) | 0.017 |
| Barthel Index at discharge from ICU | 40 (30; 60) | 55 (40; 65) | 0.063 |
| Difference (%) in Barthel Index between admission and discharge | −60 (-70; -40) | −40 (-53; -34) | 0.006 |
| MRC score upon awakening | 36 (30; 45) | 39 (30; 45) | 0.683 |
| ICU-AW upon awakening | 27 (84.4%) | 22 (78.6%) | 0.402 |
| MRC score at discharge | 40 (36; 46) | 52 (44; 56) | 0.004 |
| ICU-AW at discharge | 25 (80.7%) | 10 (35.7%) | 0.001 |
| Absolute difference in MRC between discharge and awakening | 4 (2; 6) | 10 (8; 15) | <0.001 |
| FSS-ICU score upon awakening | 12 (8; 19) | 15 (9; 23) | 0.313 |
| FSS-ICU score at discharge | 17 (12; 23) | 26 (18; 31) | 0.001 |
| Absolute difference in FSS-ICU between discharge and awakening | 4 (1; 6) | 9 (7; 11) | <0.001 |
| Delirium upon awakening | 28 (80.0%) | 17 (60.7%) | 0.080 |
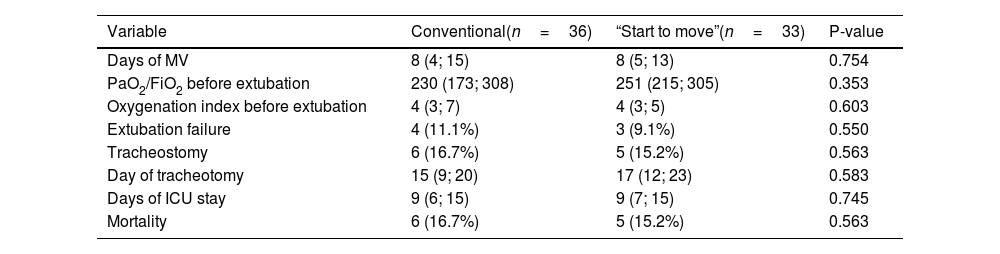
There were no significant differences between the two groups in terms of the incidence of delirium, days of IMV, days of ICU stay, or mortality at 28 days (Table 3).
Results of clinical variables and secondary outcomes between conventional treatment and the “Start to move” protocol.
| Variable | Conventional(n=36) | “Start to move”(n=33) | P-value |
|---|---|---|---|
| Days of MV | 8 (4; 15) | 8 (5; 13) | 0.754 |
| PaO2/FiO2 before extubation | 230 (173; 308) | 251 (215; 305) | 0.353 |
| Oxygenation index before extubation | 4 (3; 7) | 4 (3; 5) | 0.603 |
| Extubation failure | 4 (11.1%) | 3 (9.1%) | 0.550 |
| Tracheostomy | 6 (16.7%) | 5 (15.2%) | 0.563 |
| Day of tracheotomy | 15 (9; 20) | 17 (12; 23) | 0.583 |
| Days of ICU stay | 9 (6; 15) | 9 (7; 15) | 0.745 |
| Mortality | 6 (16.7%) | 5 (15.2%) | 0.563 |
The results of the present study evidence a significant decrease in the incidence of ICU-AW in the “Start to move” group, with a difference of 45% versus the conventional treatment group at discharge from the ICU. This is consistent with the data reported in the literature for ICUs of similar characteristics.18–20,30–33 The gain in strength between awakening and discharge from the Unit was 10 points in the “Start to move” group, with a difference of 6 points versus the conventional treatment group. This is in agreement with the results of Kayambu et al.,21 who reported a difference of 4.6 points between groups, and with those of Zang et al.,22 who reported a difference of 4.5 points on the MRC scale. However, the high ICU-AW rate observed in the conventional treatment group could be influenced by the small sample size, and should be corroborated by studies involving larger samples.
Our results indicate an increase in functionality at discharge in the “Start to move” group, with an absolute difference of 9 points versus the conventional treatment group. At present, no FSS-ICU classifying scores for determining the degree of functionality loss or gain are available for comparison against our findings. There is little evidence of the comparison of such results in ICUs of similar characteristics.
In relation to the Barthel Index, the “Start to move” group showed a 20% lesser decrease in functionality versus the conventional treatment group (60% versus 40%; p=0.006). Our data in this regard are similar to those reported by Symeonidou et al.,23 with a percentage difference of 48%. However, several studies show no significant differences in the Barthel Index scores.17,22 These results therefore could be indicative of improved performance of activities of daily living at discharge from the Unit.
On the other hand, the incidence of delirium in the “Start to move” group was 20% lower than in the conventional treatment group – though the difference failed to reach statistical significance. It should be noted, however, that the study sample for this outcome was too small to secure acceptable statistical power, thus underestimating the capacity of the test to detect significant differences between groups when such differences are present. Studies involving larger sample sizes are thus needed to assess such differences more in-depth.
With regard to the days of IMV, both groups presented a mean duration of 8 days and an absolute difference between them of 0 days (p=ns). Recent studies such as that of Zhang et al.24 have reported similar results, with an absolute difference of −0.33 days of MV (p=ns).24 However, it must be noted that the “Start to move” protocol involves a motor rehabilitation approach that does not necessarily have a direct impact on respiratory rehabilitation with the improvement of this outcome. Further studies are thus advised, with combined therapies involving respiratory muscle strengthening.
In both groups, the mean duration of ICU stay was 9 days, with an absolute difference of 0 days, in line with the results of Morris et al.25 and Kayambu et al.,21 who documented a nonsignificant absolute difference of 0.5 days (p=0.68 and p=0.43, respectively).
The mortality rate at 28 days after admission to the ICU was slightly lower in the “Start to move” group (15.2% versus 16.7%; p=ns). Recent studies such as those of Schwickert et al.,17 Kayambu et al.21 and Zang et al.22 have evaluated mortality in different periods, with differences favorable to the treatment group, but without reaching statistical significance.
Neither of the groups in our study experienced adverse events related to kinesic therapy; this is consistent with the findings published by Morris et al.25
Our study has limitations. In effect, the established sample size was not reached due to administrative issues at our center. However, the necessary post hoc statistical power adjustments were made. In turn, this was a single-center study without the admission of patients from an emergency service. Further studies are needed to address and incorporate these points. As regards the strengths of the trial, it is the only local study involving randomized patients that has been able to evidence potent physical and functional outcomes in the ICU setting.
ConclusionsThe application of the “Start to move” protocol for early rehabilitation in the ICU was associated with increased patient functionality as assessed by the FSS-ICU, a lesser incidence of ICU-AW, and greater functional independence as evidenced by the Barthel Index at discharge from the ICU. The “Start to move” protocol was not found to be useful in reducing delirium, the days of MV, the duration of ICU stay or mortality at 28 days. Application of the protocol was safe, with no adverse events being reported in either treatment group.
Author contributionsSebastián Soto: Study conception, data acquisition, drafting of the manuscript.
Paulina Vivanco: Data analysis and interpretation, drafting of the manuscript.
Rodrigo Adasme: Study design, data analysis and interpretation, final approval of the manuscript.
Paola Figueroa: Final approval of the manuscript.
Financial supportNone.
Conflicts of interestThe authors declare that they have no conflicts of interest.