Electrical impedance tomography has been described as a new method of monitoring critically ill patients on mechanical ventilation. It has recently gained special interest because of its applicability for monitoring ventilation and pulmonary perfusion. Its bedside and continuous implementation, and the fact that it is a non-ionizing and non-invasive technique, make it an extremely attractive measurement tool. Likewise, given its ability to assess the regional characteristics of lung structure, it could be considered an ideal monitoring tool in the heterogeneous lung with acute lung injury.
This review explains the physical concept of bioimpedance and its clinical application, and summarizes the scientific evidence published to date with regard to the implementation of electrical impedance tomography as a method for monitoring ventilation and perfusion, mainly in the patient with acute lung injury, and other possible applications of the technique in the critically ill patient. The review also summarizes the limitations of the technique and its potential areas of future development.
La tomografía de impedancia eléctrica se ha descrito como un nuevo método de monitorización en el paciente crítico en ventilación mecánica. Recientemente ha cobrado especial interés, debido a su aplicabilidad para la monitorización de la ventilación y la perfusión pulmonar. Su implementación continua a pie de cama y el ser una técnica no ionizante y no invasiva son propiedades particulares que la convierten en un recurso extremadamente atractivo. Asimismo, por su capacidad de evaluar las características regionales de la estructura pulmonar, podría constituir una herramienta de monitorización ideal en el heterogéneo pulmón con lesión pulmonar aguda.
En el presente artículo de revisión, se explica el concepto físico de la bioimpedancia y su aplicación clínica y se resume la evidencia científica publicada hasta la fecha en lo referido a la utilización de la tomografía de impedancia eléctrica como método de monitorización de la ventilación y de la perfusión, fundamentalmente en el enfermo con lesión pulmonar aguda, así como otras aplicaciones posibles de la técnica en el enfermo crítico. Asimismo, se resumen las limitaciones de la técnica y sus potenciales áreas de desarrollo en el futuro.
You cannot cross the same river twice, since new waters are always flowing past you. In: Teetetes (Plato).
Electrical impedance tomography (EIT) makes use of the physical principle of electrical impedance to evaluate different tissue properties. It is a diagnostic tool that examines the electrical characteristics of tissues to provide information on a noninvasive and continuous basis, at the patient bedside, and without exposure to radiation. In ventilated patients with acute lung injury (ALI), the technique is of particular interest in that it is able to afford important data on the events occurring in an axial section or slice of lung parenchyma, which by definition is heterogeneous, with zones possessing different mechanical characteristics. As a result, the global parameters afforded by the ventilator (referred mainly to pressure and volume) have different repercussions in different areas; in this sense, overdistended alveolar units can be combined with collapses alveolar units,1 and these areas moreover may present different degrees of tissue perfusion. The capacity of EIT to inform of the regional characteristics in terms of ventilation and perfusion has improved in recent years, and the technique is now considered to be potentially useful for optimizing the ventilator operating parameters. On the other hand, EIT is able to offer other important information in critical patients, such as the measurement of cardiac output, the localization of pleural occupation, or confirmation of correct positioning of the orotracheal tube. However, the limitations of EIT also must be taken into account – these fundamentally being associated to the calibration technique – and the physician using the tomograph must be able to interpret the information afforded by it in relation to the clinical changes experienced by the patient.
Physical principle and applicationImpedance is a physical variable describing the resistance characteristics of an electrical circuit in the presence of an alternating current. It reflects global opposition to the passage of current. Mathematically it is represented as a complex number comprising a real component (resistance) and an imaginary dimension (reactance). The impedance unit is the ohm (Ω).
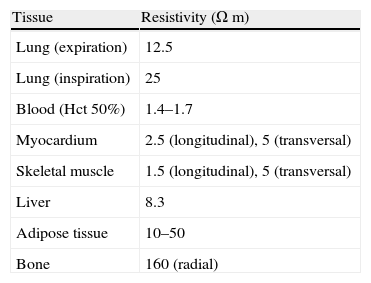
When this variable is applied to biological tissue we speak of “bioimpedance”. Different biological tissues exhibit different electrical resistances (Table 1). However, the respective figures are not absolute and can vary according to the conditions of the environment or medium (e.g., temperature). A temperature increase is associated with a decrease in impedance, secondary to changes in ion mobility. Tissue models can be developed using a two-terminal electrical circuit in which the impedance is representative of that of the tissue under study.2,3
Resistivity of different tissues (frequency 50kHz).
| Tissue | Resistivity (Ωm) |
| Lung (expiration) | 12.5 |
| Lung (inspiration) | 25 |
| Blood (Hct 50%) | 1.4–1.7 |
| Myocardium | 2.5 (longitudinal), 5 (transversal) |
| Skeletal muscle | 1.5 (longitudinal), 5 (transversal) |
| Liver | 8.3 |
| Adipose tissue | 10–50 |
| Bone | 160 (radial) |
By using multiple electrodes it is possible to obtain images of the bioimpedance of a body section, conforming what is known as electrical impedance tomography (EIT). The principle underlying this technique is based on the repeated measurement of surface voltages, resulting from the rotary injection of low-intensity alternating current between electrodes located in a circumference surrounding the studied object.4
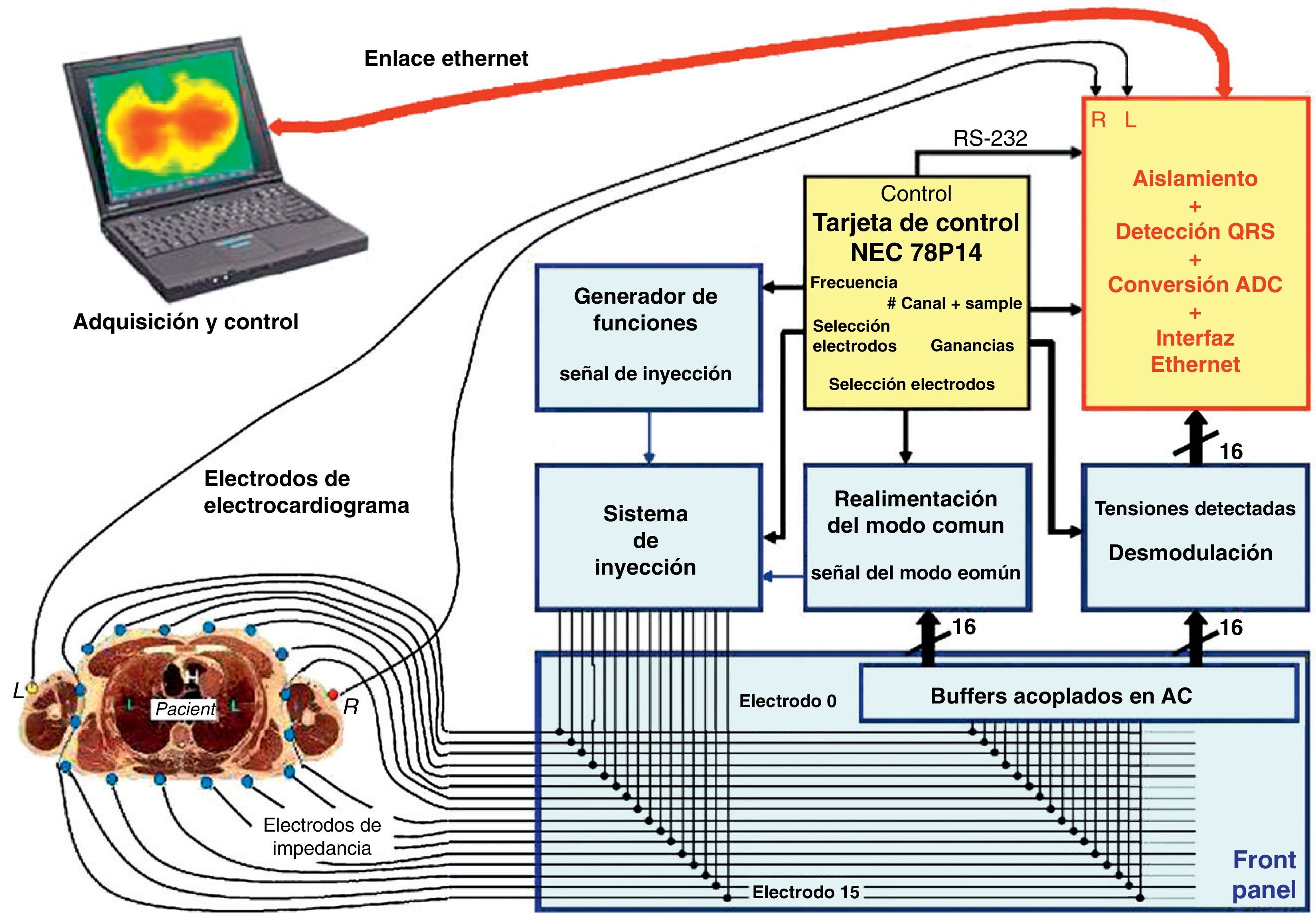
The hardware used for EIT has been extensively analyzed in the literature.5,6 In simple terms, the apparatus consists of a current injection and multicanal voltage detection system, a computer and 16 electrodes (optionally 1 or 2 neutral electrodes can be used) applied to the chest of the patient (Fig. 1). The number of electrodes can vary – the image resolution increasing with the number of electrodes used. The chest region in which the electrodes are placed is of maximum importance, given the potential interference by other structures such as the heart or diaphragm in generation of the pulmonary images. The electrodes are normally applied at the level of the sixth intercostal space. These electrodes register the information relating to impedance within a craniocaudal space of approximately 10cm.5 Generally, the measures are based on the application of a 50–80kHz alternating current (more than one frequency is possible, but is rarely employed for monitoring pulmonary impedance) of low intensity (5mApp), between two contiguous electrodes. The rest of the pairs of electrodes detect the voltage of the electrical signal, which will depend on the characteristics of the tissue through which the current has passed. This information in turn is registered by the computer. Immediately after this phase the next pair of electrodes injects current, and the resulting voltage is again recorded by the remaining pairs of electrodes. Each complete sequence of measurement of the impedance of the chest section is known as a cycle. The existing tomographs generally complete 25 cycles per second. With 16 electrodes it is possible to generate an image of the plane defined by a 1024-pixel matrix, though the true resolution with 16 electrodes is approximately 1% of the area, and is moreover not uniform throughout the section. The method used to reconstruct the information in image form is of key importance.7,8 Different reconstruction methods and algorithms have been discussed in the literature.9–12 The option most widely used in clinical practice is the Sheffield backprojection algorithm, and its later modifications.5,13,14 These reconstructions assume a rounded shape of the axial thoracic section, as a result of which they introduce a priori information in the data representation.
Bioimpedance measures can be classified into two types. The first involves determination of body tissue characteristics such as the degree of edema or the amount of adipose tissue. An increase in extracellular water content, a high electrolyte concentration, and an abundant presence of intercellular junctions reduce impedance. In contrast, adipose tissue, bone and air act as resistors, thereby increasing regional impedance. Measurements of this kind are fundamentally used in nutrition and sports medicine. The second type of bioimpedance measure involves assessment of the impedance changes fundamentally associated with the respiratory and circulatory systems. This is the type of study used in functional EIT (EITf). For reconstruction of the information, EITf uses the change in relative impedance in each pixel. This value (adimensional) is derived from the difference of the impedance of the tissue between two instants in time (Fig. 2). Reconstruction of the distribution of the absolute impedance requires knowledge of the shape of the axial section of the thorax.14 This is extremely difficult, since the section is not shaped homogeneously. Accordingly, use is made of the reconstruction based on the changes in impedance with respect to a reference, since it is assumed that the shape of the chest does not change between them. This interpretation minimizes the errors resulting from assumption of an incorrect shape of the thoracic section, and its validity has been demonstrated in recent years. Therefore, measurement of the relative impedance change allows us to compare two different physiological conditions (e.g., before and after changing the ventilator parameters, or the change between inspiration and expiration).
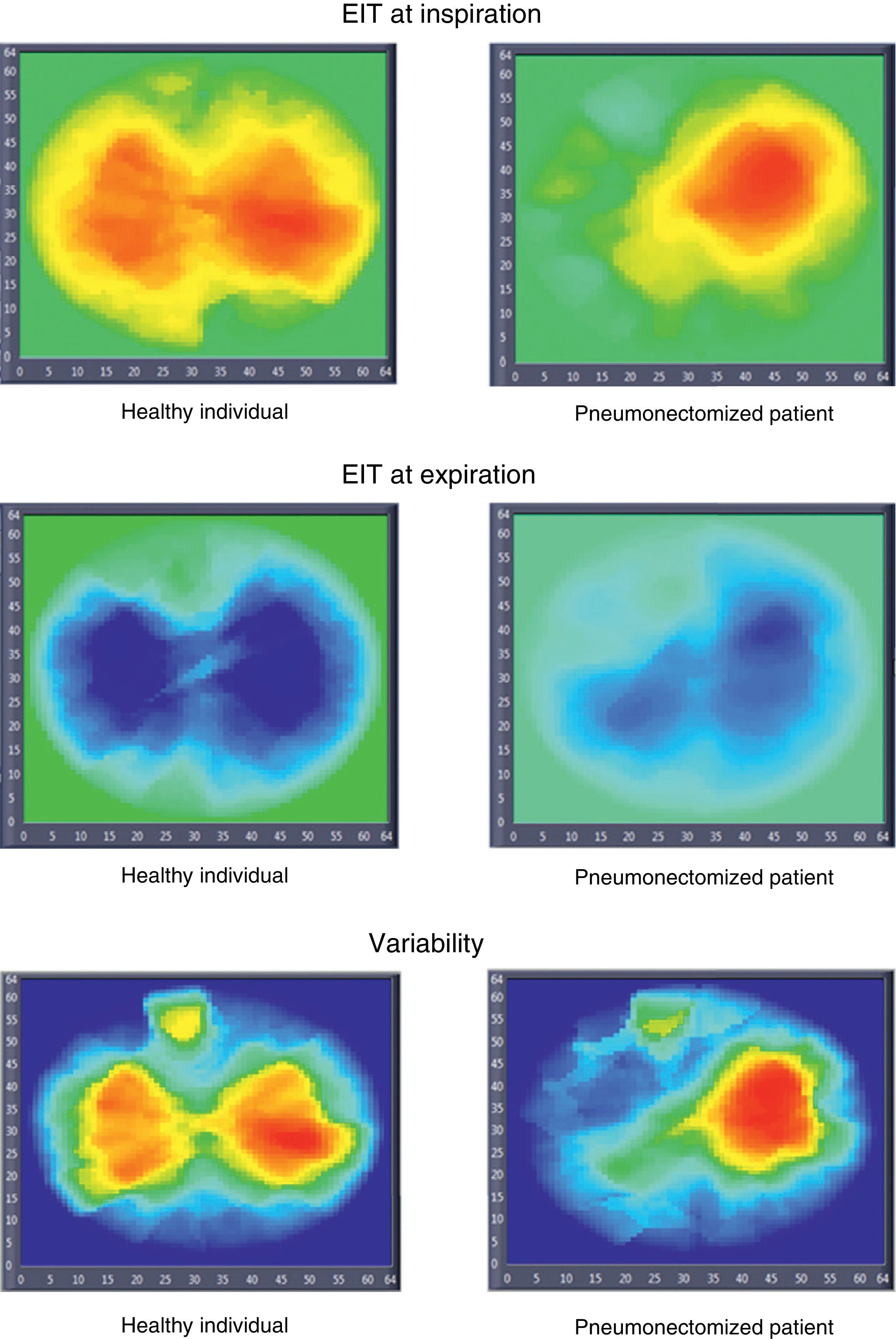
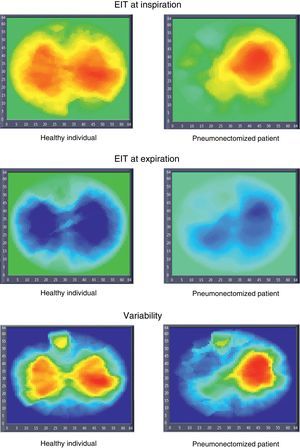
Electrical impedance tomography (EIT) images in a healthy individual and in a patient subjected to pneumonectomy (inspiration, expiration, and variability images). The inspiration and expiration images show impedance variation with respect to a reference. The variability images in turn correspond to the standard deviation of each pixel within a time interval (30s).
The change in thoracic bioimpedance is fundamentally conditioned by two cyclic mechanisms: ventilation and perfusion. The increase in air volume during inspiration, together with the increase in lung volume and the change in thoracic cage volume, leads to an impedance increment proportional to the inhaled gas volume, though the proportionality constant is dependent upon each concrete individual. On the other hand, pulmonary perfusion induces only minor changes (in the order of 3%) in thoracic impedance between systole and diastole.
Monitorization of alveolar ventilationThe lung tissues show a 5-fold greater electrical current resistance than the rest of the intrathoracic soft tissues. During the cyclic breathing process, the impedance of the pulmonary tissues changes 5% in the context of calm breathing, and up to 300% when inhaling from residual volume to total lung capacity.15 In comparison, the impedance of the chest wall remains relatively constant. By creating an image of the changes in the distribution of impedance, we can assess the distribution of ventilation.
The analysis of the changes in relative impedance in a given area of interest particular (ROI) can be used to calculate the amplitude of the change in bioimpedance (tidal volume),16,17 the minimum impedance (functional residual capacity)18 or the maximum impedance (total lung capacity).19 Such an analysis has also been used to observe the distribution of gas volume in the lung, varying the parameters of the ventilator.
There have been many EIT validation studies. To date, none have reported negative results. As early as 1995, Hahn et al. compared the technique with spirometry for validation of the changes in regional and global impedance.20 More recently, Hinz et al. compared EIT with the nitrogen wash-in/wash-out technique for the measurement of end-expiratory lung volume (EELV) in ventilated patients with ALI.21 Marquis et al. in turn used plethysmography with this same purpose.22 Regarding the validation of EIT as a method for measurement of the regional distribution of lung volume, different studies have made use of techniques such as computed tomography (CT) and positron emission tomography (SPECT and PET). Wrigge et al. used EIT to monitor recruitment in slow lung insufflation maneuvering in an experimental model of ALI.23 Likewise, Frerichs et al. compared the technique with CT to detect regional changes in lung volume resulting from changes in the ventilator parameters in healthy lung animal models—a good correlation being found between the two measures.24 Victorino et al. also used CT and EIT to explore the influence of gravity upon regional ventilation—a strong correlation being found between the two techniques.25 On the other hand, Hinz et al. validated the use of the technique for the measurement of regional ventilation, comparing the data with SPECT in an animal model of ALI.26 Recently, Richard et al. compared EIT with PET to quantify the changes in regional ventilation secondary to changes in ventilator parameters in animal models of healthy lung and ALI; in both models an excellent correlation was observed between the measurements obtained with the two techniques.27 The data obtained from all these studies suggest that the changes in relative impedance measured by EIT can be used to quantify regional ventilation with sufficient precision.
It has been seen that the global parameters, such as the pressure–volume curves or the measurement of compliance of the respiratory system, do not reliably reflect what in fact is happening in the lung structure under conditions of ALI. The intrinsic heterogeneity of the disorder, with the coexistence of collapsed and overdistended alveolar units, distorts the measurements. In this sense, EIT offers important advantages, fundamentally because of the possibility of analyzing the regional distribution of lung volume. It is essential to establish an adequate positive end-expiratory pressure (PEEP) in the ventilation strategy, particularly in the lung with acute injury. Many studies have used EIT in an attempt to establish optimum PEEP, allowing minimum de-recruitment and minimum overdistension.28–34 Kunst et al. used the technique to calculate the upper and lower inflexion points of the pressure (global)–volume (regional) curve in different ROI in animal models of ALI.35 A similar approach was adopted by Hinz et al. in ventilated patients, with the conclusion that the inflexion points of the pressure–volume curve obtained by means of global parameters are not representative of all the areas of the lung.36 Likewise, Lowhagen et al. used EIT for identification of the lower inflexion point of the pressure–volume curve in the dorsal regions in 16 ventilated patients with ALI.37 Kunst et al. in turn published a study monitoring lung recruitment and de-recruitment with different ventilatory protocols in an animal model of ALI.38 The authors concluded that the technique could be an excellent tool for choosing the optimum ventilator parameters. After different modifications in the ventilator parameters, Lowhagen et al. observed the regional changes in EELV and tidal volume distribution in the lung.39 EIT has also been used as a monitoring technique in non-conventional mechanical ventilation modalities, such as high-frequency oscillatory ventilation (HFOV). Van Genderingen et al., in an animal model of ALI, measured the distribution of lung volume with EIT during insufflation maneuvering; while the distribution was found to be non-homogeneous, increased homogeneity was noted on applying HFOV.40
Therefore, considering all these studies, EIT is seen to be an interesting monitorization method during mechanical ventilation, in view of its capacity to afford regional information, and its noninvasiveness. Another advantage of the system is that it offers continuous readings of the changing conditions of the ventilated lung, making it possible to identify at the patient bedside variations in pulmonary mechanics secondary to frequent maneuvers such as for example system depressurization after the aspiration of secretions,41 or changes in regional ventilation after modifying the patient position following mobilization.
Monitorization of lung perfusionIt has been confirmed that in addition to the impedance changes related to air volume movements, it is possible to visualize changes in impedance related to perfusion of the lung tissues. Lung perfusion is associated with a variable percentage decrease in impedance.42,43 Given the broad amplitude of the ventilatory component of the change in thoracic impedance, it is important to be able to isolate the two processes. It is possible to differentiate the changes in impedance referred to perfusion from the changes referred to ventilation by means of several maneuvers. Fagerberg et al. used an expiratory pause to evaluate the changes relating to pulmonary perfusion in 6 porcine models involving mechanical ventilation.44 Deibele et al. described a dynamic filter method for isolating the changes in impedance relative to perfusion in two individuals under conditions of spontaneous breathing, with acceptable results – though the authors concluded that further studies are needed to confirm the validity of the findings.45 Hypertonic saline injection has also been used as contrast agent in an attempt to achieve independence of the changes relative to perfusion.46
Way back in 1988, McArdle et al. observed a correlation between the EIT images and the images obtained by CT after isotope perfusion with the purpose of examining pulmonary perfusion in three patients: one with lung embolism, another with an emphysematous bulla, and the third with absence of perfusion of the left lower lobe.47 Smit et al. in turn studied pulmonary perfusion with EIT in 24 healthy individuals—the results being found to be reproducible upon analysis by two different investigators.48 The same authors investigated the effects of hyperoxia and hypoxia upon pulmonary vasoconstriction in 7 healthy volunteers and in 6 patients with chronic obstructive pulmonary disease (COPD), using EIT.49 The potential usefulness of EIT in identifying pulmonary embolism with extensive lung involvement has been demonstrated, though not so in identifying minor embolisms.50 However, to date only one validation study has been published comparing the EIT findings related to pulmonary perfusion versus a gold standard.51
Undoubtedly, this capacity to measure regional pulmonary perfusion is of great interest in the monitorization of many disease conditions. One such condition is acute lung injury (ALI). Identification of the best perfused areas can allow more efficient adjustment of the ventilator parameters, seeking recruitment of the airspace in these regions which as has been explained, the technique itself is able to help carry out. This can contribute to optimize the ventilation/perfusion ratio.44,52 Nevertheless, development of the methodology is needed in interpreting the signal exclusively related to the changes in pulmonary perfusion and in identification of the role played by the right ventricle systolic volume, the large vessels and the pulmonary capillaries in such changes.
Other applicationsThere are also other interesting applications of EIT in critically ill patients. As an example, the technique has been used to monitor the degree of lung edema.53 In this sense, an area of extraordinary potential interest is the identification of some characteristic factor capable of differentiating edema secondary to mere hydrostatic overload from edema associated to inflammatory processes. EIT has also been used to detect pleural occupation by air or fluid.54,55 The technique can detect air volumes of as little as 20ml. This possibility is extremely interesting in the ventilated critical patient with a tendency to develop pleural effusion and at risk of suffering barotrauma associated to ventilation (fundamentally if there is ALI). The immediate changes in bioimpedance associated to these findings offer the possibility of adopting early therapeutic measures, since monitorization with this technique is continuous. Likewise, a study has been published in which EIT has been used to confirm correct positioning of the orotracheal tube,56 specifically in a series of 40 patients with differential ventilation. The technique could also be used to assess correct conventional orotracheal tube placement during intubation and during the entire mechanical ventilation process—affording early identification of potential selective intubations. Other possible applications of EIT include the monitorization of unilateral lung function57 and quantification of right-side systolic volume58—comparison of the impedance cardiography technique with pulmonary artery catheter (PAC) thermodilution showing good correlation and even better reproducibility in one same patient.59 These benefits in turn are associated with the logically lesser invasiveness of EIT versus PAC.
EIT has many potential applications in the investigation of lung physiology, as has been evidenced by the studies of Lindley et al. and Frerichs et al., in which the effects of gravity upon the distribution of pulmonary volume in healthy individuals during parabolic flight have been explored.60
Limitations of the techniqueEIT obviously only affords impedance images in axial chest sections or slices, without taking into account the rest of the lung parenchyma. On the other hand, the spatial resolution of the technique referred to both ventilation (each pixel contains the information on the impedance of several alveolar units) and perfusion is still low. In this sense it must be clearly understood that EIT offers functional images, not anatomical images. It would be possible to improve the spatial resolution of the technique, though we are unlikely to reach the level of definition afforded by CT or magnetic resonance imaging (MRI).
As has been mentioned, a crucial point is the fact that calibration allows exact translation of the impedance changes into images, and of these into volume changes in a sustained manner over time. Such calibration is not simple, and errors can occur that lessen the reliability of the monitoring system. This aspect had also complicated the development of inductive plethysmography as a noninvasive method for monitoring ventilation.61
The EIT images reflect changes in the impedance of lung tissues, not absolute values. Therefore, conditions existing before monitorization with this system (e.g., consolidated areas, pleural effusion or air bullae) are not represented in them.
The potential changes in chest geometry between different maneuvers can distort the measurements obtained. This potential source of error therefore must be assumed. In addition to the changes in chest geometry, other factors can alter the measurements. For example, on measuring changes in ventilation, we may be overlapping intrathoracic fluid changes (pleural effusion, pulmonary extravascular water, pulmonary blood volume), which likewise can cause variations in the impedance results.62 It is therefore essential to associate the changes visualized by the system with the clinical changes of the patient (a circumstance not exclusive of EIT), in order to ensure their correct interpretation.
Inter-individual and even intra-individual comparison of the impedance values, particularly over long periods of time and after several changes in pulmonary conditions, is a complicated process. The correlation of the impedance changes to the changes in volume may not be totally precise, as evidenced by Bikker et al. in their recent study of 25 ventilated patients with different PEEP levels, where attempts were made to correlate EELV to end-expiratory lung impedance (EELI). These authors concluded that the linear relationship observed under conditions of normal breathing between volume changes and impedance changes cannot be used to determine EELV (which is an absolute value, not a change).63 These limitations could be partially minimized by developing adequate standardization and calibration techniques.
New areas for researchThe development of new hardware and software allowing improved spatial resolution would optimize the measurement results referred to both ventilation and perfusion. Likewise, the technique should be further developed to secure a more precise spatial distribution of the absolute impedances, and thus eliminate dependence upon relative measures—which, as has been explained above, can fail to take pre-existing conditions into account.
Likewise, it also has been explained that for the time being, EIT only affords information relating to axial sections of the thorax. The development of a system capable of generating images in three dimensions would improve the reading capacity of the technique.
Development of the technology could allow us to advance from the identification of gross changes in the studied structures to independent analysis of the findings—identifying specific particularities such as for example differentiation between hydrostatic edema and edema associated to inflammation. In this same line, EIT could be offered as an imaging technique for monitoring the course of pneumonia, and might even represent a supporting tool in its diagnostic algorithm.
Lastly, EIT could be used to study the effects upon the lungs of patients with ALI of other maneuvers associated with the treatment of refractory hypoxemia, such as prone decubitus or inhaled nitric oxide.
ConclusionsIn the current scenario of awareness of the fact that the usual therapeutic maneuvers are intrinsically able to increase the already existing damage in critical patients, monitoring of their effects upon the body is seen to be extremely important. In this sense, the very apt notion that “less is more” implies a capacity to measure. EIT offers advantages in observing what the applied treatment maneuvers (fundamentally related to mechanical ventilation) do to the lungs of patients with ALI. Its regional reading capacity, applicability at the patient bedside and safety, suggest that the technique will play an increasingly important role in minimizing the undesirable effects of maneuvering, such as the intrinsic damage caused by positive pressure ventilation upon the diseased lung.
Conflicts of interestThe authors declare no conflicts of interest.
Thanks are due to my dear family.
Please cite this article as: Riera J, et al. Tomografía de impedancia eléctrica en la lesión pulmonar aguda. Med Intensiva. 2011;35:509–17.