Monitoring is a crucial part of the care of the critically ill patient. It detects organ dysfunction and provides guidance on the therapeutic approach. Intensivists closely monitor the function of various organ systems, and the brain is no exception. Continuous EEG monitoring is a noninvasive and uninterrupted way of assessing cerebral cortical activity with good spatial and excellent temporal resolution. The diagnostic effectiveness of non-convulsive status epilepticus as a cause of unexplained consciousness disorder has increased the use of continuous EEG monitoring in the neurocritical care setting. However, non-convulsive status epilepticus is not the only indication for the assessment of cerebral cortical activity. This study summarizes the indications, usage and methodology of continuous EEG monitoring in the intensive care unit, with the aim of allowing practitioners to become familiarized the technique.
La monitorización es crucial en el cuidado del paciente crítico. Detecta disfunciones orgánicas y provee orientación en el abordaje terapéutico. Los intensivistas monitorizan habitualmente la función de varios sistemas orgánicos y el cerebro no es la excepción. La monitorización EEG continuo es una vía no invasiva e ininterrumpida para valorar la actividad eléctrica cortical con aceptable resolución espacial y excelente resolución temporal. La efectividad diagnóstica del estado epiléptico no convulsivo como causa de compromiso de la consciencia no explicable por otras causas se ha incrementado con el empleo del EEG continuo; sin embargo, no es la única indicación para valorar la actividad eléctrica cortical cerebral. Este manuscrito intenta resumir las indicaciones, modos de empleo y metodología para el empleo del monitoreo electroencefalográfico continuo en la unidad de cuidados intensivos con la finalidad que el intensivista se familiarice con el mismo.
Monitoring is defined as the act of observing the course of one or more parameters in order to find anomalies. Neurological monitoring is essential during the management of acute brain lesions and critically ill patients without neurological lesions. In general, these are the goals of monitoring: (a) establish and identify the function of an organ or system; (b) interpret pathophysiology; (c) provide physiological data for the diagnosis of anomalous situations; (d) guide therapeutics; and (e) guide prognosis. Neuromonitoring covers a wide spectrum that goes from clinical observation until the collection and acquisition of sophisticated variables for the assessment of the different aspects of brain functioning. One of these parameters is electroencephalogram (EEG) that, in a simple, continuous and noninvasive way, provides information on neuronal activity in the presence of structural and/or functional changes.1 For this reason, the use of the EEG has grown exponentially over the last few decades.2 In this manuscript we will be dealing with the utility and indications for the EEG in the critically ill patient.
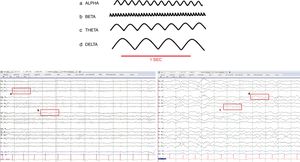
What is an electroencephalogram and how does it work?The EEG measures and records on graph paper the electrical activity in the cerebral cortex using electrodes that are placed on the cranial surface. The waves obtained represent the electrical current generated by the neurons when they communicate with each other. Usually, 21 electrodes are used, however, in some cases fewer electrodes may be used, particularly in the neurosurgical postoperative care or in the presence of ventricular drain or other neuromonitoring devices. The rate of detection of seizures is acceptably good averaging 93%, 68%, and 40% with 7, 4 and 1 electrodes, respectively.3 The electrodes are positioned under the so-called 10–20 system established by the American Clinical Neurophysiology Society (ACNS).4 By consensus, electrodes with odd numbers are positioned on the left hemisphere of the brain and those with even numbers are positioned on the right hemisphere. Small numbers correspond to parasagittal electrodes and big numbers to temporary electrodes. The letter of each electrode corresponds to the cerebral lobe, for instance, F, frontal; C, central; P, parietal, etc.4 (Fig. 1e, supplementary data).
The EEG is obtained by implementing differential amplifier technology that takes 2 electrical input voltages and then shows the output signal as the difference between both inputs voltages1,4 (Fig. 2e, supplementary data).
The EEG can be categorized based on amplitude, frequency, symmetry, and wave patterns. Amplitude is measured in microvolts and frequency in hertzs (number of waves per second). Based on their frequency, EEG waves are categorized into 4 bands: delta, zeta, alpha, and beta (Table 1, Fig. 1). The reading of different types of waves takes place in different settings too.
Normal brain waves.
| Band | Frequency (Hz) | Amplitude | Distribution |
|---|---|---|---|
| Delta | 0.1–3 | Usually>50μV | Present during physiological sleep stages iii and ivThe appearance of delta waves on a routine EEG in adult patients should be considered an abnormal finding |
| Zeta | 4–7 | Usually<15μV | It is usually seen on the frontal–central and temporal regions of the brain and its intensity increases as we grow older. It exacerbates during fatigue and hyperventilation (fairly common in children) |
| Alpha | 8–12 | Usually<50μV | It occurs during wakefulness and these waves are found at the occipital lobe. They are symmetrical in both hemispheres of the brain; asymmetries>50% are considered an abnormal finding. They show reactive wave activity: they attenuate or block with palpebral opening and mental activity and are present when we keep our eyes closed and when we are relaxed |
| Beta | 13–30Hz | Usually<25μV | It is the dominant activity of the frontal–central regions of the brain during wakefulness. It accentuates during somnolence and decreases during sleep to eventually disappear |
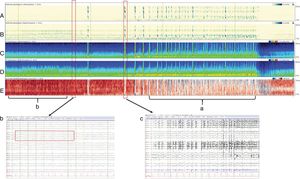
If necessary, the EEG monitoring can be extended and, in these cases, the quantitative electroencephalogram (qEEG) procedure is used. The qEEG is a digitalized alternative for EEG analysis that draws a graphical representation of the changes occurring in brain frequencies in time. This system is based on mathematical algorithms that turn a traditional EEG into a compact EEG using Fourier analysis, which allows us to compress time, reduce channels (electrodes), and make graphical representations of the waves that are coded into different colors. This is how several hours on a regular EEG can be reduced to a simple look at the screen with the values of the time-frequency axis in order to make real time observations of the compacted traditional EEG, which definitely helps in the interpretation of the EEG. This allows the detection of non-visible subtle changes without the proper training and better evaluations of continuous and repetitive changes as it is the case with the status epilepticus (Fig. 2).
Four hour-qEEG on a 56-year-old patient with abnormal mental status, fever, and rigidity at the back his neck. The patient was diagnosed with streptococcal meningitis complicated with nonconvulsive status epilepticus. Multiple electrographic seizures were recorded that originated inside the right hemisphere of the brain (confirmed by the greater amplitude of the right hemisphere compared to the left one. Panels C and D) and (a) by the relative asymmetry spectrogram (panel E) with more of a red (right hemisphere) than a blue image (left hemisphere). Also, (b) the relative asymmetry spectrogram looks red prior to the seizure due to the great interictal activity seen on the right hemisphere of the brain. Panels A and B are detectors of rhythmicity, a measure where the EEG tracing looks darker in the presence of periodic or rhythmic activity and is seen as a dark band to the frequency of the periodic activity. This spectrogram shows rhythmicity between 1 and 25Hz and, in this example shown, we can see rhythmicity asymmetry predominantly on the right hemisphere, especially in slow frequencies (delta). (c) The EEG shows one slow amplitude seizure originated at the right posterior quadrant (P4, P8, O2) that progresses diffusely over the right hemisphere of the brain with monomorphic delta activity and overlapped peaks. This activity has a wide field of action on the right hemisphere of the brain.
Since interpretation of the qEEG is easy after a quick and easy learning curve, other healthcare professionals (nurses, EEG specialists, and residents) can detect seizures on the qEEG as if they were expert neurophysiologis.5
Electroencephalogram at the intensive care unitConvulsive and nonconvulsive status epilepticusSeizures without any clinical signs and nonconvulsive status epilepticus in patients admitted to intensive care units (ICU) have been reported up to 48% and 14% respectively.6 The causes that lead to nonconvulsive status epilepticus are the same ones that lead to convulsive status epilepticus including structural lesions, metabolic disorders, toxins, epilepsy, infections, and withdrawal of drugs (i.e., baclofen) or toxics like alcohol. ICUs7 are not strangers to nonconvulsive status epilepticus especially during acute brain damage,7,8 recent convulsive status epilepticus,6 tumor postoperative care, subdural hematomas, subarachnoid hemorrhages,9 poisoning and metabolic (hypoglicemia) and electrolytic alterations (hyponatremia).10,11
The ACNS consensus statement established the indications for performing continuous EEGs (CEEG) in critically ill patients12:
- a.
Persistently abnormal mental status following generalized convulsive status epilepticus.
- b.
Acute supratentorial brain injury with altered mental status.
- c.
Fluctuating mental status or unexplained alteration of mental status without known acute brain injury.
- d.
Generalized periodic discharges on routine or emergent EEG.
- e.
Requirement for pharmacological paralysis and risk for seizures.
- f.
Clinical paroxysmal events suspected to be seizures.
Also, the ACNS recommends the use of CEEG as an additional diagnostic tool for the detection of brain ischemia in high risk populations such as in cases of subarachnoid hemorrhages.12
Recently, a group of experts agreed on the diagnostic criteria of nonconvulsive status epilepticus13 (Table 1e, supplementary data).
Critically ill patients usually show rhythmic epileptiform discharges >3Hz and minor discharges that can have a convulsive origin. Still, these discharge patterns are not always associated with brain damage or epilepsy. These so-called ictal–interictal patterns14 provide an ambiguous signification since they can be due to seizures or a marker of the severity of brain damage.14 It is adviseable to start EEG monitoring as soon as there is suspicion of nonconvulsive status epilepticus and then, at least, 24h later. This leads to the detection of up to 88% of the cases. Additionally, between 5% and 7% will be diagnosed if continuous monitoring goes on for another 48–72h.7 Approximately 25% of the patients who suffered cardiac arrest will develop seizures and it is precisely for the diagnosis of these seizures that the EEG is necessary, especially in patients who received therapeutic hypothermia.15 Similarly, the EEG helps us distinguish myoclonic status epilepticus from subcortical myoclonus, both states with a different clinical significance and a different response to treatment.
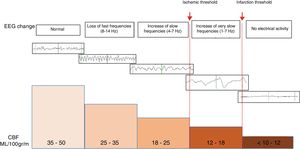
Detection of brain ischemiaThe EEG can help us in the diagnosis of brain ischemia since the morphological changes of the EEG run parallel to the changes sustained by cerebral blood flow (CBF). In general, when the CBF drops somewhere between 25 and 35mL/100g/min, the fast alpha (8–12Hz) and beta frequencies (13–30Hz) will appear attenuated on the EEG. And if the CBF drop is more accentuated and falls down even more to 17–18mL/100g/min, the waves of slow delta (0.5–3Hz) and zeta frequencies (4–7Hz) will appear enhanced on the EEG16 (Fig. 3).
The actual recommendations for the management of EEGs as a tool for the detection of brain ischemia is just limited to populations of patients with aneurysmal subarachnoid hemorrhages (ASH),3,12 especially in patients with higher risk of developing vasospasm and late ischemic deficit (poor early neurological degree, or massive subarachnoid and/or intraventricular bleeding).7 Late brain ischemia occurs in 20–30% of the patients with ASH and is defined as a clinical deterioration (new localized neurological deficit, reduced level of consciousness or both) of more than 1-h duration without a specific cause and/or new infarction seen on the brain CT scan that was overlooked on the images taken at admission or immediately after the procedure of excluding the aneurysm.17
The EEG monitoring should be initiated during the period of higher risk of ischemia, that is, 3–14 days following subarachnoid bleeding. The review and interpretation of the EEG should be a routine here to be able to detect and proceed early. It is not easy to detect late brain ischemia over long periods of time using traditional EEGs, which is why qEEGs are recommended here for monitoring purposes.12 The quantitative changes sustained by the alpha–delta relationship are the most reliable neurophysiological indicator in the diagnosis of late brain ischemia in patients with ASH. Claassen et al.18 confirmed that, compared to baseline values, a 10% reduction in the alpha–delta relationship is associated with a 100% sensitivity and a 76% specificity in the diagnosis of late brain ischemia. The quantitative changes in the alpha–delta relationship have been seen early somewhere between 5 and 60h before symptom onset and diagnosed somewhere between 24 and 48h before other additional diagnostic modalities, especially with unreliable neurological examinations.3
Monitoring of deep sedation (bispectral index)Bispectral index, popularly known as BIS, studies certain traits obtained from the analysis of the EEG spectral power in such a way that the signal generated at the frontal region is processed and converted, through the implementation of one algorithm incorporated in an internal software, into a wave and a number that objectifies the measurement.19,20
At the beginning, the BIS was designed to assess the depth of anesthesia in order to improve the titration of the drugs administered by minimizing the dose, adverse events, and costs and be able to control the wake-up process. Values go from 0 and 10019,20 (Fig. 3e, supplementary data).
Its potential applications at the ICU are:
- a.
Since it has an acceptable correlation with the different subjective scores available for the clinical assessment of sedation and analgesia, it can be used to optimize it during mechanical ventilation.21–23
- b.
It assists but cannot not replace the EEG in the monitoring of the drugs administered during the management of refractory status epilepticus (<30 is associated with burst suppression pattern in the EEC registry).20,24
- c.
It provides prognostic value for anoxic-ischemic encephalopathies on therapeutic hypothermia (<22 after cardiac arrest is associated with poor prognosis).25,26
- d.
It helps in the diagnosis of brain death.27
The BIS has different limitations that will not let us acquire a clean artifact-free registry.19,20 The studies available to this day lack the sensitivity and specificity needed and the method has not been validated universally in different populations of neurocritical patients, which is why the data available today do not support its use for the management of brain injuries.28
Use of electroencephalogram in encephalopathies and encephalitisAlthough inspecific, the use of the EEG in confused, agitated patients with an altered state of consciousness can be of great help. For instance, the EEG can confirm the presence of excessive beta activity due to the overuse of benzodiazepines; the attenuation of alpha voltages in patients with structural lesions that compromise the cerebral cortex or the presence of polymorphic delta activity in patients with lesions affecting the subcortical white matter and interrupting the ascending thalamocortical fibers.
In the case of septic patients, electroencephalographic changes keep a linear correlation with the degree of severity of encephalopathy. In the first place, we see the intermittent appearance of slow delta and zeta frequencies that will eventually turn into continuous frequencies. Later, we will see the appearance of triphasic waves and, in severe cases, the suppression of voltage or the presence of the burst-suppression pattern.29
Triphasic waves are medium-to-high-voltage complexes with a 3-phase pattern: the first phase is a negative deflection followed by a positive deflection and then another negative deflection with a delay in the anterior-posterior direction in such a way that the first deflection is visualized some ∼200ms before the last deflection. Its maximum expression is found on the central regions of the brain and it increases voltage with wake-up (Fig. 4e, supplementary data). The triphasic pattern is associated with different levels of compromise of the state consciousness and although it has been described in hepatic encephalopathies, it is also present in other encephalopaties whether metabolic and/or toxic (baclofen or lithium-induced toxicity). It has also been described in structural encephalopathies due to ischemic strokes or tumors. Uremic encephalopathy can also show triphasic waves although some experts think that the waves produced by uremia have a smaller wave amplitude (<70μV) compared to those originated by hyperamonemia.30 Also, several studies have indicated that diseases that cause cortical or white matter atrophy favor the appearance of triphasic waves in the presence of triggering events such as metabolic infections or alterations.31 Additionally, the encephalic patient can show other anomalous patterns such as the intermittent presence of frontal zeta waves, diffuse slow activity and bilateral, high-voltage paroxistic delta activity.32
The greatest anomaly seen on the EEG in cases of viral or bacterial encephalitis is diffuse delta waves. Similar to what happens with septic encephalopathy, the EEG registry will be back to normal depending on the patient's clinical progression.33,34 Patients with meningococcal meningitis and a good prognosis will show better EEGs within the first 6–9 days. If anomalies persist for more than 2 weeks, then we will have to suspect secondary complications (infarction, vasculitis).33 In herpetic encephalitis, the EEG can show the severity and location of the infection. At any time, diffuse or focal slow waves can be seen and, approximately, one week later we will see periodic or pseudo-periodic discharges.35,36 These discharges are the most characteristic ones of herpetic encephalitis and they are usually focal or unilateral, have a great amplitude and occur at a frequency of 0.5–3Hz and are predominantly of temporal lobe location.35,36 Although these discharges are not pathognomonic of herpetic encephalitis, their presence is associated with acute fever with or without focal seizures and with an analysis of the cerebrospinal fluid that is consistent with pleocytosis, strongly indicative of herpetic encephalitis.
Over the last few years we have seen an increase in the diagnosis of limbic encephalitis, which is characterized by a triad of: (1) subacute anterograde compromise of short-term memory, (2) complex partial seizures originated at the temporal lobe, and (3) psychiatric symptoms. Schmitt et al.37 recognized one single patterns in limbic encephalitis due to anti-NMDA receptor antibodies (anti-NMDA encephalitis) characterized by the presence of rhythmic delta waves of 1–3Hz with overlapped bursts of 20–30Hz beta waves over the delta waves. It is called extreme delta brush due to the similarities with the beta-delta rhythmic complexes seen in premature babies.
Use of the electroencephalogram in the prognosis of comatose patientsThe EEG can help us establish the prognosis of comatose patients, in particular of victims of anoxic-ischemic encephalopathy. Certain electrographic patterns have been associated with poor prognosis. The European Society of Intensive Care Medicine and the European Resuscitation Council establish that the absence of reactivity on the EEG in the presence of an external stimulus and the burst-suppression or status epilepticus within the 72h that follow a cardiac arrest anticipate poor neurological recoveries (severe neurological disability, persistent vegetative state or death) with false positives of up to 6%.38 Using the terminology standardized by the ACNS, a dominant burst-suppression pattern with periodic continuous discharges or a burst-suppression pattern with or without periodic discharges are predictors of poor results following cardiac arrests without false positives.39
The EEG has also been used as a prognostic tool in cases of ASH. Claassen et al.40 said that in patients without reactivity on the EEG, the presence of generalized periodic electric discharges or bilateral isolate periodic electric discharges was associated with poor prognosis. Also, patients who develop seizures during the clinical progression of subarachnoid bleeding have 3 times more chances of showing severe discapacity or dying.41 Finally, in patients with traumatic brain injuries it has been reported that non-reactivity on the EEG in the presence of auditory or painful stimuli,42 the lack of alpha rhythm on the occipital region or the stage 2 sleep pattern (N2) and the predominant presence of delta activity are all electrical traits closely associated with poor prognosis.43
Brain deathThe recommendations for the use of EEG as an additional tool in the diagnosis of brain death vary across the world. In the United States, the EEG is recommended but not mandatory. In Canada it is not recommended, and only in a few European countries it is indicated. In France,44 in the absence of sedative drugs, metabolic disorders or hypothermia, the lack of brain activity needs to be confirmed through 2 EEGs performed in 4h-intervals and with a minimum of 30min of good quality recording. In cases of brain death due to cardiorespiratory arrest, a 12h-period is recommended since the EEG can show lack of brain activity within the hours following the cardiac arrest. The electrocerebral silence seen on the EEG is defined as a lack of brain activity of over 2μV, yet despite the application of auditory and painful stimuli, to a sensitivity between 3 and 5μV/mm without a filter <70Hz (except for the 50Hz notch filter) and a filter no higher than 0.5Hz. At least 8 electrodes need to be used and other technical issues observed rigorously. Back in the 1960s, in the United States,45 the EEG was used as an additional tool to diagnose brain death but ever since then, the definition and determination of brain death has been refined and the use of EEGs for this purpose has grown thinner. Same as it happens in France, once the reversible causes of the comatose state have been ruled out, the lack of neuron activity>2μV to a sensitivity>2μV/mm with a 0–1 or 0.3Hz filter and 70Hz per 30min of good quality recording is consistent with a diagnosis of brain death.
Duration of monitoringThe answer to this question should not be controversial; it is going to depend on the indication for the monitoring and the reason why it is used or indicated.
When on suspicion of seizures without any clinical signs and/or nonconvulsive status epilepticus, the EEG should be initiated immediately and for, at least, another 24h. Diagnosis is possible in 45–58% of the cases on 30–60min of electroencephalic monitoring.12 With 24–48h of monitoring, diagnostic probability goes up to 95%.7 In general, longer monitoring (48h or more) may be necessary in comatose patients with periodic discharges or on deep sedation. However, monitoring time can be refined here. Westover et al.46 described less than 5% chances of finding seizures within the next 72h in the absence of epileptiform discharges within the first 2h of electroencephalographic monitoring.
When a diagnosis of nonconvulstive status epilepticus has been achieved, the EEG should still go on while IV anticonvulsant medications (midazolam, propofol) are being administered; up to, at least, 24h following the last seizure12; and for another day following the withdrawal of IV antiepileptic drugs.47
The optimal monitoring time in patients with encephalopathies or encephalitis is unknown. In this group of patients, monitoring time should be used individually with every patient. The use of the CEEG has not proven more useful than the routine EEG for prognostic purposes following a cardiac arrest,48 and it has the same utility in patients with anoxic-ischemic encephalopathy during the period of hypothermia.49
Obviously, there are advantages and limitations when using the CEEG. Some of the advantages that can be mentioned are:
- ∘
It provides permanent, real time, bedside information and clearly increases the chances of detecting certain anomalies such as convulsive discharges or the appearance of intermittent waves (triphasic).
- ∘
It increases the sensitivity for the detection of nonconvulstive discharges and allows us to distinguish between epileptic discharges and motor activities of a different origin.
- ∘
It provides information even when the patient is on deep sedation or neuromuscular blockade.
- ∘
It allows us to detect dynamic situations (vasospasm-related ischemia in ASH).
- ∘
It is very useful when it comes to establishing safe therapeutics and helps with the dose and time of drug administration (barbiturates, propofol) in the refractory status epilepticus.
- ∘
It has prognostic power as an additional diagnostic tool (anoxic-ischemic encephalopathy, brain death).
There are some of its limitations:
- ∘
The interpretation of results requires training and a learning curve.
- ∘
It requires greater effort and a greater work load.
- ∘
It requires the permanent supervision of electrophysiologists.
- ∘
It is subject to different patient (moves, sweating, pacemaker) and environmental (noises, screens, infusion pumps) factors that may interfere with the correct reading of the CEEG tracing.
- ∘
The continuous monitoring generates gigabytes of data that are not easy to store and keep.
The traditional systems of EEG monitoring allow us to store a huge amount of data when they are digitalized. We have already mentioned that in order to facilitate analysis and interpretation, several quantitative modalities have been developed that compress information and facilitate the understanding of all the phenomena observed by neurophysiologists. We have already talked about the qEEG here. Variants of this last modality are under development now and they are being tested to facilitate the reading of the registry such as1:
- -
Color density spectral array built in a chart x/y that measures frequency and time that are later compressed and then put in a chart.
- -
Topographic mapping of the EEG spectral power for asymmetry assessment purposes.
- -
Assessment of new trends in different characteristics of traditional EEGs.
- -
Integration of wave amplitude.
- -
EEG video.
On the other hand, the lack of assistant specialists, EEG machines, and trained neurologists restricts monitoring adding difficulties such as the tedious process of having to place the electrodes and the time needed to perform the actual monitoring. All this limits the immediate use of EEGs in urgent situations and in cases of nonconvulsive status epilepticus. Several devices with limited numbers of electrodes have been developed in recent years and they can be placed very quickly and without the presence of a specialist. These are highly sensitive devices for the diagnosis of seizures (98–100%), they are easy to use and interpret by different healthcare providers,50 and they can help distinguish immediately nonconvulsive status epilepticus and see the response to early therapies.
ConclusionThe use of CEEG as an additional tool of neuromonitoring purposes at the ICU is considered an important tool today for the management of critically ill patients regardless of the presence of primary brain injuries. The nonconvulsive status epilepticus is highly prevalent and is associated with greater morbimortality.51 Also, the nonconvulsive status epilepticus can be misdiagnosed due to poor interpretations and poor EEG classifications as epileptiform activity, yet this can be avoided in observance of the international recommendations and the standardized terminology established by the ACNS. A great number of clinical studies have already been published and they support the use of the CEEG at the ICU setting, yet the CEEG is still underused. Although the studies published so far do not have a large number of patients and they have not been interpreted by expert neurophysiologists, the CEEG can be used to alert the ICU team on any possible changes in a patient's neurological status, assist during therapeutic management, see the response to drugs, make prognostic assessments, and diagnose the patient. Yet despite all this, we need additional studies to have enough evidence on how the CEEG impacts the results of patients monitored during their ICU stay.
Conflicts of interestNone whatsoever.
Please cite this article as: Rubiños C, Godoy DA. Monitorización electroencefalográfica en el paciente crítico: ¿qué información útil puede aportar? Med Intensiva. 2020;44:301–309.