To examine the predictive value of an early transcranial Doppler ultrasound (TCD), a study was performed in the emergency department for patients with spontaneous subarachoniod hemorrhage (SAH) in good neurological condition, in order to know which patients are at high risk of developing delayed cerebral ischemia (DCI).
DesignA descriptive observational study was carried out involving a period of 3 years.
SettingCritical Care and Emergency Department.
PatientsThe study consecutively included patients with SAH of grades I–III on the Hunt and Hess scale.
Variables of interestDCI (decrease of 2 points in GCS or focal deficit), mean velocity (MV) of middle cerebral arteries (MCA), Lindegaard Index (IL). Sonographic vasospasm pattern (SVP) was considered if MCA-MV >120cm/s and IL >3.
ResultsThe mean age of the 122 patients was 54.1±13.7 years; 57.3% were women. SVP was detected in 24 patients (19.7%), although high velocities patterns (HVP) were present in 38 patients (31.1%). DCI developed in 21 patients (MV183±49cm/s), all with previous SVP. In this group MV increased 22±5cm/s/day during the first 3 days. The group without HVP (84 patients/MV of 67±16.6cm/s), compared with DCI group, showed differences in highest MV (p<0.001), and also ΔMV/day (8.30±4.5cm/s vs 22±5cm/s) during the first 3 days (p=0.009). In our series, ROC analysis selected the best cut-off value for ΔMV/day as 21cm/s (p<0.001).
ConclusionDuring the first 3 days, an increase of 21cm/s/24h in MCA-MV was associated with the development of symptomatic vasospasm. TCD is a useful tool for the early detection of patients at risk of DCI after SAH.
Establecer el valor predictivo, para desarrollar deterioro neurológico tardío de origen isquémico (DNI), de un estudio doppler transcraneal (DTC) en pacientes con hemorragia subaracnoidea espontánea (HSA) en buena situación neurológica.
DiseñoEstudio descriptivo-observacional desarrollado durante 3 años.
ÁmbitoCuidados Críticos y Urgencias.
PacientesSe incluyeron de forma consecutiva aquellos pacientes con HSA en buena situación neurológica (Hunt-Hess I-III).
Variables de InterésDNI (disminución en 2 puntos del GCS o déficit focal), velocidad media (VM) en arterias cerebrales medias, índice de Lindegaard (IL). Se consideró patrón sonográfico de vasoespasmo (PSV) cuando la VM fue>120cm/s y existía un IL>3.
ResultadosLa media de edad de los 122 pacientes fue de 54,1±13,7 años. El 57,3% eran mujeres. Se detectaron 24 pacientes con PSV (19,7%) encontrándose VM elevadas en 38 pacientes (31,1%). 21 pacientes desarrollaron DNI (VM 183+/-49cm/s), todos presentaron PSV. En los pacientes con DNI se detectó un aumento de VM de 22+/-5cm/s/24h durante los 3 primeros días. Al comparar aquellos pacientes que no presentaron VM elevadas (85 pacientes/VM 67+/−16,6cm/s) con respecto a los que desarrollaron DNI encontramos diferencias en las VM (p<0,001) y en el ΔVM/24h (8,30+/−4,5cm/s Vs 22+/−5cm/s) durante los 3 primeros días (p=0,009). Mediante curvas ROC, se fijó que el ΔVM/día de 21cm/s (p<0,001), era el que mejor predecía el DNI.
ConclusiónDurante los 3 primeros días un incremento en la VM de 21cm/s/24h se asoció con el desarrollo de vasoespamo sintomático. El DTC es una herramienta útil para la detección de aquellos pacientes con HSA en riesgo de desarrollar DNI.
Hemorrhagic cerebrovascular disease of aneurysmal origin accounts for about 80% of all cases of bleeding within the subarachnoid space secondary to non-traumatic causes. Spontaneous subarachnoid hemorrhage (SAH) represents 5–10% of all cases of stroke, with a mean incidence of 6–10 cases/100,000 inhabitants, and is the most common cause of sudden death due to stroke.1–4 An avoidable mortality rate of up to 20% has been recorded following SAH.5 One of the main complications, responsible for the ominous outcome and particularly for the neurological sequelae, is symptomatic vasospasm or delayed cerebral ischemia (DCI).6,7 This occurs in 26.5–46% of all patients between days 4 and 14 following the initial event,8,9 and manifests clinically as diminished consciousness or the appearance of focal neurological defects not attributable to repeat bleeding or hydrocephalia. The underlying etiopathogenesis and physiopathology are not well known. The diagnosis is established by arteriography, revealing a reduction in arterial caliber, or by ultrasound – with the recording of an increased flow velocity. In this context, mean velocity (MV) values of over 120cm/s in the middle cerebral artery (MCA) are significantly correlated to vessel narrowing.10
Due to the frequency and seriousness of DCI, many attempts have been made to establish an early prediction of the disorder, using a range of approaches such as bleeding volume determined by computed axial tomography, clinical severity, different hematological parameters, and transcranial Doppler ultrasound (TCD).11–17
While the usefulness of TCD for the diagnosis and follow-up vasospasm (symptomatic or otherwise) in SAH has been well established, its validity in predicting DCI due to vasospasm remains to be determined.18–23 To date, studies have been made of different elements such as the absolute MV value, its increment, or the application of ultrasound techniques allowing us to measure the auto-regulatory capacity of the brain.17,24–26 However, the contradictory results obtained explain the current lack of recommendations for performing emergency TCD.26–29 For this same reason, the possibility of using the technique in deciding which SAH patients in good neurological condition should receive closer care and monitoring has not been explored to date. Establishing in the first hours of patient management which cases of non-serious SAH belonging to a group at high risk of suffering DCI could benefit from admission to the Intensive Care Unit (ICU) would be essential as well as efficient in prescribing early and aggressive prophylactic treatment in the presence of brain ischemia secondary to vasospasm.
We postulate that emergency TCD, performed in the first 24h following admission to hospital, could predict symptomatic vasospasm in the anterior cerebrovascular territory, since arterial spasm secondary to SAH is progressive, not sudden, and takes place in the large arteries of the circle of Willis. To this effect, we must study the velocity increment/24h during the first 3 days, not the absolute values of MV in the MCA, in clinically homogeneous groups. The present study explores whether TCD performed during the first 3 days in SAH patients in good neurological condition is able to predict the ulterior appearance of DCI.
Material and methodA longitudinal descriptive study was made in the Department of Critical and Emergency Care Medicine (Virgen del Rocío University Hospital, Seville, Spain) during the period 2005–2007, following the guidelines of the Research Committee of our center.
We included all cases of spontaneous SAH of low clinical severity corresponding to grades I–III according to the scale of Hunt and Hess (HH) at the time of admission to hospital.12 We excluded traumatic SAH, incidental aneurysms, patients lacking a TCD window, and spontaneous SAH corresponding to HH grades IV and V. We recorded the time elapsed from symptoms/signs onset to patient arrival in hospital.
In all cases the diagnosis of SAH was established by computed axial tomography (CAT) or lumbar puncture when CAT proved negative and the clinical manifestations were suggestive of SAH. The severity of bleeding was estimated from the tomographic study following the Fisher scale.11 After arriving in hospital, the patients remained for 24–48h in the observation area of the Emergency room. The patients were kept under fasting conditions to wait for emergency arteriography. From the start, treatment was provided in the form of intravenous nimodipine at a dose of 0.5mg/h, with gradual dose increments over the next 6h up to 2mg/h, in the absence of adverse hemodynamic effects. Posteriorly, treatment was continued via the oral route at a dose of 60mg/4h, provided there were no contraindications.
TCD exploration (Doppler Multidop-P, DWL, Germany) was carried out with a 2MHz ultrasound probe through the temporal window, as described by Aaslid et al.30 A TCD study was carried out in the first 24h and again after 48h (minimum). Ultrasound was performed between days 4 and 14 following SAH, except if the first study was performed during this period and proved normal. The studies were made by only 7 physicians with extensive experience in TCD. In both hemispheres we recorded the MV of the proximal middle cerebral artery (M1). If the MV obtained exceeded two standard deviations (SD) for the age of the patient–reaching 120cm/s or not–ultrasound exploration was made of the homolateral extracranial internal carotid (ICA-ex) at submandibular level, with calculation of the Lindegaard index (IL): MV MCA/MV ICA-ex. The patients with an MV MCA/MV ICA-ex ratio of >3 were classified as subjects with sonographic vasospasm, while patients with MV >120cm/s and IL <3 were diagnosed with increased cerebral blood flow.30–32 If the patient required ventricular drainage due to hydrocephalia in the first 24h, the registry regarded as valid was that obtained after evacuation of the cerebrospinal fluid (CSF). Symptomatic vasospasm (DCI) was defined by a worsening of the level of consciousness of two or more points on the Glasgow Coma Scale (GCS), or by the appearance of a focal defect between days 4 and 14 after the bleeding event, not attributable to repeat hemorrhage, hematoma, hydrocephalia or metabolic alterations. Vasospasm secondary to vascular anomaly exclusion was considered when the previously cited clinical manifestations showed a time relationship to the surgical intervention, except for a consequence of incidental surgical clipping.
Cerebral arteriography for clarifying the etiology of SAH was performed in all the patients. This exploration was repeated after 14 days if the first study proved negative or when the condition did not correspond to peri-mesencephalic SAH. In those cases where needed, cerebral arteriography was carried out to establish a diagnosis of angiographic vasospasm. In the case of arteries not subjected to ultrasound study (basilar artery and anterior cerebral artery), the existence of angiographic vasospasm was not counted as such to the effects of our study.
Patient information and data confidentialityThe recommendations of the Declaration of Helsinki referred to research were followed. The patients were informed of the interest of their case, and permission was verbally requested for the collection of information and inclusion in the study. No express written consent was needed, since the study involved no medical intervention, and no extraordinary biological samples were collected for analysis, other than those forming part of usual clinical practice. Patient confidentiality was guaranteed by assigning a code to each patient, consisting of two initial digits corresponding to the code of the hospital first attending the case, followed by the clinical history number (comprising up to 7 digits). The code list was kept in a safe place by the study supervisors.
Statistical analysisThe SPSS version 17.0 statistical package was used for the statistical analysis. Statistical significance was considered for p<0.05. Continuous variables were reported as the mean and standard deviation. Student's t-test or Mann–Whitney U-test was used for the comparison of means, as required. Qualitative variables in turn were compared using the chi-squared test or Fisher exact test. Excluding the patients who developed hyperemia, the receiver operating characteristic (ROC) curve was used to calculate the optimum cutoff point corresponding to the increase in MV/24h during the pre-vasospasm period.
ResultsA total of 123 SAH episodes were recorded during the study period. One patient was excluded from the study due to lack of a sonographic window. A total of 122 patients were studied: 70 females (57.3%) and 52 males (42.6%), with a mean age of 54.1±13.7 years.
Most of the patients (70.4%) were admitted to hospital in the first 24h after the bleeding episode and were diagnosed with SAH in under 24h after the onset of symptoms. In 36 cases (29.5%) the diagnosis was delayed more than 24h, either because the patient did not seek medical help before then, or because the process had not been identified earlier. Evaluation of the degree of altered consciousness upon arrival in hospital showed 87.6% of the patients to have a GCS score of 14–15. The mean delay in performing the arteriographic studies was 57h. The distribution according to clinical severity as determined from the HH scale, the etiology of SAH, the clinical course and the treatment provided are reflected in Table 1.
Clinical characteristics and evolution of the 122 patients with SAH.
| Clinical variables | |
| GCS, n (%) | |
| 15 | 78 (63.9) |
| 14 | 29 (23.7) |
| 13 | 12 (9.8) |
| 12 | 0 (0) |
| 11 | 2 (1.6) |
| 10 | 1 (0.8) |
| Hunt-Hess scale, n (%) | |
| Grade I | 16 (13.1) |
| Grade II | 68 (55.7) |
| Grade III | 38 (31.1) |
| Grades IV and V | 0 (0) |
| Fisher scale, n (%) | |
| Grade 1 | 5 (4.1) |
| Grade 2 | 21 (17.2) |
| Grade 3 | 38 (31.14) |
| Grade 4 | 58 (47.5) |
| Etiology of SAHa, n (%) | |
| No findings | 35 (28.6) |
| Aneurysm | 83 (68) |
| Middle cerebral artery | 11 (13.2) |
| Anterior cerebral artery | 9 (10.8) |
| Posterior cerebral artery | 1 (1.2) |
| Anterior communicating artery | 27 (32.5) |
| Posterior communicating artery | 18 (21.6) |
| Posteroinferior cerebellar artery | 1 (1.2) |
| Common carotid artery | 6 (7.2) |
| Ophthalmic artery | 5 (6.1) |
| Vertebral artery | 1 (1.2) |
| Basilar artery | 4 (4.8) |
| AVM | 3 (2.4) |
| Others | 1 (0.8) |
| Definitive treatment, n (%) | |
| Endovascular | 46 (52.8) |
| Neurosurgical | 14 (16.1) |
| Both procedures | 7 (8.1) |
| None | 20 (23.0) |
| Complications, n (%) | |
| Repeat bleeding | 14 (11.4) |
| DCI | 21 (17.2) |
| Hydrocephalia | 16 (13.1) |
| Medical treatment, n (%) | |
| Nimodipine | 102 (82.9) |
| Triple H after DCI | 18 (85.7) |
| GOS, n (%) | |
| 1. Death | 16 (13.0) |
| 2. Vegetative state | 3 (2.4) |
| 3. Severe disability | 5 (4.1) |
| 4. Moderate disability | 15 (12.2) |
| 5. Good recovery | 83 (68.3) |
GCS, Glasgow Coma Scale; AVM, arteriovenous malformation; DCI, delayed cerebral ischemia; GOS: Glasgow Outcome Scale; Triple H (at least one of the following: hypertension, hemodilution, hypervolemia).
A total of 17.7% of the patients with vascular anomalies presented arteriographic vasospasm at the time of diagnosis. The time to embolization was 3.8±2.6 days and to surgery 9.6±9 days. Seventy-six patients were admitted to the ICU (62.2%). No antifibrinolytic agents or corticosteroids were used. Oral or intravenous nimodipine was administered to 102 patients (83.6%), and 99% received analgesics in the emergency room. During emergency care, antihypertensive medication was prescribed in 20 patients (16.3%).
In 18 patients with DCI (85.7%), at least one of the “triple H” therapy components (hypertension, hemodilution and hypervolemia) was present. Repeat bleeding in the course of hospital stay was recorded in 14 patients (11.4%). External ventricular drainage due to hydrocephalia was decided in 16 patients.
Cerebral ultrasound in the form of TCD was performed in all the patients. During the first 3 days we carried out a total of 146 TCD explorations. A high-velocity sonographic pattern was identified at some point during the clinical course in 38 patients (31.1%). Fourteen patients presented high MV values bilaterally, together with IL <3, attributable to cerebral hyperemia.
In 24 patients we obtained sonographic vasospasm patterns (SVPs) with high MV values unilaterally or bilaterally, together with IL elevation. All but one of the patients presented maximum MV >120cm/s (MV 177±45cm/s). The exception (maximum MV 112cm/s) presented moderate hydrocephalia not requiring ventricular drainage.
Three patients showed a sonographic vasospasm pattern without the ulterior appearance of DCI. The maximum MV was 171±41, with no statistically significant differences versus the maximum MV in the group with DCI.
A total of 21 patients presented DCI (17.1%). Prior to appearance of the neurological defect, all the patients developed a sonographic vasospasm pattern. The maximum MV in this group was 183±49cm/s, and the average day of appearance of the highest velocity value was day 10. The mean time to onset of sonographic vasospasm was 7±3.9 days. On excluding the patients with vasospasm following bleeding or ischemic complications, post-aneurysmal exclusion, the mean day of onset was found to be earlier (5.7±3). The mean velocity increment during the first 3 days after SAH, in the group of patients who developed DCI, was 22±5cm/s in 24h (Table 2).
Comparison of the transcranial cerebral Doppler ultrasound (TCD) recordings between the patients with sonographic vasospasm pattern (SVP) and those with a normal Doppler pattern.
| TCD pattern | DCI | n | MV (SD) cm/s | IL | ΔMV/day (SD) cm/s |
| SVP | Yes | 21 | 183 (49)* | >3 | 22 (5)** |
| No | 3 | 171 (41)* | >3 | ||
| Normal | No | 84 | 67.98 (16.6)* | n.a. | 8.3 (4.5)** |
DCI, delayed cerebral ischemia; n.a., not applicable; SD, standard deviation; MV, mean velocity.
Five of the 21 patients with DCI and sonographic vasospasm arrived in hospital after the third day following SAH (mean 6 days, range 4–11 days). All had initial MV values in the middle cerebral artery (MCA) of >120cm/s (mean 217±48cm/s). These findings were independent of the age of the patients. Two patients presented DCI on the day of admission. In the remaining three subjects sonographic vasospasm preceded DCI by a number of days.
A total of 84 patients presented no DCI or high-velocity pattern due to vasospasm or other causes. Nevertheless, in this group we identified two patients with arteriographic vasospasm. The maximum MV in any MCA and day of evolution was 67.98±16.6cm/s. The difference with respect to the maximum MV in the group with sonographic vasospasm proved statistically significant (p<0.001). The increase in velocity/24h in the group with a normal pattern in the TCD registry was 8.30±4.5cm/s. The difference in the increase in velocity/24h, during the same period, versus the group presenting DCI and sonographic vasospasm was significant, both on including (p<0.01) and excluding the postoperative vasospasms (p<0.001).
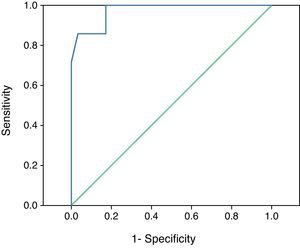
In the ROC curve analysis, in order to establish the velocity increment that best identified the patients at risk of DCI, we included 88 TCD explorations corresponding to those patients with at least two ultrasound registries in the first 72h of evolution and with no ulterior development of hyperemia. We found that the MV increments in the MCA could differentiate those patients at risk of suffering DCI (area under the curve (AUC): 0.973, p<0.001) (Fig. 1). On analyzing the coordinates, we found the best cutoff value for the MV increment in the MCA during the first three days to be 21cm/s, with a sensitivity of 85.7%, a specificity of 96.6%, a positive predictive value of 85.7%, and a negative predictive value of 97.2%.
DiscussionOur results indicate that an increase in mean velocity of 21cm/s/24h in the MCA, in the first 3 days after SAH is significantly correlated to the development of symptomatic vasospasm.
The maximum MV recorded in the MCA of our patients with symptomatic vasospasm exceeded 120cm/s in all cases and in a homogeneous manner in all age intervals, except in one patient with hydrocephalia-buffered velocity readings. It is therefore not necessary to use other lower cutoff points, such as normal mean velocity for the age plus two standard deviations, which complicate the calculations without affording any advantages.25,33
Our study specifically explores the usefulness of TCD in predicting the development of symptomatic vasospasm in patients with SAH and in good neurological condition, during the first 72h after the bleeding event, since upon admission to the emergency room it is necessary to determine which patients regarded as mild cases according to the HH clinical score should receive more exhaustive follow-up during critical care–as it is done with HH scores 4–5. Our results not only confirm the usefulness of the increase in MV in the MCA as a predictor of DCI, but also the need to use IL for adequate identification of the ultrasound patterns.34
Those authors describing a low predictive value of TCD offer a series of arguments. In the pre-vasospasm period, covering the first 72h after SAH, the MV values in the MCA and anterior cerebral artery (ACA) are usually within normal limits. Consequently, considering the absolute values of MV in the MCA during the early phase of SAH would be of no use.17,25,26 In contrast, other authors consider that although there is no close correlation between elevated MV in the MCA and the appearance of DCI, the flow velocity increment/day must be taken into account.21,26 Accordingly, a rapid increase in flow velocity of 50cm/s in 24h would be predictive of the ulterior appearance of clinical manifestations of vasospasm,24,26 though the cited MV increment in the anterior vascular territory, while compatible with our present and previous findings,34 is manifestly greater. Nevertheless, the clinical heterogeneity of the patients included in most such studies, with no distinction of severity levels, causes the detected velocity increment prior to symptomatic vasospasm to be non-comparable with our own results. It should be noted that the mentioned increment was only evaluated in relation to the 24h preceding the development of symptomatic vasospasm, not during the first days after SAH. Likewise, the authors made no distinction between MCA and ACA–a fact that could generate alterations in the recorded mean values, due to the mixing of vessels with different velocities and inter-communication systems (ACA)–and no mention was made of the presence or absence of arteriovenous malformations that would alter the flows and ultrasound recordings.
Due to the significant morbidity-mortality (50%) associated to SAH, and the important probability of clinical deterioration, early treatment of the acute neurological and medical complications is essential. In this sense, TCD is a key tool for the management of these patients. Accordingly, the American Academy of Neurology (AAN) recommends TCD for the diagnosis and follow-up of vasospasm, with level of evidence type A, class II, and the technique has been included on a routine basis in the clinical practice guides.35,36 The sensitivity of TCD in detecting symptomatic vasospasm has been found to be comparable to that of angiography.29 Nevertheless, during the vasospasm period (days 4–14), many patients with DCI show normal MV values in MCA (false negative results). Furthermore, a considerable number of patients with ultrasound values indicative of vasospasm (>120cm/s) never develop DCI (false positive results).25,26 As an explanation for the absence of a statistically significant correlation between MV in MCA and DCI in this stage, it has been postulated that the false positive cases may be characterized by increased collateralization of the cerebrovascular tree, which would avoid ischemia thanks to the blood supply from non-spasmodic vessels, and by the impact of other factors such as the presence of epileptiform activity, for example. In the false negative cases, DCI would depend on the narrowing of vessels (arterioles) not amenable to ultrasound evaluation. In addition, in neurologically affected patients, intracranial hypertension would buffer the velocity values, producing false normalization. In concordance with the recommendations of the AAN,35 the patients in our series with velocities >120cm/s presented a high probability of developing symptomatic vasospasm.
The maximum MV in MCA recorded in our patients with symptomatic vasospasm exceeded 120cm/s in all cases, independent of the age interval. Nevertheless, we wish to point out that the diagnostic reliability of TCD in detecting vasospasm only from MV in MCA is low.31,37 Using only this value would produce false positive results due to the inclusion of hyperemia and hyperperfusion phenomena. However, use of the full definition of sonographic vasospasm, IL >3 in patients with elevated MV in MCA, offers high specificity (94–100%) in detecting vasospasm in MCA.38 In our series, maximum MV >120cm/s in MCA and IL >3 allowed us to identify 100% of the patients who ulteriorly developed DCI secondary to vasospasm from among the individuals with elevated MV values.
The aneurysmal SAH rate in our series was similar to the rates described in other European series, with values of 75–80%.1 The large volume (48%) of patients with Fisher grade 4 probably can be attributed to strict adherence to the tomographic grading protocol. In our case, in the presence of blood in the ventricular system (independently of the amount), we assigned the maximum Fisher grade, even if the amount of blood evidenced in the subarachnoid space was small. Application of the modified Fisher scale39 probably would have allowed more specific grading of the tomographic lesions in our sample.
The severity scales used to date for classifying patients with SAH are not only mainly clinical, and afford global prognostic information on the patients, but also provide an estimate of the risk of developing vasospasm.11,12,14 However, despite the great interest in the use of such scales, they do not allow continuous evaluation of the patients. Follow-up using TCD would increase the capacity to identify those individuals who may develop late vasospasm despite a good initial post-bleeding condition. In our study, it must be underscored that the initial clinical condition of the patients was good. The non-inclusion of patients with SAH corresponding to HH grades IV and V causes our results to center on patients in whom the usual clinical scales are less useful, since such individuals do not always evolve favorably, due to the appearance of symptomatic vasospasm.12
Thus, according to our results, TCD during patient management in the emergency room or in equivalent settings (intermediate care units, etc.) would allow us to identify those patients with SAH without neurological deterioration who are at risk of developing symptomatic vasospasm, and would allow us to establish the following classification: high risk group and low risk group. The high-risk group would include those patients with an MV increment in MCA >21cm/s/24h during the first 72h post-SAH, and patients with MV in MCA >120cm/s and IL >3 from day 4 onwards. In turn, the low risk group would comprise those patients with no substantial MV increment/24h in MCA during the first 3 days after SAH, or with normal MV readings from day 4 onwards. Therefore, during the first 72h after SAH, patients admitted to observation or equivalent areas should be subjected to two TCD studies. Once identified, the patients classified as being at high risk should be prioritized for emergency diagnostic-therapeutic arteriography. These individuals should be admitted to the ICU or to intermediate or semi-critical care units for exhaustive vigilance, with the introduction of aggressive prophylactic treatment against brain ischemia secondary to vasospasm (“triple H”: hemodilution, hypervolemia, hypertension). This strategy possibly could reduce the incidence of DCI and its sequelae. In turn, those patients identified as presenting low risk could be moved to a conventional hospitalization ward.
Positive selection in the first days after SAH of those patients at risk of developing DCI, even where presentation is late, would moreover avoid inefficient use of the limited beds available in the ICU.
Our study has some limitations. Firstly, the clinical condition of the patients, with a practically intact level of consciousness, would explain the existence of higher MV values in MCA compared with neurologically impaired, sedated and mechanically ventilated patients24,26,38 However, this circumstance did not affect the high velocity cutoff point defined as 120cm/s. Secondly, we consider that there are other recently introduced techniques such as color duplex transcranial ultrasound that allow two-dimensional (2-D) ultrasound evaluation of the brain, color visualization of the arteries, and their Doppler spectral analysis.40 In our center the technique used is blind TCD, which requires adequate training both for obtaining the registries and for interpreting them. Although the former technique may prove simpler, in relation to the localization and identification of the arteries of the circle of Willis, our team is highly qualified and can perform and evaluate the results of TCD in a precise manner–a circumstance that may not be extendable to other centers lacking specifically trained personnel.36 As a third limitation, our ultrasound studies were exclusively centered on the middle cerebral arteries, due to their greater accessibility, as a preliminary phase in the investigation of the usefulness of TCD as a predictor of sonographic post-SAH vasospasm in other vascular territories. The rest of the circle of Willis, while subjected to ultrasound evaluation to the effects of clinical follow-up, was not taken into account in the present study. It should be mentioned that the velocity increments recorded in MCA in our series were only obtained from patients arriving in hospital in under 3 days after the bleeding episode, and in whom two TCD explorations could be performed. Lastly, the data collected and analyzed correspond to a single center. A broader study involving other hospitals therefore would be needed to validate our findings.
As strengths of our study, mention should be made of the considerable number of patients included and their clinical homogeneity–all being in good neurological condition after the bleeding episode, or after CSF evacuation in those individuals with secondary symptomatic hydrocephalia. Another aspect worth mentioning is the exhaustive patient follow-up made, allowing the early detection of DCI despite presentation in the late evolutive phase.
ConclusionsThe anticipation of ischemic complications due to vasospasm can be established by monitoring the evolution of cerebral blood flow velocity in SAH patients in good neurological condition. In this group of patients, an MV increment in the TCD registry of 21cm/s/24h in the MCA during the first 3 days of evolution, or the detection of MV >120cm/s with IL >3, allows us to identify those patients at risk of developing symptomatic vasospasm over the following days.
Financial supportThis study has been supported by a grant from the Consejería de Salud de Andalucía 2002 (Dossier reference 30/01).
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Muñoz-Sanchez MA, et al. Ultrasonografía doppler transcraneal urgente: utilidad predictiva del vasoespasmo sintomático en la hemorragia subaracnoidea espontánea en pacientes con buena situación neurológica. Med Intensiva. 2012;36:611–8.