The benefit of enteral nutrition in critically ill patients has been demonstrated by several studies, especially when it is started early, in the first 24–48h of stay in the Intensive Care Unit, and this practice is currently advised by the main clinical guidelines. The start of enteral nutrition is controversial in patients with hemodynamic failure, since it may trigger intestinal ischemia. However, there are data from experimental studies in animals, as well as from observational studies in humans that allow for hypotheses regarding its beneficial effect and safety. Interventional clinical trials are needed to confirm these findings.
El beneficio de la nutrición enteral en el paciente crítico ha sido demostrado en varios estudios, especialmente si esta es iniciada precozmente, en las primeras 24-48h de ingreso en la Unidad de Cuidados Intensivos, y en la actualidad esta práctica es recomendada por las principales guías de práctica clínica. El inicio de nutrición enteral en el paciente crítico con inestabilidad hemodinámica es una decisión controvertida, fundamentalmente debido al potencial riesgo de isquemia intestinal asociado a su empleo. Sin embargo, existen datos procedentes de estudios animales y de estudios observacionales en humanos que permiten plantear la hipótesis sobre su efecto beneficioso y seguridad. Son necesarios ensayos clínicos de intervención que establezcan una relación causa-efecto.
- -
To understand the physiopathology of the gastrointestinal tract in the critical patient with hemodynamic instability and the potential benefits of enteral nutrition (EN).
- -
To review the available evidence on the benefits and risks of EN in patients of this kind.
- -
To summarize the recommendations of the main clinical practice guides.
- -
To offer practical ideas and the point of view of a group dedicated to research in this field.
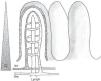
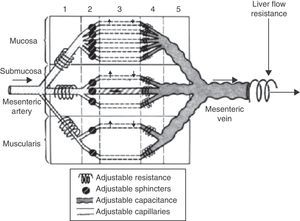
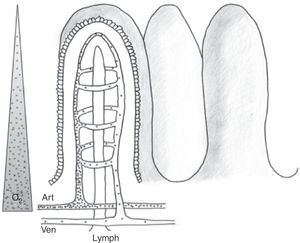
The physiology of the splanchnic circulation is complex, but has been known for some years, and a basic understanding of its characteristics is essential in order to correctly interpret the alterations that occur in critical patients with hemodynamic instability. Under resting and normal conditions, 20–25% of cardiac output is located in the splanchnic circulation. In turn, the metabolic activity in this region accounts for 30% of overall body oxygen consumption. When eating, the blood flow in this zone can double thanks to a phenomenon known as postprandial hyperemic response. This response is basically mediated by local factors, giving rise to notorious splanchnic vasodilatation.1,2 The anatomical configuration of the intestinal microvascularization is complex, with arterial and venous plexuses at mucosal and submucosal level, and in the muscularis propria. This system is able to redistribute blood flow in the case of decreased intravascular volume within the systemic circulation, allowing precise regulation of splanchnic blood flow (Fig. 1). In the intestinal villi, the arterial and venous vessels run parallel to each other, though with flow in opposite directions. This anatomical distribution allows the direct passage of low-molecular weight molecules (such as oxygen) from the arterial to the venous circulation, conforming a “countercurrent” exchange mechanism. Although the latter is not relevant under physiological conditions, in the presence of hypoperfusion it generates a decreasing tissue oxygen pressure gradient from the base to the tip of the villus (Fig. 2). As a result, the tip of the intestinal villus is particularly sensitive to tissue hypoxia.
Intestinal microvascularization (i). Schematic representation of the anatomical distribution of the intestinal microvascularization. Regulation can be made at several levels (precapillary, capillary and postcapillary), thus allowing blood flow redistribution.
Intestinal microvascularization (ii). Schematic representation of the anatomical distribution of the intestinal microvascularization (artery, vein and lymphatic vessel) at villus level. The passage of low-molecular weight molecules (dots) and the countercurrent mechanism are shown. The triangle at left represents the oxygen concentration gradient from villus base to tip.
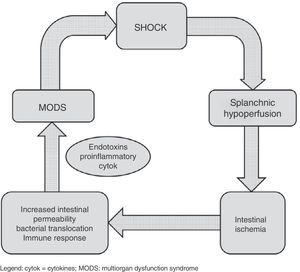
Shock is characterized by blood flow redistribution with vasoconstriction at splanchnic circulatory level and in peripheral tissues, in an attempt to maintain crucial brain and coronary perfusion.4 This can give rise to an imbalance in the oxygen supply/demand ratio at intestinal level, with resulting ischemia. This situation in turn leads to depletion of the cellular ATP reserves, with rupture of the tight junctions between the epithelial cells. Consequently, the solute concentration gradients between the apical and basolateral compartments are lost, producing intracellular edema, necrosis and apoptosis. The end result of this alteration of the intestinal microcirculation is rupture of the intestinal epithelial barrier, favoring bacterial translocation phenomena. Such persistent alteration of the microcirculation in shock patients5 can perpetuate the proinflammatory response and favor progression toward multiorgan dysfunction syndrome, which in turn results in increased splanchnic hypoperfusion – thereby completing a vicious circle that can increase the probability of a fatal outcome (Fig. 3). In addition, in this complex scenario, different patient interventions such as vasoactive and inotropic support,6 mechanical ventilation or the use of hemodynamic support systems, act upon the splanchnic circulation, with consequences that are hard to predict. Considering the above, nutritional support in these patients becomes a genuine challenge. From a conceptual and physiopathological point of view, enteral nutrition (EN) administered to the critical patient with hemodynamic instability would induce an increase in the need for oxygen at intestinal level and in splanchnic blood flow due to vasodilatation at this level. In this respect, predominance of an increased oxygen demand would predispose to intestinal ischemia, while an increase in splanchnic perfusion could counter the microcirculatory alteration found in patients in shock.2,7
Benefits of enteral nutrition in the unstable patientIn the last 15 years experimental models in animals and also human clinical studies have produced evidence of a beneficial effect of EN in the critical patient with hemodynamic instability.
Animal modelsThe existing body of information is large, and comprises models of hemorrhagic shock, septic shock, burns and ischemia-reperfusion.2 Most of these studies offer similar results referred to the alterations at intestinal microcirculatory level induced in situations of hemodynamic instability, and to the effects of EN. Such alterations persist despite normalization of the “classical” hemodynamic parameters such as mean arterial pressure, following initial resuscitation. There are a number of reasons for this: increased concentrations of endogenous vasoconstrictors (e.g., endothelin I or angiotensin II), a decrease in vasodilators (e.g., nitric oxide), and ischemia-reperfusion damage at microcirculatory level.8 It has been demonstrated that the decrease in splanchnic blood flow is associated to ischemic damage to the intestinal mucosa, bacterial translocation and multiorgan dysfunction.9,10 The great majority of the experimental studies offer results in the same line: EN is adequately tolerated, without the induction of feared intestinal ischemia. In fact, due to the hyperemic response induced by EN, the latter is able to revert splanchnic ischemia11,12 and even lessen bacterial translocation phenomena.13,14 In contrast, some studies involving the induction of extreme intestinal hypoperfusion (complete obstruction of the superior mesenteric artery) have found the start of EN to worsen splanchnic ischemia.15
All these studies have provided detailed insight to the physiopathology of splanchnic circulation in shock patients and have contributed to postulate the beneficial effects of EN. However, there are methodological and clinical applicability limitations that restrict the usefulness of the results and the drawing of conclusions.
Clinical studies in patientsThe great majority of existing clinical studies in humans are of an observational nature and involve patients in the postoperative period of heart surgery. This fact conditions interpretation of the results, and although associations can be identified, no firm causal relationships can be established. Table 1 summarizes the most important findings of the main studies published to date in relation to EN support in the critical patient with hemodynamic instability. In the present review mention will be made only of the most relevant studies. In the year 2005, the group led by Chiolero published its experience with the utilization of EN in 70 post-heart surgery patients, based on a prospective observational study.16 All the patients presented hemodynamic instability, and 18 moreover required mechanical support such as intraaortic balloon counterpulsation. Based on previous studies of the energy requirements of patients of this kind, involving indirect calorimetric measurements, a calorie target of 25kcal/kg body weight and day was established, to be reached over 4–6 days. Enteral nutrition, started in the first 2–3 days of admission to the Intensive Care Unit (ICU) according to a previously established protocol with prudent and progressive escalation of the energy supply in the first days of EN, was not found to be associated to serious complications such as intestinal ischemia. However, the energy supplied was variable (mean 1360±620kcal/day, corresponding to 70±35% of the target level). In fact, the energy balances were clearly negative. Another interesting finding was the fact that energy provision decreased as the noradrenaline and dopamine doses increased. The main conclusions of this study have been recently reproduced by an independent research group17 that prospectively assessed early EN (started in the first 48h of stay in the ICU in accordance with a previously established EN protocol) in 37 critical patients subjected to heart surgery and with hemodynamic instability. All the included patients required vasoactive drug support in the first 48h of admission, and 11 needed intraaortic balloon counterpulsation, mechanical circulatory assistance and/or venoarterial extracorporeal membrane oxygenation due to refractory cardiogenic shock. The energy target was 25kcal/kg/day, to be gradually reached by day four of EN. The conclusions were analogous to those of the group of Chiolero: early EN proved feasible in these patients and was not associated to serious complications. However, EN alone was unlikely to reach the nutritional objectives. Indeed, the energy targets were only reached in 40% of the patients, and the cumulative energy balances–particularly from day 7 of stay in the ICU–were manifestly negative. These observations point to the potential indication of complementary parenteral nutrition in these patients. In contrast to the study of Chiolero, no clear association was observed between the enteral energy supply and the administered vasoactive drug dose. Regarding the complications, and although no cases of intestinal ischemia were detected, the frequency of complications associated to EN was high (62% of the patients)–the most frequent problem being constipation (46%), followed by diarrhea (27%). Most of these complications were mild, and in only 24% of the cases was temporary EN suspension required. Both studies underscored the need for an EN protocol, with careful control of the energy supplied and of the energy balance, as well as close monitoring of the alarm signs of intestinal ischemia.
Summary of the main studies in critical patients with early enteral nutrition and hemodynamic instability.
| Author | Year | Design | n | Patients included | Main conclusions | Complications |
|---|---|---|---|---|---|---|
| Berger et al.40 | 2000 | Observational, prospective | 45 | POHS (23 with hemodynamic instability). Six healthy controls | Intestine maintains function and absorptive capacity | Not described |
| Revelly et al.41 | 2001 | Observational, prospective | 9 | POHS. All required vasoactive drug (doses not described) | Hemodynamic improvement after start of early EN | Not described |
| Kesek et al.42 | 2002 | Observational, prospective | 73 | POHS or chest surgery. Hemodynamic situation not described | Early EN adjusted on an individualized basis is feasible, with few complications | No serious complications observed |
| Berger et al.16 | 2005 | Observational, prospective | 70 | POHS. All hemodynamically unstable. Eighteen required IAoBC | EN proved possible, though supply was usually insufficient | No serious complications observed |
| Lukas et al.43 | 2010 | Observational, retrospective | 48 | Patients with ECMO (35 VA and 13 VV due to respiratory failure). Thirty-nine with hemodynamic instability | EN proved possible in patients with ECMO. Only 55% of the nutritional target was reached | No serious complications observed |
| Khalid et al.18 | 2010 | Observational, retrospective | 1.174 | Clinical patients with vasoactive drug requirements | Lesser mortality in the early EN group. Greater effect with greater severity and number of vasopressor drugs | Not described |
| Rai et al.44 | 2010 | Observational, retrospective | 43 | Septic patients. Thirty-three with septic shock. APACHE II 20±8. Vasoactive drug doses not described | Despite slowing of gastric emptying, EN according to protocol should be considered in septic shock | Not described |
| Umezawa Makikado et al.20 | 2013 | Observational, prospective | 7 | POHS with severe hemodynamic failure and need for VA ECMO | Early EN is feasible and safe under adequate medical supervision | No serious complications observed |
| Ferrie et al.19 | 2013 | Observational, retrospective | 86 | Thirty-one patients with VA ECMO. Fifty-five patients with VV ECMO | 79.7% of energy target reached. Eighteen patients required complementary PN | No serious complications observed |
| Flordelis-Lasierra et al.17 | 2013 | Observational, prospective | 37 | POHS with hemodynamic instability | Early EN is feasible. The scope of the nutritional target is difficult to establish | No serious complications observed |
| Mancl and Muzevich27 | 2013 | Observational, retrospective | 259 | 48% septic shock, 23% cardiogenic shock | Most patients receiving EN and vasopressors tolerate EN | 3 cases (0.9%) of bowel perforation/intestinal ischemia |
APACHE: Acute Physiology and Chronic Health Evaluation; IAoBC: intraaortic balloon counterpulsation; ECMO: extracorporeal membrane oxygenation; EN: enteral nutrition; PN: parenteral nutrition; POHS: postoperative period of heart surgery; ARDS: acute respiratory distress syndrome; NGT: nasogastric tube; VA: venoarterial; VV: venovenous.
In the year 2010, Khalid et al. published a retrospective analysis18 fundamented upon a large database of the Society of Critical Care Medicine (IMPACT Project). The data were entered in a prospective and multicenter basis. A total of 1174 critical patients subjected to mechanical ventilation during at least 48h and with hemodynamic instability requiring vasoactive medication were included. The most frequent cause of admission was severe respiratory failure, followed by sepsis. The patients were divided into two groups: early EN (started in the first 48h of mechanical ventilation) and late EN. The study excluded those patients with an absolute or relative contraindication to EN at the time of admission: gastrointestinal obstruction or bleeding, ileus, gastroparesis, acute pancreatitis, peritonitis, ischemic colitis or esophageal rupture. Patients receiving parenteral nutrition before intubation were also excluded. A multivariate statistical analysis was performed, adjusting for confounding variables, and a survival analysis was carried out to evaluate the effect of EN upon patient mortality. The early EN group showed significantly lesser mortality and a shorter ICU stay than the late EN group, after adjusting for variables such as the severity scores or patient age in the multivariate analysis. The effect was greater in those patients with greater hemodynamic instability–this being understood as a greater need for vasopressor treatment (number of drugs and duration of vasopressor therapy). The study design (observational, retrospective and without randomization to the respective study groups) did not allow the drawing of conclusions referred to causality, and the results must be interpreted with caution.
Two case series have been published in the last year: a retrospective series involving 31 patients, and a prospective study of 7 patients. Early EN was prescribed, defined as starting in the first 24–48h of admission to the ICU, in critical patients with refractory cardiogenic shock requiring mechanical assistance with venoarterial extracorporeal membrane oxygenation as a bridge to heart transplantation or as a bridge to recovery.19,20 Once again, EN was provided according to a pre-established protocol. Both series came to the conclusion that early EN is possible, and is not associated to serious complications.
All these studies are of an observational nature, which implies limitations in design and the need for cautious interpretation of the results. An interventional clinical trial is needed to assess the efficacy and safety of EN in critical patients with hemodynamic instability, with a view to confirming the hypotheses raised.
Risks of early enteral nutritionThe most feared complication of EN support in the hemodynamically unstable patient is intestinal ischemia, particularly of a non-occlusive nature, produced as a consequence of increased oxygen consumption in a proportion not adjusted to the increase in splanchnic blood flow, with consequent alteration of the supply/demand ratio at intestinal mucosa level. This is an infrequently described complication, though the associated mortality rate is high: between 11 and 27% in the postoperative period of heart surgery.21 Little is known of the incidence of intestinal ischemia. A retrospective study based on a registry of 4311 critical trauma patients22 reported an incidence of 0.3%, with an associated mortality rate of 44%. The condition was most often detected in the second week of admission to the ICU. In an attempt to identify a “risk profile” for the development of intestinal ischemia, the authors evaluated a series of clinical and laboratory test parameters, and came to the conclusion that none of them was sufficiently specific. Furthermore, some parameters, such as abdominal bloating, were of late onset. Thirty-one percent of the cases had significant abdominal trauma. The study did not specify the precise moment in which EN was started: the authors only indicated that EN began after the initial resuscitation phase. Likewise, no mention was made of whether EN was administered according to an established protocol in all cases, or of the hemodynamic situation of the patients at the time of starting EN support.
In recent years there have been reports of non-occlusive intestinal ischemia after EN, particularly following the use of postpyloric feeding tubes and with the utilization of nutritional formulas rich in non-fermentable fiber.23 However, in most of these studies EN was not provided on an early basis, and important details are missing, such as the hemodynamic situation of the patients at the start of nutritional support or the way in EN was provided, and daily monitoring of the clinical condition of the patients.24
Daily and careful monitoring of the possible alarm signs of intestinal ischemia in these high risk patients is of crucial importance. Such signs can be of a clinical, laboratory or radiological nature (Table 2). None of them are sufficiently sensitive or specific to establish a firm diagnosis, though their presence should cause us to consider temporary suspension of EN support until this serious complication has been ruled out. Clinical signs such as increased gastric residue, a rise in intraabdominal pressure to over 15mmHg (particularly when associated to recent oliguria), or sudden worsening of the hemodynamic situation of the patient, should be regarded as possible indicators of intestinal ischemia. Laboratory test measurements such as lactacidemia or leukocytosis can be useful, though they tend to manifest late in the context of intestinal ischemia. Regarding the use of imaging techniques, the presence of dilated and thickened bowel loops on the abdominal X-rays, intestinal pneumatosis, or the presence of air in the portal vein or peritoneal cavity, should cause us to suspect this complication. Techniques such as Doppler ultrasound, magnetic resonance angiography and arteriography are very useful for the diagnosis of occlusive intestinal ischemia, but their role in non-occlusive intestinal ischemia–which is more prevalent in critical patients with hemodynamic instability–is more limited.25 The introduction of new multi detector computed tomography systems may help confirm the diagnosis.26
Alarm signs of intestinal ischemia.
| Classification | Signs | Comments |
|---|---|---|
| Clinical | Gastric residue >500ml | |
| Abdominal bloating | ||
| Intense abdominal pain | Less reliable in sedated patients | |
| Intraabdominal pressure >15mmHg | ||
| Ileus | Nonspecific, late | |
| Oliguria | Nonspecific, late | |
| Shock | ||
| Laboratory | Hyperlactacidemia | All nonspecific, and often late |
| Metabolic acidosis | ||
| Leukocytosis | ||
| Radiological | Without significant alterations (20–25%) | All nonspecific. May require patient transfer outside the ICU. Multidetector CT may be useful for diagnostic purposes. MR angiography, Doppler ultrasound and angiography contribute information in occlusive ischemia. Less useful in non-occlusive intestinal ischemia |
| Intestinal pneumatosis | ||
| Free fluid | ||
| Dilated and thickened bowel loops | ||
| Air in portal vein or pneumoperitoneum |
CT: computed tomography; MR: magnetic resonance imaging; ICU: Intensive Care Unit.
Table modified from the book chapter published by Umezawa Makikado et al.45
Mancl and Muzevich27 have recently published a retrospective analysis of 259 critical patients requiring vasoactive drugs and the concomitant administration of EN. Although positive conclusions were drawn regarding the tolerance of EN on the part of the patients, the authors described three cases of mesenteric ischemia/intestinal perforation. This frequency (0.9%) is considered to be similar to that of spontaneous intestinal perforation described in the critical patient receiving EN. The description of these three cases may be useful for knowing the clinical characteristics associated to the development of non-occlusive mesenteric ischemia or intestinal necrosis associated to EN. The study indicates that a specific EN protocol was not applied in all the Units; instead, multidisciplinary management was provided in accordance with the current clinical practice guides. This may have resulted in variability among different professionals in starting nutritional support and in monitoring its efficacy and safety. In fact, substantial differences are noted regarding the way in which EN was provided among the different patients. Likewise, no definition is provided of the different complications or monitored clinical parameters, or of the frequency with which they were monitored. On the other hand, in the Discussion section of their article, the authors pointed out the need to implement a specific EN protocol in their center. As data requiring special consideration, it should be mentioned that in the first described case of non-occlusive intestinal ischemia, full-dose EN was started and was not interrupted upon detecting abdominal bloating. In this regard, it should be remembered that abdominal bloating in these patients is an alarm sign, particularly when accompanied by an intraabdominal pressure of over 15mmHg. This should have led to the suspension of EN. Furthermore, given the previously described high risk circumstances, EN support, in concordance with the previously cited prospective studies,16,17 should be started on a gradual and stepwise basis – thereby allowing closer monitoring of patient tolerance, and its immediate interruption if any alarm sign is detected. Likewise of note in the second described clinical case is the fact that nutritional support was not interrupted despite sudden and significant worsening of the hemodynamic situation of the patient following good initial tolerance of EN.
In conclusion, the diagnosis of non-occlusive intestinal ischemia is complex and must be based on the combination of strong clinical suspicion and the laboratory test results and imaging study findings.28
Review of the clinical practice guidesThe current clinical practice guides are not sufficiently clear in addressing the start of EN in critical patients with hemodynamic instability. The guides published by the American Society for Parenteral and Enteral Nutrition,29 and those published by the European Society of Parenteral and Enteral Nutrition,30 state that EN should not be started in hemodynamically unstable patients until complete resuscitation has been achieved and/or the patients are hemodynamically stable. These recommendations are based on the results of non-randomized clinical studies, studies with historic controls, case series and expert opinions. Furthermore, the definition of hemodynamic stability is not addressed in detail –a fact that reduces the practical applicability of the recommendations. The Canadian guides31 in turn recommend the early introduction of EN in the critical patient, with reference to the study published by Khalid et al. in 2010,18 but do not clearly specify the approach to hemodynamically unstable patients.
The guides published by the Spanish Society of Intensive Care and Coronary Units (SEMICYUC) and by the Spanish Society of Parenteral and Enteral Nutrition move one step further in their recommendations compared with the rest of the guides. In effect, they recommend the start of EN in the septic patient32 following resuscitation or, at least, once a state of “stable shock” has been reached, with adequate perfusion pressure (stabilized vasoactive drug doses, stabilized and/or decreasing metabolic acidosis and lactate levels, and a mean arterial pressure of ≥60mmHg). These guides moreover indicate that close monitoring for signs of intestinal intolerance is required. Regarding cardiac patients,33 they describe that when cardiac function is greatly impaired, the introduction of EN is possible, though because of caution due to the risk of intestinal ischemia, it is usually postponed beyond 24–48h. In both cases, the recommendations are based mainly on expert opinions.
Apart from the main clinical practice guides described above, it should be mentioned that the review published by Turza et al.34 in 2009 explored a practical approach to EN support in patients of this kind, following four steps: evaluation of the case history and nutritional antecedents of the patient; evaluation of the current physiological situation of the patient; organization of the logistics for starting EN; and clinical monitoring once EN has been started. Likewise, a very recent review has examined EN support in patients with extracorporeal membrane oxygenation.35
Practical issuesBased on the experience gained from their different studies, the authors of the present review consider it interesting to describe the way in which EN support was started in their post-cardiac surgery patients, together with the monitoring of efficacy and safety, with a view to offering readers some orientation in this respect. The EN protocol of the Department of Intensive Care Medicine of our center was followed, and was required to be known by the medical, nursing and auxiliary personnel. The mentioned protocol detailed the way to start EN, as well as daily monitoring of the different complications associated to EN. The readers are invited to consult the critical care nutritional interventional algorithms published by the SEMICYUC for further information.36
What route should be used for starting enteral nutrition?As a general rule, EN support was provided via the nasogastric route.
What calorie target should be established?Based on previous studies, we established a calorie target of 25kcal/kg body weight and day. However, for patients with a body mass index (BMI) of under 20kg/m2, the target was calculated as 25kcal/kg of ideal weight. In turn, in the case of patients with BMI >30kg/m2, we considered 25kcal/kg of ideal weight plus 30%.37
How should enteral nutrition be started?Enteral nutrition was started after checking patient tolerance of liquids. To this effect, 100ml of water was administered via the nasogastric tube at three-hour intervals. In the presence of a gastric residue of less than 200ml on two consecutive attempts, liquid tolerance was considered to be positive. We then started a standard EN formula, with progressive increments of 25% until reaching 100% of the target calorie supply by day four of EN. The gastric residue was monitored every 6h on the first day of EN, every 12h on the second day, and daily over the following days. In order to minimize the risk of aspiration and ventilator associated pneumonia, the patient headrest was kept raised more than 30 degrees during the administration of EN.
When should enteral nutrition be started?According to the latest guidelines of the SEMICYUC,32 EN should be started after the patient resuscitation phase, when stabilization of the vasoactive and inotropic drug doses, and of the signs of hypoperfusion, has been achieved.
How should enteral nutrition efficacy be monitored?As an orientation, and taking into account that the scope of this issue falls beyond the aims of the present review, the authors used the energy balance, calculated as the difference between the calories supplied and the target calories, on a daily basis and accumulated in the course of patient admission. Likewise, we calculated nutritional tolerance as the ratio between the calories supplied and the target calories. Based on these efficacy results, the advisability of complementary parenteral nutrition should be assessed individually.
How should enteral nutrition safety be monitored?A protocolized definition of the main complications of EN should be established: increased gastric residue, abdominal bloating, diarrhea associated to EN, diet aspiration, etc. Emphasis must be placed on the importance of monitoring for alarm signs of intestinal ischemia.
Key ideas
- -
The indication of early EN support in the critical patient with hemodynamic instability is subject to controversy.38
- -
There is evidence from experimental studies in animals and observational studies in humans (mostly in the post-heart surgery context) that allows us to hypothesize about its beneficial effects, tolerance and safety.37
- -
To date, no interventional studies in humans have been carried out with a view to determining a possible cause-effect relationship.
- -
Due to their physiopathological characteristics, hemodynamically unstable critical patients are at a high risk for intestinal ischemia. This complication is serious and involves important mortality.
- -
The application of predefined protocols in EN is essential, with prudent and progressive initiation of the energy supplied, and close monitoring of the alarm signs (clinical, laboratory and radiological) of intestinal ischemia.
- -
The scope of the nutritional needs with EN alone in patients of this kind is difficult to establish. Daily monitoring of the calories supplied and of the energy balance is therefore crucial. The role of complementary parenteral nutrition39 in these patients has not been defined to date.
The authors declare that they have no conflicts of interest. The instructions for authors and ethical responsibilities have been taken into account.
The authors thank the personnel of the Cardiological ICU of the Department of Intensive Care Medicine (Doce de Octubre University Hospital) for their contribution to this study. Thanks are also due to Aurora Ruiz for her help in drafting Fig. 2.
Please cite this article as: Flordelís Lasierra JL, Pérez-Vela JL, Montejo González JC. Nutrición enteral en el paciente crítico con inestabilidad hemodinámica. Med Intensiva. 2015;39:40–48.