To evaluate the effects of esophageal pressure monitoring in adult patients with mechanical ventilation requirements in the Intensive Care Unit.
DesignA systematic review (PROSPERO Register CRD42018118583) was carried out.
SettingIntensive therapy. Critical care.
Patients or participantsAdults with mechanical ventilation requirement in the Intensive Care Unit.
InterventionsEsophageal pressure monitoring.
Outcomes of interestIntensive Care Unit mortality and length of stay, mechanical ventilation days, adverse events.
ResultsFour studies with 301 participants were included. Esophageal pressure monitoring during mechanical ventilation had little or no effect on mortality in intensive care at 28 days (RR 0.74; 95% CI 0.31–1.76; participants 261; studies 2; I2: 68%), with little or no differences in ICU length of stay (MD 0.48; 95% CI −1.90 to 2.85; participants 284; studies 3; I2: 7%) or impact upon adverse events (RR 0.74; 95% CI 0.50–1.09; participants 261; studies 2; I2: 0%). There is uncertainty about whether esophageal pressure monitoring reduces the duration of mechanical ventilation.
ConclusionsEvidence of low or very low certainty indicates that esophageal pressure monitoring during mechanical ventilation would produce little or no effect on Intensive Care Unit mortality, Intensive Care Unit length of stay, days on mechanical ventilation or adverse events.
Evaluar los efectos de la monitorización de la presión esofágica en pacientes adultos con requerimiento de ventilación mecánica en la Unidad de Terapia Intensiva.
DiseñoRevisión sistemática (Registro PROSPERO CRD42018118583).
ÁmbitoTerapia intensiva. Cuidados críticos.
Pacientes o participantesAdultos con requerimiento de ventilación mecánica en la Unidad de Terapia Intensiva.
IntervencionesMonitorización de la presión esofágica.
Variables de interés principalesMortalidad en terapia intensiva, días de hospitalización en terapia intensiva, días de ventilación mecánica, eventos adversos.
ResultadosSe incluyeron 4 estudios con 301 participantes. La monitorización de la presión esofágica durante la ventilación mecánica produciría poco o ningún efecto sobre la mortalidad en terapia intensiva a los 28 días (RR 0,74; IC 95% 0,31 a 1,76; participantes 261; estudios 2; I2: 68%), poca o ninguna diferencia en los días de hospitalización en terapia intensiva (DM 0,48; IC 95% −1,90 a 2,85; participantes 284; estudios 3; I2: 7%) o los eventos adversos (RR 0,74; IC 95% 0,50 a 1,09; participantes 261; estudios 2; I2: 0%). Existe incertidumbre sobre si la monitorización de la presión esofágica reduce los días de ventilación mecánica.
ConclusionesLa evidencia de certeza baja o muy baja indica que la monitorización de la presión esofágica durante la ventilación mecánica produciría poco o ningún efecto sobre la mortalidad en terapia intensiva, los días de hospitalización en terapia intensiva o ventilación mecánica y los eventos adversos.
Mechanical ventilation (MV) is one of the most widely used procedures. Also, it is one of the therapeutic pillars of intensive medicine.1,2 In order to preserve gas exchange and minimize the work of breathing in an attempt to reduce oxygen consumption by the tissues, MV is indicated for a wide variety of conditions that go from scheduled surgical procedures to acute respiratory failure,3 neuromuscular diseases or even coma. The monitorization of MV is one of the critical functions in the modern4 intensive care unit (ICU) setting because, although MV can save the lives of patients who may need it, its inappropriate use can lead to pulmonary injuries.5 The causal mechanism of MV-induced pulmonary damage is varied. However, it is possibly associated with transpulmonary pressure (TPP),6 that is, the pressure at the airway opening (Pao) minus the pressure in the pleural space. Pleural pressure measurements, estimated from esophageal pressures (Pes) by inserting a balloon catheter connected to a pressure transducer, allow us to analyze the distensibility of the thoracic and pulmonary wall, the work of breathing, the function of respiratory muscles, and the presence of diaphragm paralisis.7 At the same time, the absolute values of the Pes can be influenced by the mechanics of breathing, the tidal volume, the weight of the mediastinum, the abdomen, and the position and tone of the esophageal smooth muscle wall.6 A Pes-guided ventilation strategy would allow us to reduce pulmonary stress and individualize the parameters of the ventilator by obtaining positive TPPs during exhalation and avoiding excessive values (<27 cmH2O) of the latter to avoid alveolar overdistension, repetitive cycles of opening, and the alveolar collapse that is often associated with MV-induced pulmonary damage.8,9
There are different types of esophageal catheters available. Depending on their diameter and length each requires a specific filling with a certain amount of air to measure Pes correctly. These devices can be kept for long periods of time, as long as they remain properly positioned.6 Pes has been measured over the last half a century to draw the physiology and mechanical characteristics of the respiratory system. Although the use of these measuring techniques has occurred in the lab to improve our understanding of the basic physiological mechanisms, they have also been used in the clinical context.4 Still, the clinical implementation of these measuring techniques is still limited probably due to the technical difficulties and the lack of knowledge.7
For the time being, no systematic reviews have assessed the effect of Pes monitorization in the main outomes at the ICU setting. The results of a systematic review on this matter may be useful for the investigators, decision-makers, healthcare professionals, and patients since it would bring extra knowledge to the impact Pes monitorization has on different hard endpoints.
The objective of this systematic review is to assess the effect Pes monitorixation has in adult patients requiring MV with respect to mortality at the ICU setting, days at the ICU, days on MV, and adverse events compared to standard airway pressure (Paw) monitorization.
MethodsFor the systematic review the Cochrane Handbook for Systematic Reviews of Interventions,10 and the methodological expectations of the Cochrane Intervention Reviews were used.11
Results are reported following the recommendations established by the declaration of Preferred Reporting Items for Systematic Reviews and Meta-Analyses –PRISMA–.12 The protocol of this review was registered in the International Prospective Register of Systematic Reviews –PROSPERO– (CRD42018118583).
Randomized and semi-randomized controlled clinical trials were included. Observational studies as additional or supplementary evidence to the clinical trials would have been included too. However, this would have been limited to prospective cohort studies with simultaneous group comparison.13 No restrictions based on language, date or status of publication were applied. The studies included recruited men and women over 18 requiring MV at the ICU setting with Pes monitorization compared to standard airway pressure monitorization and ventilator pressure-volume curves. The methods used for non-randomized trials were not implemented since no observational studies were found that would meet the inclusion criteria.
The following endpoints were studied:1 mortality at the ICU setting, days on MV, days at the ICU, and adverse events. These endpoints were not considered inclusion criteria for the selection of the studies.
A research on the main electronic databases (MEDLINE, EMBASE, LILACS, The Cochrane Library; see Appendix B Annex A) and on the grey literature was conducted until October 2019. Also, searches were conducted in prospective clinical trials registries (ClinicalTrials.gov, World Health Organization International Clinical Trials Registry Platform, ControlledTrials.com). The bibliographic reference lists of the studies included were reviewed and subject matter experts were asked to identify studies that may have been omitted. The transcripts from the meetings held by the Society of Critical Care Medicine, the American Academy for Respiratory Care, and the European Respiratory Society were reviewed manually.
Two evaluators independently assessed the eligibility of the studies and collected the characteristics of each. Two evaluators independently assessed the risk of bias for every randomized clinical trial included following the recommendations established by the Cochrane Collaboration.10 Any disagreements on these processes were solved by an independent third evaluator.
Result data for every study were included in the RevMan software14 to assess the effects of treatment. Dichotomic results were presented using the relative risk with 95% confidence interval (95%CI). Mean differences and the 95% confidence interval were used to present the continuous results. The heterogeneity of effect sizes was assessed through visual inspection of the forest plots to assess the amount of confidence interval overlapping, also using the chi-square test. P values < 0.10 were considered indicators of a potentially significant heterogeneity.10 The I2 statistic was also used. The Cochrane Handbook for Systematic Reviews of Interventions was used to interpret it.10 In case of clinical heterogeneity, the studies were not combined.10 Publication bias would have been studied by the production of funnel plots but the number of studies was not big enough.
Regarding the studies included the effect of the intervention in the different subgroups was studied based on the underlying diagnosis (acute respiratory distress syndrome [ARDS] vs patients without ARDS defined according to the definition established by the American-European Consensus Conference15 or the Berlin Definition16), and the presence of obesity (defined as a BMI > 30 kg/m2).17 The analysis of sensitivity included an assessment of the effect of the results of interest and excluded studies with uncertain or high risk of bias in the domains of allocation concealement and random sequence generation. Two independent reviewers assessed the degree of confidence on the level of evidence using the 5 domains established by the Grading of Recommendations Assessment, Development and Evaluation –GRADE– working group.18 The degree of confidence on the level of evidence was scored as “high”, “moderate”, “low” or “fairly low” using GRADEpro GDT.19 Discrepancies were solved by an independent third evaluation.
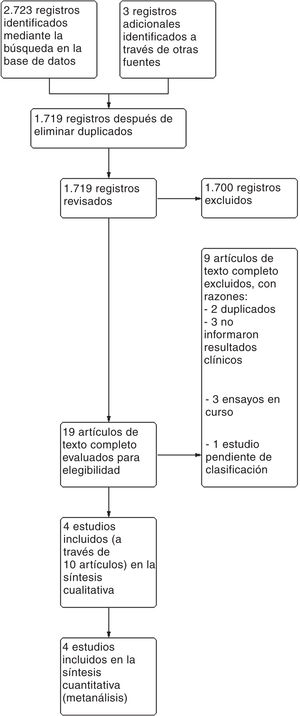
ResultsA total of 2726 references were found after a digital dig into databases and trial registries. A total of 1719 of these references were studied after removing the duplicate ones after inspecting titles and abstracts. We though that 19 of these references were potentially eligible, and the entire text was analyzed. Nine of these were eventually discarded. A total of 3 ongoing studies and 1 study pending classification were identified (published as an abstract only) (see table with the characteristics of the studies excluded and pending classification in Appendix B Annex B). Finally, 4 studies represented by 10 articles were eventually included (see the PRISMA flow chart on Fig. 1).
A total of 4 clinical trials from 10 articles with 301 participants were included since we found secondary references for the studies conducted by Talmor et al.,20 Beitler et al.,21 and Obi et al.22 Also, clinical trial registries of the aforementioned studies were used to collect the data (see a summary of the main characteristics on Table 1; Appendix B Annex B shows the characteristics of each study included). The studies were conducted mainly in adult patients with ARDS20,21,23 except for 1 study that was conducted in patients with obesity.22 Both the severity and the oxygenation disorders of the patients included were highly variable among the studies. The size of the sample was predominantly small with studies like the ones conducted by Obi et al.,22 and Wang et al.23 that included 25 and 23 participants, respectively or the study conducted by Talmor et al.20 that included 61 participants. Only the study conducted by Beitler et al.21 included 200 participants. Most studies monitored Pes as positive end-expiratory pressure titration to keep an end-expiratory TPP somewhere between 0 and 10 cmH2O. Other studies also used end-inspiratory TPPs < 20 cmH2O or 25 cmH2O as the threshold. Most studies were conducted in countries with high GDPs like the United States, and Canada.20–22 Only 1 study was conducted in a country with a medium-high GDP like China.23 Two of the studies included were funded by the National Heart, Lung, and Blood Institute of the United States.20,21 Another study received no funding for research purposes, authorship or publication,22 and the remaining study did not disclose its source of funding.23
Main characteristics of the studies included.
| Study | Country | Participants | Procedure | Comparator | Follow-up |
|---|---|---|---|---|---|
| Beitler et al.21, 2019 | United States | N = 200 | Pes-guided PEEP for end-expiratory TPP between 0 and 6 cmH2O, and end-inspiratory TPP ≤ at 20 cmH2O | PEEP based on the empirical PEEP-FiO2 table from the OSCILLATE PEEP trial according to the best static complacency | 28 days |
| Canada | ARDS | 60 days | |||
| Obi et al.22, 2018 | United States | N = 25 | PEEP to keep end-expiratory TPP between 0 and 10 cmH2O | 30 days | |
| Morbid obesity | |||||
| Patients on MV | |||||
| Talmor et al.20, 2008 | United States | N = 61 | MV with certain adjustments after the early measurements of Pes to keep the end-expiratory TPP between 0 and 10 cmH2O, and the end-inspiratory TPP < 25 cmH2O | MV based on the strategy used by the ARDSnet trial | 28 days |
| ARDS | 180 days | ||||
| Wang et al.23, 2019 | China | N = 23 | PEEP was adjusted based on Pes so that the end-expiratory TPP maintained a smaller positive number within 0−10 cmH2O | MV based on the strategy used by the ARDSnet trial | Unreported |
| ARDS due to trauma |
ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure; Pes, esophageal pressure; TPP, transpulmonary pressure.
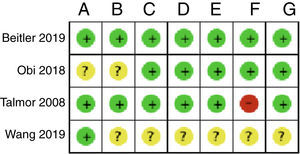
Fig. 2 shows the individual assessment of the risk of bias of the studies included. Appendix B Annex B shows a detailed description of the judgements behind the assessment of the risk of bias found on the table with the characteristics of the studies included. Three of the studies included did not disclose enough information in their articles to be able to pass judgement, which is why all the necessary information had to be obtained by contacting the authors.20,22,23 Only 1 of the studies was considered to have a low risk of bias for all the domains assessed with the Cochrane tool.21
Summary of the risk of bias: review of the authors’ judgement on each risk of bias for every study included. A: random sequence generation; B: randomization concealement; C: blinding of participants and personnel; D: blinding of the result assessment; E: data of incomplete results; F: selective reporting; G: other.
Pes monitorization during MV in adult critically ill patients could reduce mortality at the ICU setting at the 28-day follow-up (relative risk, 0.74; 95% CI, 0.31–1.76; participants, 261; studies, 2; I2, 68%), however, the 95% CI would be suggestive that there is little or no inter-group difference at all. The degree of confidence on the level of evidence was low due to inaccuracy and risk of bias (Fig. 3). The summary of the findings is shown on Table 2. There is uncertainty on whether Pes monitorization during MV in adult critically ill patients reduces the days on MV (mean difference, −4.13; 95% CI, −8.87 to 0.61; participants, 301; studies, 4; I2, 68%) since the degree of confidence on the level of evidence was assessed as very low due to inaccuracy, inconsistency, and risk of bias (Fig. 4). Pes monitorization during MV in adult critically ill patients can produce little or no difference at all in the number of days spent at the ICU (mean difference, 0.48; 95% CI, −1.90 to 2.85; participants, 284; studies, 3; I2, 7%). The degree of confidence on the level of evidence was low due to concerns associated with inaccuracy and risk of bias (Fig. 5). Pes monitorization during MV in adult critically ill patients can produce little or no difference at all in the occurrence of adverse events (relative risk, 0.74; 95% CI, 0.50–1.09; participants, 261; studies, 2; I2, 0%). The degree of confidence on the level of evidence was low due to inaccuracy and concerns associated with the risk of bias (Fig. 6). None of the studies disclosed information on the analysis of the resulting costs associated with the intervention. The subgroup and sensitivity analyses did not show any differences in the primary analyses (Appendix B Annex C).
Summary of the findings.
| Esophageal pressure monitorization during MV compared to standard airway pressure monitorization and ventilator pressure-volume curves for adult critically ill patients | |||||
|---|---|---|---|---|---|
| Patient or population: Adult critically ill patients | |||||
| Procedure: esophageal pressure monitorization during MV | |||||
| Comparison: Standard airway pressure monitorization and ventilator pressure-volume curves | |||||
| Outcomes | Number of participants (studies) | Quality/Certainty of the evidence (GRADE) | Relative effect (95%CI) | Anticipated absolute effects | |
| Risk with standard airway pressure monitorization and ventilator pressure-volume curves | Risk difference with esophageal pressure monitorization during MV | ||||
| Mortality at the ICU setting, 28-day follow-up | 261 (2 RCA) | ⊕⊕○○ Lowa,b,c | RR 0.74 (0.31–1.76) | 326 per 1000 | 85 less per 1000 (225 lowest to 247 highest) |
| Days on MV | 301 (4 RCA) | ⊕○○○ Very lowd,e,f | – | The mean days on MV goes from 10.5 to 14.5 | MD, 4.13 less (8.87 lowest to 0.61 highest) |
| Days at the ICU setting | 284 (3 RCA) | ⊕⊕○○ Lowf,g | – | The mean hospitalization days at the ICU setting went from 9.5 to 20.9 | MD, 0.48 higher (1.9 lowest to 2.85 highest) |
| Adverse events | 261 (2 RCA) | ⊕⊕○○ Lowc,g | RR 0.74 (0.50–1.09) | 302 per 1000 | 79 less per 1000 (151 lowest to 27 highest) |
| Risk in the intervention group (and its 95%CI) is based on the risk assumed in the comparison group and on the intervention relative effect (and its 95%CI) | |||||
95%CI, 95% confidence interval; MD, mean difference; MV, mechanical ventilation; RCA, randomized clinical trial; RR, risk ratio.
Lower level of evidence due to high risk of bias in the domain of selective result reporting in one of the studies included in the analysis.
There is substantial heterogeneity that may be partially explained by the differences seen in the comparator between both studies included in the analysis. Since no differences in the effect size were reported the levels of evidence did not go down.
Lower level of evidence due to the presence of few events and the early interruption of one of the trials.
Lower level of evidence due to risk of uncertain bias in the domain of random sequence generation, and high and uncertain risk of bias in the domain of randomization concealement.
Lower level of evidence due to differences in the effect size: there is substantial heterogeneity that may be partially explained by the differences seen in the population and the comparators among the studies included in the analysis (subgroup analyses could not explain it).
The evidence analyzed shows that Pes monitorization during MV would produce a small or null effect on mortality, days at the hospital, days on MV or on the adverse events. However, the number of studies was limited, and the analysis of publication bias could not be conducted. Also, certain specific populations were more represented in the studies like patients with ARDS, which limits the applicability of these results to other populations or to the general population that may require MV. These results are consistent with the routine clinical practice where Pes monitorization is often spared for this type of patients who are more critically ill and with more altered breathing mechanics.4,7
The main methodological limitations of the studies included are associated with the risk of bias since only 1 study had a low risk of bias in all the domains analyzed. Results were consistent among the different studies conducted regarding mortality at the ICU setting, days at the hospital, and adverse events. However, there was a serious inconsistenty among the studies regarding days on MV since one of the studies included in the analysis showed a tendency towards benefit for the control group,21 while the remaining studies showed this tendency for the experimental group. Since we could not explain this inconsistency, we decided to bring the degree of confidence on the level of evidence down using the GRADE approach.
Th inaccuracy surrounded all the endpoints of interest was obvious since very few studies with events were included in the analysis. Particularly, one of the studies included was stopped early for showing benefits20 simply because it may be overestimating the effect size of Pes monitorization. Some statistical models show that clinical trials interrupted early for showing benefits systematically overestimate the effects of treatment,24 especially when the number of events in the study is <200.25,26 The tendency of the trials interrupted early of overestimating the effects of treatment is especially dangerous because the apparently convicing results of these trials are often published in a rush in prestigious journals, rapidly spread to the media, and incorporated fast to the clinical practice guidelines and quality improvement initiatives. This automatically puts an end to further investigations on the issue at stake.25,26
The main limitation of our review is the small number of studies found on the issue at stake. An experienced clinical research associate in the process of systematic reviews conducted the search on the medical literature available. However, the number of studies included was limited because this review based its analysis on hard endpoints at the ICU setting. Also, although Pes monitorization is still being used to improve our understanding of the basic physiological mechanisms, its clinical implementation, is still limited.7 The strength of our review was that it followed a predefined protocol and documented all the changes and methods implemented (Appendix B Annex D).
This is the first systematic review ever conducted to study the effect of Pes monitorization in patients requiring MV at the ICU setting. Some of the studies included favored the adoption and implementation of Pes monitorization at the ICU setting and guided its implementation in the clinical context. However, the impact of Pes monitorization to guide MV has not been studied that much out of the physiological variable setting.4,7 A consensus expert suggests the monitorization of Pes and tidal volume as potentially useful tools in more critical patients with ARDS to determine recruitement and the possibility of atelectrauma.27 Also, this expert consensus suggests integrating the monitorization of multiple variables into the healthcare process of critically ill patient with respiratory failure. Also, that the monitorization of both the ventilator pressure–volume curves and the static respiratory varibles provides limited information on work of breathing and recruitment, thus emphasizing the need for dynamic respiratory monitorization, where Pes monitorization plays a key role.27
Most of the clinical trials included analyzed oxygenation parameters as primary endpoints. It may be possible that the oxygenation changes triggered by the MV guided by Pes monitorization don’t have any effects on other hard endpoints like mortality at the ICU setting, days on MV or adverse events, which would turn oxygenation into an inadequate endpoint. Probably, to this date, in this review, there is still no consensus on the causal relation between oxygenation changes and hard endpoints.28,29
ConclusionsPes monitorization in adult patients requiring MV would have little or no effect at all on mortality, days of ICU hospitalization, days on MV or advers events. The adoption of Pes monitorization is a useful tool for healthcare professionals for the management of patients regarding MV guidance because it provides additional information that is difficult to obtain otherwise. Although, to this date, this practice has not proven beneficial and there is still uncertainty on the incidence rate of adverse events and their implications in the use of resources. More studies are needed on the use of Pes monitorization in patients requiring MV from the beginning and throughout the entire process including patients from the general population at the ICU setting with different diagnoses and severity while assessing significant endpoints like mortality, days on MV or days at the hospital.
FundingThis study was funded by the University Institute School of Medicine of the Italian Hospital (IUHI): Cochrane Grant for Perfecting Systematic Reviews and Evidence-based Medicine (IUHI affiliate Cochrane Center).
AuthorsLuis Ignacio Garegnani: study design and idea, data mining, data analysis and interpretation, draft/writing of the manuscript, and critical revision of the manuscript intellectual content.
Pablo Rosón Rodriguez: data mining, data analysis and interpretation.
Juan Victor Ariel Franco: draft of the manuscript, critical revision of the manuscript intellectual content, and final approval of the manuscript latest version.
Camila Escobar Liquitay: design and implementation of the bibliographic search strategy, and data mining.
Conflicts of interestThe authors declared no potential conflicts of interest regarding the research and/or authorship of this manuscript.
We wish to express our gratitude to Dr. Lic. Sacha Virgilio, and Dr. Agustín Ciapponi for their collaboration developing the study protocol.
Please cite this article as: Garegnani LI, Rosón Rodriguez P, Franco JVA, Escobar Liquitay C. Monitorización de la presión esofágica durante la ventilación mecánica en pacientes críticos adultos: revisión sistemática y metaanálisis. Med Intensiva. 2021;45:387–394.