The series of updates on methodology for research in critically ill patients has addressed the difficulties caused by the characteristics of patients of this kind, bioethics, the interpretation of results, and methodological error. New statistical methods for causality research, meta-analysis and big data analysis have also been described. The last update refers to the horizon for new research in the field of critical care. We close the series with the hope of having provided a global vision of the statistical methods oriented toward the future, with the aim of promoting statistical culture. In parallel, we have analyzed the evolution in complexity of the methodological analysis in the journal.
La serie de actualización en metodología para la investigación en el enfermo crítico ha tratado las dificultades inherentes a las características de este tipo de enfermo, la bioética, la interpretación de resultados y los errores metodológicos. Además, se han mostrado nuevas técnicas estadísticas para la investigación de la causalidad, se ha tratado el metaanálisis y se ha profundizado en el análisis de big data. Este último abordaje es hacia donde van las nuevas investigaciones en el campo del enfermo crítico. La serie que cerramos ha pretendido dar una visión de conjunto de los métodos estadísticos orientada hacia el futuro, con el fin de mejorar la cultura estadística. De manera paralela, hemos valorado la evolución de la complejidad de los análisis metodológicos en la revista.
With this special paper we put an end to the series on methodology in Intensive Care Medicine that we initiated back in April 20181 and that included 8 papers that we hope stimulated your interest on statistical analysis when preparing articles to be published in our scientific journal.
The series included 4 papers on general issues. The first one was about the importance of methodology in the clinical research of the critically ill patient,2 including a description of the difficulties that are inherent to this type of patients when it comes to clinical research, and the disappointments that follow therapeutic failures – sometimes triggered by implementing wrong designs. Another paper exposed some of the key elements when it comes to interpreting statistical results,3 such as facilitating the understanding of common concepts in methodology that are not always fully understood by healthcare providers or researchers. The third review discussed ethics in clinical research,4 and clarified fundamental aspects of bioethical issues that need to be taken into account when conducting studies, which, by the way, today is a prerequisite of any clinical research. The fourth paper discussed common errors in the use of statistics5 warning readers on the slips or even frauds that are sometimes found in researches published and that may affect the way these clinical researches are implemented in the routine clinical practice. All papers discussed all these issues from the perspective of critically ill patients, their peculiarities and determinant factors.
On the other hand, we also discussed new statistical methodology tools. A review of new techniques in the management of causality in observational studies6 presented us with different ways of analysis beyond conventional multivariate logistic regression analyses. Another review on alternative statistical methods7 showed us options different from the usual techniques to be able to explore the truth behind the data. We also presented an introduction to this study by using big data,8 the tool that will probably change our way to analyze health biological reality forever and, particularly, the way we analyze the critically ill patients’ biological reality, in whom the abundance and complexity of data open a huge field for the implementation of these techniques. Lastly, we included a great article on meta-analysis,9 one of the main sources of evidence-based knowledge not only for our clinical practice but also for the detection of uncertainty to guide future researches.
In the article that introduced the entire series, we analyzed the density of using statistical tools in the original articles we publish regularly on Medicina Intensiva, and compared it to that of the other two major journals in intensive care, Intensive Care Medicine and Critical Care Medicine.1 We saw significant differences in the presence of multicenter studies, in the amount of tests used by the original paper and, especially, in the use of analysis of greater statistical complexity. Probably, higher quality during the research process facilitates the incorporation of more complex tests and even a larger amount tests which, in turn, facilitates publishing in higher impact journals.
In order to improve all these indicators, the editorial committee intends to follow two parallel lines.10 On the one hand, the intend to increase our impact factor by encouraging citing our journal by improving its accessibility and circulation (changes in the web, app), by having excellent researchers publishing with us, by facilitating publication in English, and by promoting the responsibility of Spanish-speaking intensivists in the publication of our journal. On the other hand, we understand that the editorial process needs to be demanding and constructive in order to improve the scientific quality of studies. The role of the reviewing team is essential here, will bring quality and complexity to the statistical analyses during the entire peer-review process, and give more consistency and credibility to the studies that we publish. We strongly believe this will bring more chances of external citations to our journal.
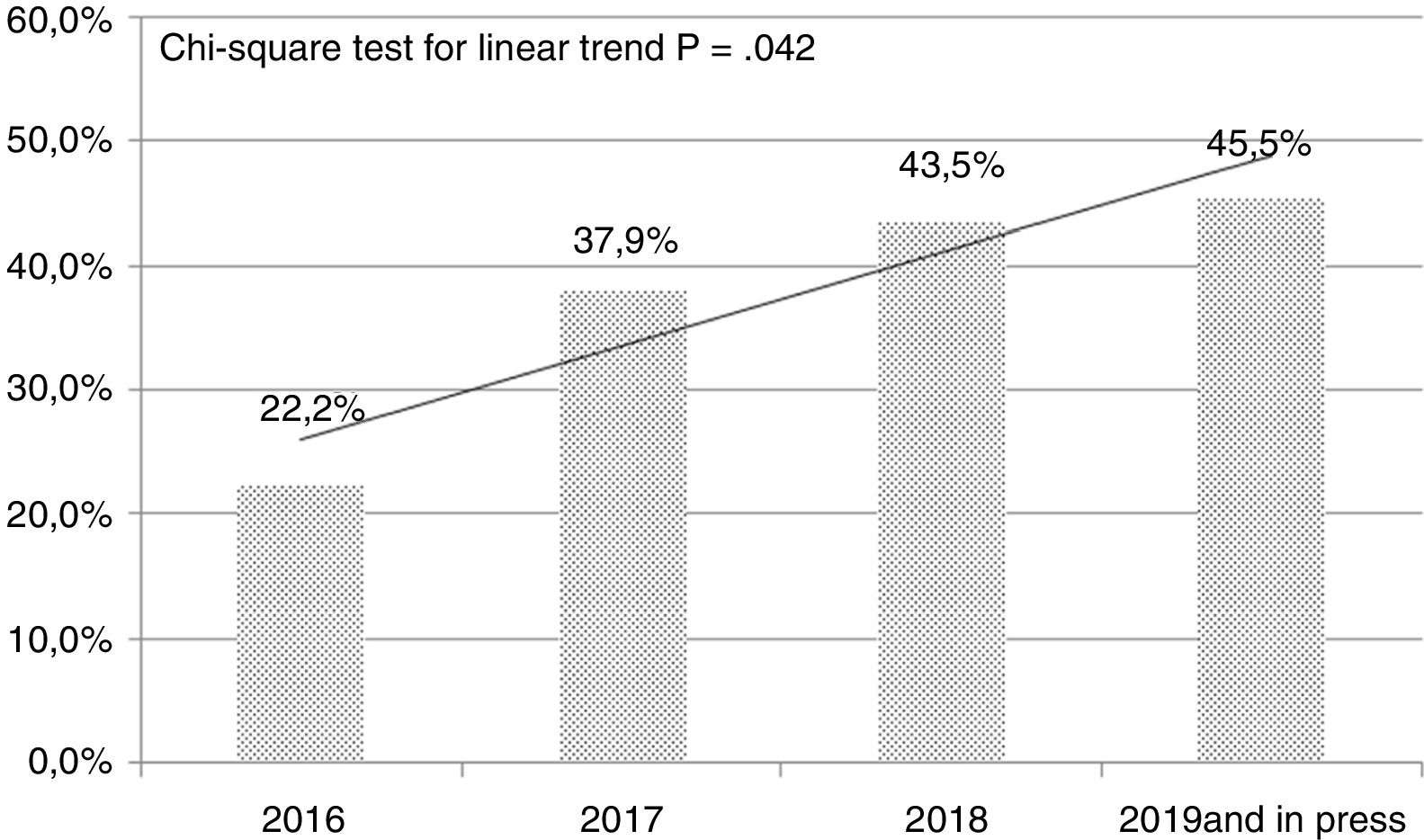
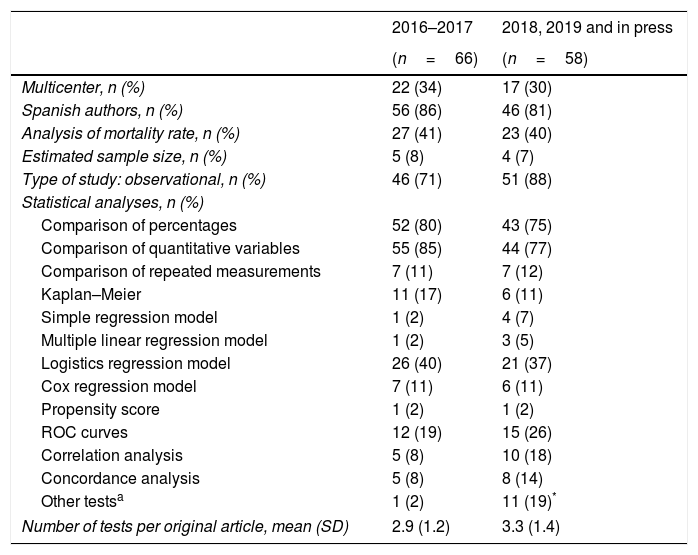
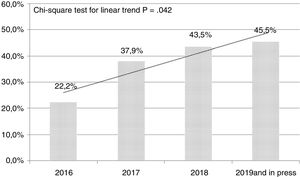
In the follow-up we conducted on the methodological quality of the original papers published in our journal we can see a certain evolution during the last few years. Table 1 shows a new analysis that draws a comparison between the originals published in 2016 and 2017 and the originals published back in 2018 plus those published online (as of November 2018). We have to say that we basically publish observational studies, and have a low rate of multicenter studies, and a scarce estimate of sample sizes (Table 1). We should also mention here that in this short period of time we saw a clear increase in the amount of unusual tests that were incorporated into our papers to the point that they appeared in almost 20% of all original papers. Also, during the last few years we have seen a significant increase in the percentage of studies that incorporate more than 3 statistical analyses (chi-square test for linear trend; P=.042) (Fig. 1). On the other hand, the number of systematic reviews and meta-analyses has gone up from 0 to 5 during the second period examined, and we have published two original papers based on big data. All this suggests a greater and better methodological elaboration in the original papers published. We understand that a greater statistical complexity does not guarantee the interest of readers on clinical research, but it is a prerequisite for its development as other scientific journals have proven in the past.11
Comparative analysis of the statistical methodology applied to original papers published in Medicina Intensiva in two consecutive periods of time.
| 2016–2017 | 2018, 2019 and in press | |
|---|---|---|
| (n=66) | (n=58) | |
| Multicenter, n (%) | 22 (34) | 17 (30) |
| Spanish authors, n (%) | 56 (86) | 46 (81) |
| Analysis of mortality rate, n (%) | 27 (41) | 23 (40) |
| Estimated sample size, n (%) | 5 (8) | 4 (7) |
| Type of study: observational, n (%) | 46 (71) | 51 (88) |
| Statistical analyses, n (%) | ||
| Comparison of percentages | 52 (80) | 43 (75) |
| Comparison of quantitative variables | 55 (85) | 44 (77) |
| Comparison of repeated measurements | 7 (11) | 7 (12) |
| Kaplan–Meier | 11 (17) | 6 (11) |
| Simple regression model | 1 (2) | 4 (7) |
| Multiple linear regression model | 1 (2) | 3 (5) |
| Logistics regression model | 26 (40) | 21 (37) |
| Cox regression model | 7 (11) | 6 (11) |
| Propensity score | 1 (2) | 1 (2) |
| ROC curves | 12 (19) | 15 (26) |
| Correlation analysis | 5 (8) | 10 (18) |
| Concordance analysis | 5 (8) | 8 (14) |
| Other testsa | 1 (2) | 11 (19)* |
| Number of tests per original article, mean (SD) | 2.9 (1.2) | 3.3 (1.4) |
In sum, we hope that this series on methodology has pleased all our readers. We hope it was useful to understand the different aspects of statistical design and interpretation and to present complex issues that may be difficult but essential for the future development of clinical research. And if we did not deliver, at least we take pride in the fact that it stimulated researchers to incorporate new tools to the arsenal of petitions to statisticians. If this helps increase the quality and the possibility of citations of the studies already published, then we can happily say mission accomplished.
Conflicts of interestThe author declares no conflicts of interest whatsoever.
Please cite this article as: García Garmendia JL. Evaluación y cierre de la serie sobre metodología en Medicina Intensiva. Med Intensiva. 2019;43:121–123.