The use of extracorporeal membrane oxygenation systems has increased significantly in recent years; given this reality, the Spanish Society of Critical Intensive Care Medicine and Coronary Units (SEMICYUC) has decided to draw up a series of recommendations that serve as a framework for the use of this technique in intensive care units. The three most frequent areas of extracorporeal membrane oxygenation systems use in our setting are: as a cardiocirculatory support, as a respiratory support and for the maintenance of the abdominal organs in donors. The SEMICYUC appointed a series of experts belonging to the three working groups involved (Cardiological Intensive Care and CPR, Acute Respiratory Failure and Transplant work group) that, after reviewing the existing literature until March 2018, developed a series of recommendations. These recommendations were posted on the SEMICYUC website to receive suggestions from the intensivists and finally approved by the Scientific Committee of the Society. The recommendations, based on current knowledge, are about which patients may be candidates for the technique, when to start it and the necessary infrastructure conditions of the hospital centers or, the conditions for transfer to centers with experience.
Although from a physiopathological point of view, there are clear arguments for the use of extracorporeal membrane oxygenation systems, the current scientific evidence is weak, so studies are needed that define more precisely which patients benefit most from the technique and when they should start.
El empleo de sistemas de oxigenación con membrana extracorpórea se ha incrementado significativamente en los últimos años; ante esta realidad, la Sociedad Española de Medicina Intensiva Crítica y Unidades Coronarias (SEMICYUC) ha decidido elaborar una serie de recomendaciones que sirvan de marco para el empleo de esta técnica en las Unidades de Cuidados intensivos. Los tres ámbitos de empleo de oxigenación con membrana extracorpórea más frecuentes en nuestro medio son: como soporte cardiocirculatorio, como soporte respiratorio y para el mantenimiento de los órganos abdominales en donantes. La SEMICYUC nombró una serie de expertos pertenecientes a los tres grupos de trabajo implicados (Cuidados Intensivos Cardiológicos y RCP, Insuficiencia Respiratoria Aguda y Grupo de trabajo de Trasplantes de SEMICYUC) que tras la revisión de la literatura existente hasta marzo de 2018, elaboraron una serie de recomendaciones. Estas recomendaciones fueron expuestas en la web de la SEMICYUC para recibir las sugerencias de los intensivistas y finalmente fueron aprobadas por el Comité Científico de la Sociedad. Las recomendaciones, en base al conocimiento actual, versan sobre qué pacientes pueden ser candidatos a la técnica, cuándo iniciarla y las condiciones de infraestructura necesarias de los centros hospitalarios o en su caso, las condiciones para el traslado a centros con experiencia.
Aunque desde un punto de vista fisiopatólogico, existen claros argumentos para el empleo de oxigenación con membrana extracorpórea, la evidencia científica actual es débil por lo que es necesario estudios que definen con más precisión qué pacientes se benefician más de la técnica y en qué momento deben iniciarse.
The use of extracorporeal membrane oxygenation (ECMO) systems in adult patients has been growing during the last decade with promising results. The technological advances made in the design of the pump with more simple, compact equipments and, above all, the use of much more biocompatible and efficient membranes for gas exchange has enabled longer periods of use and safer techniques which in turn has contributed to the widespread use of these ECMO systems.
Several task forces and research teams have revealed favorable results in selected patient although, when these results have been put to the test with strict criteria, it has been difficult to draw definitive conclusions with a high degree of certainty. Generally speaking, the studies do not have an adequate control group or have a high level of heterogeneity among them due to differences in the criteria established for starting the ECMO or in the protocols for the management of patients, which shows just how weak scientific evidence is in this field.1,2
Although it is an invasive technique for critically ill patients with a high rate of complications, the impact that these complications have on mortality is limitated.3 Thus, since its use is already a reality and although there is controversy on several issues concerning the utility of ECMO systems and their practical application, the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) has designed a series of general recommendations for the use of ECMO in ICU settings.
Traditionally, the use of ECMO has been seen as means of cardiocirculatory or respiratory support; however, during the last few years its use has been oriented toward the maintenance of normothermia in abdominal organs prior to their extraction in organ donors. These three areas of applications keep a close correlation with Intensive Medicine, which is why we have come up with three different sets of recommendations: ECMO as cardiocirculatory support; ECMO as respiratory support; ECMO for the maintenance of abdominal organs in donors.
Thee use of ECMO has a series of requirements and conditions that go far beyond this paper though. The Extracorporeal Lung Support Organization (ELSO) official website (https://www.elso.org/) provides information on other aspects such as the technique; training needs both for physicians and nurses and other significant issues for the appropriate and rational use of this technique. This web also shows information on the activity and results obtained by the hospitals that offer ECMO systems, which is a valuable piece of information. We wish to appeal to all those centers specialized in this technique to collaborate in this registry.
MethodologyThe decision to elaborate a series of recommendations on the use of ECMO at the ICU setting was made by the SEMICYUC Board of Directors after seeing the importance of this issue. The board decided to designate one coordinator (EFM) to write this document on how to use ECMO systems at the ICU setting. The coordinators from the tree task forces or research teams involved were contacted and asked to designate experts from each group to set up the team that would eventually write the document. This writing committee had to analyze all the references and bibliographical entries, plan the recommendations and act as the liaison with other team members who were given the first drafts in order to collect their modifications or suggestions of the document. Back in October 2016 the writing committee was created with these members: ML, OP and TG from the group of Acute Respiratory Failure; MPF, JLPV and MSB from the group of Cardiovascular Intensive Care and RCP and JJR and JMPV from the SEMICYUC Transplant team. The members from this writing committee requested the collaboration from other experts whenever they deemed it necessary (Annex).
Back in November 2016 the first video call was held so that each group could establish the setting and format of the recommendations and agree on the methodology that would be used. Through the usual Boolean operators, each group conducted a structured bibliographic search until March 2018 on Medline/PubMed with the following keywords: Circulatory Assistance, Cardiogenic shock, Cardiac transplantation, Transportation in ECMO, Venoarterial extracorporeal membrane oxygenation, acute respiratory distress syndrome, refractory hypoxemia, protective ventilation, ultraprotective ventilation, extracorporeal membrane oxygenation, extracorporeal CO2removal, ECMO referral, Outcome, Non heart beating donation, Normothermic abdominal perfusion. This search has been complemented with a list of known relevant bibliography.
When it came to the writing of recommendations, all the agreements were made by consensus, initially among the members of the writing committee and then holding four different video calls. If something had not been decided unanimously during the video call, discussion continued through the e-mail until the final writing was accepted by the entire group. Then, the first draft was sent to the difference research teams, so they could come up with ideas or suggestions.
The initial recommendations were presented at the LII SEMICYUC National Congress where all members and attendees made comments and gave their suggestions on this regard.
After the initial writing, the document was reviewed by the SEMICYUC Scientific Committee and then published on the SEMICYUC official website for 15 days so that all members could give their feedback. After these fifteen days, the final writing was sent for publication.
ResultsECMO recommendations as assisted circulatory supportRecommendation #1ECMO as circulatory support is indicated in situations of refractory cardiogenic shock with the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS 1) scale and always as a bridge to a goal: recovery; heart transplant; until a therapeutic decision has been made or until a different long-term assist device has been implemented.
The use of ECMO as circulatory support should be part of a therapeutic strategy and have a clear-cut target in mind. As a bridge to recovery in potentially reversible diseases, as a bridge to heart transplant or other types of long-term or definitive assist device, or until a decision is made on whether more tests are needed before considering the patient eligible for the heart transplant.4–6
The refractoriness of the state of shock is defined as the persistence of hypotension and/or tissue hypoperfusion due to low cardiac output (<2.2l/min/m2) yet despite optimal volemia, the use of inotropic and/or vasoactive drugs at high doses. High doses of these drugs are associated with high mortality rates and, after the initiation of ECMO, they can be titrated or withdrawn. The following ones are considered elevated doses of inotropic or vasoactive drugs: dopamine >10μg/kg/min, noradrenaline >0.5μg/kg/min, adrenaline >0.1μg/kg/min, dobutamine >10μg/kg/min, milrinone >0.5μg/kg/min.7
Hypoperfusion is defined as oliguria (<30ml/h or <0.5ml/kg/h); cold limbs; mental disorders and/or high levels of serum lactate (>2.0mmol/l).
The cardiac output should be measured using calibrated and reliable methods (a catheter through the pulmonary artery or transpulmonary thermodilution) and/or through echocardiography.8
Recommendation #2The indications for circulatory ECMO should be well-defined to avoid implants in pre-mortem situations, in futile cases or patients in whom this technique is contraindicated.
The ECMO technique is not a technique to be used as a last therapeutical resort in pre-mortem situations or terminally ill patients, since in these cases the mortality rate is 100%. To avoid inadequate uses of this technique, we should know what indications and contraindications the ECMO technique has2,9 (Tables 1 and 2).
Indications for venoarterial ECMO.
| • Cardiogenic shock due to myocardial infarction that remains unresponsive to therapy after revascularization through coronary intervention has been performed |
| • Patients on a heart transplant waiting list who experience hemodynamic deterioration |
| • Fulminant or acute myocarditis |
| • Clinical decompensation of exacerbated acute or chronic heart failure unresponsive to treatment that leads to planning an etiology-solving initiative |
| • Acute poisoning with cardiodepressant drugs |
| • Patients with electrical storm who remain unresponsive to antiarrhythmic therapy and/or radiofrequency ablation |
| • Pulmonary thromboembolism with severe dysfunction of right ventricle and shock |
| • Myocardial dysfunction due to sepsis in very particular cases |
| • Patients who, after cardiac surgery, cannot be disconnected from the extracorporeal circulation despite an adequate surgical correction |
| • Refractory post-cardiotomy cardiogenic shock where there is a reasonable possibility of recovery or surgical reintervention |
| • Patients with graft primary failure following the heart transplant |
| • As circulatory support while certain procedures are being conducted in high-risk patients (percutaneous coronary interventions, percutaneous aortic endoprosthesis placement) |
| • Cardiac arrest in very particular casesa |
| • Patients with severe acute respiratory failure who would need veno-venous ECMO but show associated uni- or biventricular dysfunction |
To this day, this is a highly controversial indication. It is important to know when the cardiac arrest occurred, and when the witnesses started the CPR maneuvers. The most benefited ones are patients with cardiac arrests due to defibrillation rhythm and in-hospital cardiac arrests with immediate access to this technique. If no effective rhythm recovery is possible, ECMO should be considered as the preservation system for organ perfusion for donation purposes.10
Contraindications for venoarterial ECMO.
| Absolute | Relative |
|---|---|
| • Terminal chronic disease | • Agea |
| • Uncontrolled neoplasm | • Absolute contraindications for anticoagulation |
| • Irreversible neurological damage diagnosed | • Morbid obesity (BMI >40kg/m2) |
| • Severe aortic failure | |
| • Non-repaired aortic dissection | |
| • Sepsis with multiple organ failure (defined as ≥2 organs with at least ≥2 points in the SOFA scale not including the cardiovascular one) | |
| • Non-recoverable and non-transplant-eligible heart or for any other type of ventricular support |
BMI: body mass index; SOFA: Sequential Organ Failure Assessment Score.
ECMO should not be the first-line therapy for the management of cardiogenic shock, but it has been confirmed that if implanted early it improves the results.
Before the ECMO, the optimal medical and/or surgical therapy should have already been used, since the ECMO technique is no stranger to complications.11–13
It is hard to tell what the optimal time to implant the ECMO really is, but as a rule of thumb we can say that it should happen before an established multiple organ failure. This means that an accurate monitoring and follow-up of certain clinical and analytical variables should be essential for its early implementation.14
The SAVE scale (www.save-score.com) recommended by the ELSO assigns scores to different parameters to predict mortality once the ECMO has been implanted. It may be useful in the decision-making process.15
The pre-ECMO parameters associated with poor prognosis are:
- •
Weight (<65kg or >90kg).
- •
Age (>53 years, above all, patients over 63).
- •
Hemodynamic: pulse pressure (≤20mmHg), diastolic blood pressure (>40mmHg), cardiac arrest.
- •
Respiratory: peak inspiratory pressure (>20mmHg), mechanical ventilation (with more h less survival).
- •
Renal: acute renal failure, chronic renal failure, HCO3 (<15mmol/l).
- •
Other organ failure: central nervous system dysfunction, renal failure, liver failure.
ECMO as circulatory support should be implanted in tertiary referral centers to secure the results. These should be centers experienced in the use of this technique and other medical-surgical and laboratory specialties to handle whatever complications may occur following the use of circulatory support systems or cardiogenic shock.
The ECMO consists of an extracorporeal mechanical circulation that is no stranger to complications, meaning that the personnel in charge needs to be fully experienced in the management of the patients who undergo this technique.16 Knowledge, training and experience are required at all time: to establish the indication; the optimal time to start the therapy; choose the right optimal cannulation17; the daily management of the technique; the prevention and early detection of complications and the most appropriate resolution. ECMO systems require one multidisciplinary team from different medical specialties led by specialists in the management of critically ill patients; one trained nursing team; techniques and services available at the centers 24/7 all year round. This team should also be in charge for the continuing medical education (CME) programs of the personnel, the protocols, the checklists and a registry of all the cases with further review and analysis.
Once implanted, one individualized and multidisciplinary therapeutical strategy should be used with every patient: how and when should ECMO be disconnected when the myocardial function has been recovered; indicate whether the patient should be in the heart transplant waiting list or contraindicate it on a temporal or definitive basis; indicate the need for a long-term assist system (as a bridge both to recovery and the transplant) or a definitive assist device when the transplant is contraindicated. Choosing the best option for every patient requires training and experience from the entire team. This is something we have learned from the large number of cases where ECMO systems have been used.
The ELSO recommends that ECMO centers should be tertiary referral centers with a minimum amount of annual cases so that the technique can be considered coste-effective.16 The number of ECMO/center/year has not been determined precisely, but according to the latest expert recommendations it should be over thirty procedures (including veno-venous ECMOs, but with a significant amount of veno-arterial ECMOs).18,19
Recommendation #5The transfers from the ECMO-free center to the tertiary referral center will occur after evaluating the patient's clinical stability on whether to implant the ECMO prior to the transfer. The transfers from ECMO-capable tertiary referral centers to heart transplant centers will occur with transplant-eligible patients only and after clinical stabilization with circulatory support systems.
Inter-hospital transfer programs are necessary to guarantee the accessibility of this technique to all individuals and provide optimal therapy regardless of the treating hospital. The possibility of accessing these ECMO programs should be seen as a guarantee of the principle of equality, however, given the characteristics of this technique, it needs to be conducted in a specialized center and with a series of requirements and services to improve the patients’ prognosis.20
If a patient in cardiogenic shock is ECMO-eligible and there are no contraindications, he should be transferred early to his tertiary referral center to undergo this technique.21 Then the clinical and hemodynamic state of the patient should be evaluated to decide whether he can be transferred without any assist device. Once at the tertiary referral center, he will be re-evaluated before deciding whether to finally implant the ECMO. In the presence of clinical instability, the assist device should be placed beforehand for a safe transfer, which is why the ECMO team will travel with both the equipment and the personnel required to the center where the patient remains hospitalized.
Once the ECMO has been initiated and the patient has been stabilized, he will be transferred to the tertiary referral center to move on with the therapy and other critical care. When it comes to choosing the means of transportation that we will use with patients on ECMO (ground or aerial transportation are the most common ones, sea transportation if necessary) we need to be aware of the availability of out-of-hospital emergency resources; distance; weather conditions; the existence of a heliport at the referring hospital and certain characteristics of the patient such as his height, weight and clinical situation). Once at the target center, the indication will be re-evaluated, and a decision will be made on whether to implant bedside ECMO.
It has been confirmed that ECMO transfers with experienced teams are safe and possible and have minimal complications. Also, survival is similar to the group of patients at the tertiary referral center.22
ECMO-capable centers without a heart transplant program as required by the patient will need to come to terms with the transplant-capable center what is the optimal moment to perform the transfer and proceed to enter the patient in the transplant waiting list.
It is necessary that each region or autonomous community creates a program establishing the flow of circulatory support-eligible patients for ECMO-capable centers and/or transplant centers. Also, there should be consensus on the management of these patients (pre- and post-ECMO) among the different centers involved in this process (local, ECMO centers and ECMO-transplant centers).
Support from healthcare institutions is required here if we want Spain to have mobile ECMO programs available for all individuals, so access to healthcare can be the same for everyone.23
Recommendations for the use of ECMO in cases of respiratory failureRecommendation #6Veno-venous ECMO is one complex high-flow technique that should be considered early in critically ill patients with respiratory failure refractory to other therapies.
VV-ECMO is one extracorporeal system for oxygenation purposes, CO2 purification and to facilitate protective or ultra-protective mechanical ventilation (MV) using two thick venous cannulae22–30 to allow the necessary blood flow. Double lumen cannulae can be used here but their use in the hypoxemic respiratory failure is limited by the flow. These flows go from 3 to 5l/min, but lower flows are needed to purify the CO2 and perform protective or ultra-protective ventilation, and up to 5–7l/min in cases of hypoxemia refractory to other therapies.24 The VV-ECMO can associate multiple complications being hemorrhages the most common of all and reported in up to 60% of the patients (less common and serious with the use of new systems) followed by infections.1,3
To this day, it is considered a “bailout therapy” for the management of refractory respiratory failure and only when other therapies have failed that, by the way, should always include the use of protective MV: tidal volume (TV): 4–8ml/kg of ideal weight; plateau pressure (Pplat) ≤30cmH2O) and at least a change to the decubitus prone (DP) position unless contraindication25 and the use of neuromuscular blockers for at least 48h.26 There should be a predictable recovery of the disease that triggers respiratory failure, or the VV-ECMO should be established as a bridge to lung transplant.
The optimal time to implant ECMO is still controversial and has not been established yet, but we know that implanting it before multiple organ failure improves the pronosis.27
The results from the recently published EOLIA study28 show that the early use of ECMO for the management of severe cases of acute respiratory distress syndrome (ARDS) reduces 60-day mortality, although not significantly compared to conventional approaches that include ECMO as a bailout therapy (35% versus 46%; p=0.09). Both groups were homogeneous when it comes to severity (SOFA 10.8±3.9 ECMO group; 10.6±3.5 in the control group); randomized, on average, after 34h on MV and with a PaO2/FiO2 ratio of 73±30 and 72±24 respectively). However, 28% of the patients from the control group ended up receiving ECMO due to refractory hypoxemia, and 57% of the patients died. This subgroup of more critically ill patients had a lower PaO2/FiO2 ratio, higher lactate levels; greater cardiac damage and pre-ECMO cardiac arrest in 9 cases.
The ELSO indicates ECMO for the management of hypoxemic respiratory failure with a PaO2/FiO2 ratio <100 with FiO2 >0.9 and/or Murray scale scores ≥3, oxygenation index >80 despite the use of optimal therapy for 6 or less hours.29 Other groups establish PaO2/FiO2 ratios <50 with FiO2 1 despite the use of protective MV and, at least, a change to the DP position for 3h.25 In cases of hypercapnic respiratory failure, one PaCO2 ratio >80mmHg or the impossibility to ventilate keeping Pplat >30cmH2O25 or CO2 retention despite Pplat >30cmH2O25 would be other indications proposed by the ELSO.
Once the ECMO support has been initiated, it is recommended to maintain protective or ultra-protective mechanical ventilation (TV 3–4ml/kg; Pplat <25cmH2O).30
The indications and contraindications of ECMO as respiratory support are shown in Table 3 being most of them relative contraindications.
Indications and contraindications for VV-ECMO.
| Indications for VV-ECMO |
|---|
| ARDS: pneumonia of any etiology, aspiration syndromes, alveolar proteinosis, obstetrics pathology, inhalation syndromes |
| Obstruction of the airway, pulmonary contusion, bronchopleural fistula |
| LT: bridge, respiratory support in the intraoperative period, GPF (<7 days) |
| Status asthmaticus |
| Pulmonary hemorrhage or massive hemoptisis |
| Hypercapnia (pH <7.20) and/or PaCO2 >80mmHg Impossibility to maintain Pplat <30cmH2O |
| Pulmonary vasculitis |
| Contraindications for VV-ECMO |
|---|
| Pulmonary disease without predictable recovery of the pulmonary function if LT is not indicated |
| Contraindications for anticoagulation |
| Age >65 years (evidence more limited in this age group). It is a relative contraindication |
| MOF with SOFA >15 points |
| MV >7days (special consideration with Pplat >30cmH2O, impossibility for PEEP >10cmH2O, FiO2 >0.9). It is a relative contraindication |
| Severe drug-induced immunosuppression (neutrophils <400/mm3) |
| Coma after cardiac arrest |
| Comorbidities: active malignant disease, chronic heart disease, non-recoverable and non-transplant pulmonary disease, chirrosis with ascites, irreversible neurological condition |
| Hemorrhagic or potentially hemorrhagic lesions of the CNS |
| Impossible cannulation |
GPF: graft primary failure; MOF: multiple organ failure; ARDS: acute respiratory distress syndrome; CNS: central nervous system; SOFA: Sequential Organ Failure Assessment Score; LT: lung transplant; MV: mechanical ventilation.
The VV-ECMO is recommended as part of a global management algorithm that must include the use of protective MV and, at least, a change to the decubitus prone position.
The use of VV-ECMO for the management of acute respiratory distress syndrome in adult patients should be considered as a bailout therapy and indicated in each case. The technological advances made on this technique have made it a safer easy-to-use technique and allowed a more liberal use. However, the implantation of the VV-ECMO should happen methodically and in a very organized way according to several organizations. Also, and in the light of the present data, its use should be evaluated in advance because it is a risky procedure and any wrong indications should be prevented.31,32
All studies – published and ongoing include patients with severe ARDS and certain clinical characteristics as shown in Table 4.
Gasometric and ventilatory criteria used by different studies.
| French consensus25 | ELSO29 | CESAR33 | EOLIA28 | |
|---|---|---|---|---|
| Gasometric criteria | PaO2/FiO2 <50 with FiO2=1 for >3h | PaO2/FiO2 <150 with FiO2 >0.9 and/or Murray 2–3 score | SaO2 <90% with FiO2 >0.9 for 12 or more hours | PaO2/FiO2 <50 with FiO2 >0.8 for more than 3h |
| PaO2/FiO2 <80 with FiO2=1 for >6h | PaO2/FiO2 <80 with FiO2 >0.9 and/or Murray 3–4 score | pH <7.20 of respiratory or metabolic causes | PaO2/FiO2 <80 with FiO2 >0.8 for more than 6h | |
| pH <7.20 for more than 6h | PaCO2 >80mmHg with Pplat <30cmH2O | pH <7.25 for more than 6h with HR >35rpm | ||
| Ventilatory criteria | TV: 4–8ml/kg | – | TV: 4–8ml/kg | TV: 6ml/kg |
| PEEP (elevated) | – | – | PEEP ≥10cmH2O | |
| Plateau pressure ≤30cmH2O | – | Plateau pressure | Plateau pressure ≤32cmH2O | |
| Recruitment maneuvers: yes, prone decubitus position | – | ≤30cm H2O | Recruitment maneuvers: yes, prone decubitus position |
Also, patients who have received an adequate protective mechanical ventilation have been included in these studies and recruitment maneuvers have been conducted among patients in the decubitus prone position before implanting the VV-ECMO.34
Recommendation #8The potential indications for the VV-ECMO as a bailout therapy include the severe hypoxemic and/or hypercapnic acute respiratory failure with predictable recovery of the lung function.
The VV-ECMO can be used in patients with severe acute respiratory failure in whom mechanical ventilation cannot maintain oxygenation or with an acceptable and maintained CO2 washout (Table 3). This clinical situation can be seen in the ARDS or status asthmaticus.35–37 The VV-ECMO can also be used in patients in whom the effort to maintain an adequate oxygenation has a high risk of ventilator-induced pulmonary lesion. In this case, the use of VV-ECMO leaves the “lung at rest” reducing pressure on the airway and tidal volume.38 Due to the complications associated with this technique and the lack of studies confirming its effectiveness, patients need to be selected with care.
The absolute contraindications are: dying patients with established multiple organ failure; patients with poor shot-term prognosis or serious comorbidities; or advanced neurological disease. The relative contraindications are: mechanical ventilation of seven-day duration with high pressure on the airways; elderly patients; difficulty managing vascular accesses and contraindications for anticoagulation.39 Also, there are scales to measure risk assessment and predict mortality risk in these patients.40,41
Recommendation #9It is advisable to transfer these patients to tertiary referral centers or centers where the VV-ECMO is an essential part of the global management of patients with respiratory failure.
The management of patients on ECMO in tertiary referral centers has lower mortality rates,33,42–44 which is why the transfer of ECMO-eligible patients to tertiary referral centers or centers experienced on the use of this technique should be taken into consideration. Transfers to these centers should be conducted following top safety measures and evaluating the possibility of clinical deterioration if the transfer is done with conventional ventilation. In this case, the ECMO transfer should be kept in mind.45
ECMO transfers are always safe as long as they performed by expert medical teams.46–48 The percentage of complications and mortality rates are similar between patients transferred on ECMO support and those in whom the ECMO system was implanted at the ECMO-capable center.49
The criteria for immediate consultation for transfers to tertiary referral center in the management of respiratory failure include: severe hypoxemia with a PaO2/FiO2 ratio <60mmHg or a PaO2/FiO2 ratio <100mmHg and/or PaCO2 >100mmHg for more than 1h after the administration of the optimal therapy and further failure of other therapies.50 Other criteria have been established as well for transfer-eligibility to ECMO-capable centers51: pH <7.2; Murray scale scores >2.5, FiO2 not higher than 0.8 for 8 days and Pplat not higher than 30cmH2O for 7 days.
Recommendation #10The ECCO2R systems (CO2purification systems) are low-flow devices capable of purifying CO2effectively. They should have very precise indications such as to facilitate protective ventilation, but there is still no evidence to establish its indications.
ECCO2R systems (CO2 purification systems) are one extracorporeal life support technique with low-flow devices for the purification of PaCO2 effectively. They should have very accurate indications such as to facilitate protective ventilation, but there is still no evidence to establish its indications. The ECCO2R systems are easier devices to use compared to ECMO and they are considered partial respiratory support techniques. Compared to ECMO, these systems require lower blood flows between 250ml/min and 1.5–2l/min, have a smaller membrane area and require systemic anticoagulation.52,53 They can be arteriovenous systems without pump or veno-venous systems, although the actual tendency are veno-venous systems with single double-lumen cannula since they associate fewer vascular complications.54,55 They allow the effective purification of CO2 and do not contribute directly to oxygenation.56 In patients with chronic obstructive pulmonary disease these devices could be used for the management of hypercapnic respiratory failure to avoid invasive mechanical ventilation, facilitate its withdrawal, in unresponsive patients to non-invasive mechanical ventilation and to facilitate ventilation per se,56,57 although they are no stranger to complications.58 Evidence is more limited for the management of ARDS and its use is more oriented toward controlling hypercapnia during the use of protective or ultra-protective MV (TV 3–4ml/kg; Pplat <25cmH2O) for the management of moderate57 and moderate-to-severe ARDS although we are still waiting for the results from experimental clinical trials.59,60
In conclusion, ECCO2R systems are one promising adjuvant therapeutic strategy for the management of patients with severe exacerbations of chronic obstructive pulmonary disease and for the application of protective or ultra-protective mechanical ventilation in patients with ARDS without potentially fatal hypoxemia. However, given the observational methodology of most clinical trials available and the differences seen in both the technical characteristics and performance of the actual devices, the benefit-risk ratio for and against ECCO2R systems in these populations of patients is still controversial.
Recommendations for the use of ECMO in organ donationRecommendation #11Normothermal abdominal perfusion with ECMO (NAP-ECMO) should be considered as an in situ preservation technique in donors of abdominal organs in controlled asystole.
The use of normothermal abdominal perfusion with ECMO as a method to preserve donors’ abdominal organs in controlled asystole has been gaining momentum in our country61 following the excellent findings published about international62,63 and national64 experiences alike. Its use is compatible with lung donation where ultrafast surgery with cold perfusion65 is commonly used and, in recent experiences, even with heart preservation and extraction.66 This technique allows us to recover abdominal organs from ischemic damage – especially the liver.67 Also, it reduces the urgency of the surgical procedure and the iatrogenia sometimes caused by ultrafast surgery.68 The logistics associated with the entire process is easier and it allows us to easily assess suspicious or marginal organs, since it facilitates active interventions on the organ preservation environment and gives us the capacity to determine viability and quality markers during maintenance. Also, it allows us to perform biopsies and analyze intraoperative findings (nodules, steatosis, etc.).
Recommendation #12If this technology is not available, agreements should be reached with hospitals that have this technology in its mobile version.
In the intensive care setting, the use of the extracorporeal membrane oxygenation has grown exponentially during the last few years following the advances made on ECMO circuits and due to the experience accumulated in special circumstances such as in the H1N1 pandemics.33,69 It is still a complex procedure that should be conducted in tertiary referral centers according to the recommendations made by the Extracorporeal Life Support Organization.16
On the other hand, according to data from the National Transplant Organization, to this day, our country has 91 hospitals with donation programs in controlled asystole.70 Obviously, very few of these hospitals have ECMO programs available in their intensive care units or for normothermal abdominal perfusion purposes in controlled asystole donors.
The experience accumulated from inter-hospital transfers of mobile support teams for abdominal organ preservation in Andalucía71 and the creation of a mobile team from the Madrid Healthcare Administration72 confirms the benefits of having transplant coordinator intensivists experienced in the management of ECMO systems and controlled asystole donation and the utility of promoting inter-hospital alliances to increase the donation potential of our ICUs.
Recommendation #13Before initiating ECMO after the donor's death and while the procedure is being performed, the perfect closure of the thoracic aorta needs to be secured.
The possibility of recovering the donor's pulse after death has been declared when normothermal abdominal perfusion with ECMO is being used can be disturbing from the ethical point of view.73 This situation can only occur when the closure of the thoracic aorta has not been secured.
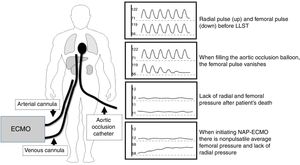
The adequate inflation of the balloon and the correct placing of the aortic occlusion catheter is essential not only to avoid any hypothetical pulse recoveries but also to secure the adequate perfusion of the organs and restrict preservation to the abdominal territory (Fig. 1).74,75
The following steps need to be observed here.76
- •
Before limiting life support therapy, one left radial artery should be catheterized together with the arterial and venous cannulae and the aortic occlusion catheter.
- •
Fill the occlusion balloon catheter and keep it for a few seconds while monitoring the radial and femoral pressures. The femoral pulse wave should disappear, and the radial pulse wave should be present. The filling volume should be written down as the minimal volume of aortic occlusion to be used during normothermal abdominal perfusion.
- •
Afterwards, check the right position of the occlusion catheter at the level of thoracic aorta and fix it adequately.
- •
After death has been declared, fill up the occlusion balloon and initiate ECMO.
- •
During normothermal abdominal perfusion both the radial and the femoral pressures should be monitored. The femoral line should measure the nonpulsatile pressure achieved with the ECMO flow and the radial line should focus on the post-cardiac arrest baseline values.
- •
If pressure on the radial artery goes up parallel to the femoral pressure increase or in the presence of electrical activity, ECMO should be immediately terminated, the right position of the occlusion catheter checked and normothermal abdominal perfusion resumed after a 5-min observation period.
Hypovolemia, anemia, acidosis or any other alteration of the internal environment and preservation of the donor as we would do with any critically ill patient.
The following preservation parameters should be observed:
- 1.
Circuit temperature: 37°C.
- 2.
pH: 7.35–7.45.
- 3.
PaO2: 100–150mmHg.
- 4.
Hematocrit levels >20%.
- 5.
Sodium, potassium, glucose, lactate within lab normal range.77
It is advisable to use charts to register all these variables tested and make the right corrections when it comes to repositioning volume, blood, bicarbonate and keeping normalcy in other parameters such as sodium, potassium, glucose, or lactate to have as much information as possible on the quality of preservation.74
Recommendation #15NAP-ECMO should be kept as long as necessary to allow the recovery of the liver after ischemic stress and an adequate viability assessment should be conducted prior to the transplant.
How long should the preservation of normothermal recirculation be is a fundamental though still controversial issue. There is not such thing as an established useful minimal time to achieve the desired cell recovery and the question on for how long should donors be preserved has been decided empirically by different groups. Very short times would lead to incomplete cell recovery after ischemic stress and to a possible inadequate assessment of the graft. On the contrary, very long times would lead to destabilize the system and prolonged recirculation of harmful elements.74
The average time recorded according to national and international experiences is close to 90–120min.77
DiscussionThe actual recommendations on the use of ECMO in Intensive Medicine show the horizon of the actual or potential practice of using this technique in adults. The lack of randomized studies in many areas leads to recommendations based on expert opinions and the consolidated experienced of some centers, so we cannot rule out that in future editions, and based on scientific evidence, this or that recommendation will sustain major changes.
In the present manuscript, recommendations are given for three different situations: circulatory support (venoarterial ECMO), respiratory support veno-venous ECMO) and organ preservation in donors (regional ECMO). As part of the ECMO recommendations as respiratory support, one recommendation on the use of ECCO2R has been included and it is a controversial one since it is obvious that the ECCO2R is a CO2 purification technique rather than an oxygenation technique. In any case, we are talking about a respiratory support system with extracorporeal circulation that shares practical aspects with ECMO systems which somehow justifies its inclusion in this manuscript.
Although the manuscript has been structured by separating the indications for circulatory and respiratory support, on some occasions, we may find both situations. In these circumstances, the experience from the team is crucial to decide the ECMO technique and strategy to use. On the other hand, the degree of consolidation of the technique is different in each of the three scenarios. The use of ECMO as circulatory support has been a relative common thing in specialized centers and traditionally it has been accepted as a useful resource as a bridge to heart transplant, fulminant myocarditis and other situations of pump failure. On the contrary, when it comes to respiratory failure, the use of ECMO had two important setbacks with the publication of two negative clinical trials.78,79 Ever since, its use has been very restricted for four decades to just a few centers here and there that have accumulated experience in this field.80 After the H1N1 pandemic declared back in 2009 and the promising results of the use of ECMO during such pandemic31,65 there has been a resurgence of this technique posing scientific and ethical challenges for different reasons (lack of quality scientific evidence, accessibility to the technique in circumstances of limited resources, uncertain risk-benefit ratio/therapeutic futility, risk of complications). As a matter of fact, in recent guidelines on the management of patients with ARDS, the use of ECMO is not a definitive recommendation due to the lack of evidence.81 With the publication of the EOLIA trial,28 there are still doubts on its effectiveness.
The EOLIA trial is a randomized multicenter trial that included intensive care units from France, the U.S. and Canada. In total, 124 patients with severe ARDS treated with veno-venous ECMO associated with conventional mechanical ventilation were recruited, plus 125 patients with ARDS treated with mechanical ventilation only (mechanical ventilation of lung protection with tidal volumes of 6ml/kg of predicted body weight, use of neuromuscular relaxants, ventilation in the decubitus prone position for long periods of time and recruitment maneuvers). The crossing of patients was allowed from the control group to the ECMO group with persistent refractory hypoxemia despite the measures used or following the treating physician best possible judgment. The primary outcome was 60 day-mortality through an intention-to-treat analysis.
The authors found a 35% crude mortality rate at 60 days (44/124 patients) in the ECMO group and a 46% crude mortality rate (57/125) in the control group showing a 10% absolute risk reduction (ARR) (confidence interval [CI] – 2% to 22%) but the trial was terminated after including 75% of the 331 patients scheduled. The conclusion from the authors was that “ECMO does not reduce mortality significantly compared to conventional mechanical ventilation where the use of ECMO as a bailout therapy is included”. However, we should keep in mind that 28% of the patients from the control group crossed to the refractory hypoxemia group at an average 6.5±9.7 days after randomization (mean, 4 days, interquartile range, 1–7). These patients showed significantly higher values compared to the patients from the control group when it comes to the average initial plateau pressure (31.7±5.5 versus 28.5±4.1cm of water), which is suggestive that they were patients with more severe ARDS when they crossed than the patients who received ECMO. The 60 day-mortality rate of the patients who crossed to the intervention group with ECMO was 57% (20/35 patients) among the control group patients who crossed to ECMO versus 41% (37/90 patients) among the remaining control group patients (relative risk [RR] 1.39; 95% CI, 0.95–2.03). The frequency of complications did not change significantly between the two groups except for the fact that there were more hemorrhagic events that required blood transfusions in the ECMO group compared to the control group (in 46% versus 28% of the patients, ARR, 18%; 95% CI, 6–30) and more cases of severe thrombocytopenia (in 27% versus 16%, respectively; ARR, 11%, 95% CI, 0–21).
For all these reason, and yet despite the disappointing findings from this multicenter clinical trial (relative risk 0.76; 95% CI, 0.55–1.04; p=0.09), the study recruitment density (0.058patients/month/center in 100 participating units), the high percentage of crossing from patients to receive ECMO and the early termination of the study, the study statistical power may have been underestimated since it would take more than 8 years recruiting patients with severe ARDS to detect a statistically significant 11% absolute difference. Therefore, the use of ECMO as a bailout therapy can be considered in selected patients with severe ARDS in tertiary referral centers.
With the publication of the actual recommendations we are not promoting a liberal or disproportionate use of ECMO and by no means we are trying to trivialize this technique but encourage its rational use based on the existing knowledge. In this sense, we have heard critical voices speaking against the huge widespread use of this invasive technique and the disproportionate use of resources. However, this criticism does not lean on indisputable scientific support.82 That is why we wish to draw everyone's attention to the fact that its indications are always assessed by experienced personnel who should accurately evaluate the risk-benefit ratio individually since this is a technique that can lead to serious complications. Also, given the uncertainty of some indications, conditions of use are a necessity based on the updated knowledge of the actual bibliography with additional case registries to evaluate the results.
In sum, ECMO is a technique that can be indicated for the management of patients who experience cardiac or respiratory failure and for the preservation of donor's organs. Although from a physiopathological point of view, several arguments recommend its use, we still need more studies before defining precisely what patients are ECMO-eligible and when is the optimal time to start this therapy. Given the characteristics of this technique, its implementation requires solid institutional support and multidisciplinary personnel well-trained in all possible scenarios. A national registry of the cases included would also be desirable to know the results and detect any possible deviations.
Conflicts of interestMPFC has organized the ECMO courses that have been partially funded by Maquet, Thoratec and Cardiolink. MLS has organized the ECMO courses that have been partially funded by MAQUET, and JLPV has organized the ECMO courses that have been partially funded by Maquet and Fresenius. The remaining authors declare no conflict of interests whatsoever.
- -
Victoria Boado Varela, MD, Intensive Care Unit, Hospital Universitario de Cruces, Bilbao, Spain
- -
Ricardo Gimeno Costa, MD, Intensive Care Unit, Hospital Universitari i Politècnic La Fe, Valencia, Spain
- -
Rocío Gómez López, MD, Intensive Care Unit, Hospital Quirónsalud, Pontevedra, Spain
- -
Miguel Domínguez, MD, Intensive Care Unit, Hospital Quirónsalud, Pontevedra, Spain
- -
Rubén Jara Rubio, MD, Intensive Care Unit, Hospital Universitario Virgen de la Arrixaca, Murcia, Spain
- -
Luis Martín Villén, MD, Intensive Care Unit, Hospital Virgen del Rocío, Sevilla, Spain
- -
M. Ángeles Rodríguez Esteban, MD, Intensive Care Unit, Hospital Universitario Central de Asturias, Oviedo, Spain
- -
M. Isabel Rubio López, MD, Intensive Care Unit, Hospital Universitario Marqués de Valdecilla, Santander, Spain
- -
Jordi Riera, MD, Intensive Care Unit, Hospital Universitario Valle Hebrón, Barcelona, Spain
- -
Eduardo Miñambres García, MD, Intensive Care Unit, Hospital Universitario Marqués de Valdecilla, Santander, Spain
- -
Francisco del Río Gallegos, MD, Intensive Care Unit, Hospital Clínico San Carlos, Madrid, Spain
Please cite this article as: Fernández-Mondéjar E, Fuset-Cabanes MP, Grau-Carmona T, López-Sánchez M, Peñuelas Ó, Pérez-Vela JL, et al. Empleo de ECMO en UCI. Recomendaciones de la Sociedad Española de Medicina Intensiva Crítica y Unidades Coronarias. Med Intensiva. 2019;43:108–120.