To determine the predictive value of the inotropic score (IS) and vasoactive-inotropic score (VIS) in low cardiac output syndrome (LCOS) in children after congenital heart disease surgery involving cardiopulmonary bypass (CPB), and to establish whether mid-regional pro-adrenomedullin (MR-proADM) and cardiac troponin I (cTn-I), associated to the IS and VIS scores, increases the predictive capacity in LCOS.
DesignA prospective observational study was carried out.
SettingA Paediatric Intensive Care Unit.
PatientsA total of 117children with congenital heart disease underwent CPB. Patients were divided into two groups: LCOS and non-LCOS.
InterventionsThe clinical and analytical data were recorded at 2, 12, 24 and 48h post-CPB. Logistic regression was used to develop a risk prediction model using LCOS as dependent variable.
Main outcome measuresLCOS, IS, VIS, MR-proADM, cTn-I, age, sex, CPB time, PIM-2, Aristotle score.
ResultsWhile statistical significance was not recorded for IS in the multivariate analysis, VIS was seen to be independently associated to LCOS. On the other hand, VIS>15.5 at 2h post-CPB, adjusted for age and CPB timepoints, showed high specificity (92.87%; 95%CI: 86.75–98.96) and increased negative predictive value (75.59%, 95%CI: 71.1–88.08) for the diagnosis of LCOS at 48h post-CPB. The predictive power for LCOS did not increase when VIS was combined with cTn-I >14ng/ml at 2h and MR-proADM >1.5nmol/l at 24h post-CPB.
ConclusionsThe VIS score at 2h post-CPB was identified as an independent early predictor of LCOS. This predictive value was not increased when associated with LCOS cardiac biomarkers. The VIS score was more useful than IS post-CPB in making early therapeutic decisions in clinical practice post-CPB.
Estudiar el valor predictivo de la escala inotrópica (IS) y la escala vasoactiva-inotrópica (VIS) en el síndrome de bajo gasto cardiaco (SBGC) en niños poscirugía de cardiopatías congénitas mediante bypass cardiopulmonar (BCP). Determinar si adrenomedulina (MR-proADM) y troponina cardiaca-I (cTn-I) asociadas con IS y VIS incrementan su capacidad predictora de SBGC.
DiseñoEstudio prospectivo y observacional.
ÁmbitoCuidados intensivos pediátricos.
PacientesCiento diecisiete pacientes pediátricos con cardiopatías congénitas corregidos mediante BCP, clasificados en función de la presencia o no de SBGC.
IntervencionesLos datos analíticos y clínicos se midieron a las 2, 12, 24 y 48h post-BCP. Las principales variables se analizaron mediante regresión logística multivariante, considerando SBGC como variable dependiente.
Variables de interés principalesSBGC, IS, VIS, MR-proADM, cTn-I, edad, sexo, BCP, PIM-2 y escala Aristóteles.
ResultadosEl IS no alcanzó significación estadística en el estudio multivariante; sin embargo, el VIS se asoció independientemente a SBGC. El VIS>15,5 a las 2h del ingreso en CIP, ajustado por edad y tiempo de CEC, muestra alta especificidad (92,87%; IC 95%: 86,75-98,96%) y alto valor predictivo negativo (75,59%; IC 95%: 71,10-88,08) para predecir SBGC a las 48h post-BCP. La capacidad predictora no se incrementa al incorporar cTn-I>14ng/ml a las 2h y ADM>1,5nmol/l a las 24h del postoperatorio.
ConclusionesEl VIS a las 2h post-BCP es un predictor independiente precoz de SBGC. Este valor no se incrementa al asociarse biomarcadores cardiacos de LCOS. La escala de VIS fue más útil que la escala de IS en la toma de decisiones terapéuticas tras la cirugía cardiaca.
Current research focuses on preventing mortality and reducing the risk of morbidity in children who undergo congenital heart disease (CHD) surgery with cardiopulmonary bypass (CPB). In order to accomplish these objectives, it is essential to monitor the diverse variables influencing these potential outcomes, such as the levels of inotrope score (IS) and/or of vasoactive-inotropic score (VIS), and their prognostic value in the post-operative period.1–3 Nevertheless, the association between VIS and the post-operative course of the patient remains controversial.
Wermousky et al.1 have quantified the degree of inotrope support received by neonates after surgical repair of transposition of the great arteries, and have reported that the maximum IS within the first 48h was an indicator of poor prognosis, of higher incidence of prolonged mechanical circulatory support, renal replacement therapy, neurological damage, of cardiac arrest and death. In addition to this, IS was also associated with longer duration of mechanical ventilation (MV), paediatric intensive care unit (PICU) and hospital stay.2 On the other hand, other authors have been unable to establish an association between VIS and the early post-operative outcomes in neonates who underwent heart surgery with CPB.4 Therefore, elevated VIS levels could not be associated with prolonged MV duration and PICU stay. Likewise, these authors have found that low cardiac output syndrome (LCOS) was not associated with MV duration or length of PICU stay.
Neonates undergoing CPB are more likely to present increased risk of morbimortality compared with other age groups. Thereby they might be considered a special group. Gaies et at.5 have studied 391 children (only one-third were neonates) who underwent CHD surgical repair, in which VIS>15 within the first 24h post-operatively was considered empirically high. A high VIS level was strongly associated with mortality at 30 days following surgery, with incidence of cardiac arrest, need for mechanical circulatory support, dialysis, neurological damage, MV duration and length of PICU stay. In another work on VIS as a short-term outcome biomarker, a maximal VIS on the second post-operative day predicted adverse outcomes in adolescents following cardiac surgery.6
There are other studies regarding the paediatric population that centre on cardiac biomarkers, such as the β-type natriuretic peptide (BNP),7 mid-regional pro-atrial natriuretic peptide (MR-proANP),8 pro-adrenomedulina (MR-proADM),9 cardiac troponin-I (cTn-I)10 and copeptin11 as early predictors of LCOS to monitor the post-operative period after CHD surgery with CPB. To date, there are no published studies that relate IS, VIS and cardiac biomarkers levels with the prediction of LCOS in children during the post-operative period after CHD surgery requiring CPB.
In order to address this knowledge gap, we have performed a study of CHD children to evaluate whether IS and VIS scores improve the predictive power for LCOS.IS and VIS have been estimated in the immediate post-operative period and during LCOS development and have been associated with the cardiac markers above mentioned predictors of LCOS in the paediatric population. These could be useful in aiding in therapeutic decision-making in clinical practice after CPB, and in improving the prognosis of the patient.
Patients and methodsStudy design and populationThis prospective, observational study was conducted at a single referral hospital during a 2-year period. The current study enrolled 117 consecutively only patients (aged 10 days to 15 years) who had undergone CHD surgery requiring CPB, and who had been admitted to a PICU for monitorisation. The exclusion criteria included: (a) immediate death following PICU admission (with in the first 2h), (b) infection, (c) renal failure requiring haemofiltration, (d) polymalformative syndromes, (e) congenital metabolic defects, and (f) chronic diseases, different from the original cardiac pathology of the patient. The patients who met some of the exclusion criteria or those who died during surgery were excluded from the study.
Following PICU admission, the management and monitoring of the patients were performed in accordance with current protocols.12,13 Upon admission to the PICU, all of the patients were administered milrinone as LCOS prophylaxis14 and subsequently, according to their course, the consecutive order of administration of vasoactive drugs was dopamine, adrenaline and noradrenaline. This order of inotropic drugs administration could differ depending on the specific pathophysiology of each heart disease, as well as preload and peripheral resistance patterns in each case.
The Aristotle score15 and the Paediatric Index of Mortality (PIM-2)16 were assessed in all of the patients. Measurements of IS and VIS scores were performed at 2, 12, 24 and 48h post-operatively and following the formulae described below:
IS: Dopamine (mcg/kg/min)+Dobutamine (mcg/kg/min)+100×adrenaline (mcg/kg/min1)
VIS: IS+10×Milrinone (mcg/kg/min)+10000×vasopressin (mU/kg/min).2
All of the patients received mechanical ventilation and were sedated with midazolam and fentanyl. Post-operative haemodynamic monitoring included: (a) insertion of an arterial line: a PiCCO catheter was placed into the femoral artery. In patients weighing 5–15kg, a 3-F catheter (PV 2013 L07 Pulsiocath) was used and a 4-F catheter (PV 2014 L08Pulsiocath, Pulsion Medical Systems AG, Munich, Germany) was inserted in the patients who weighed more than this body-weight threshold. Because of the problems that catheterisation might cause, we dispensed with the placement of a PiCCO catheter in the patients weighing <5kg; (b) insertion of a central venous catheter (tip either in or close to the right atrium), which was checked by a chest radiograph, and (c) occasionally, another catheter was placed into the left atrium of the patients at risk of post-operative left ventricular dysfunction.
In the post-operative period, the patients were divided into 2 groups according to presence or absence of LCOS. LCOS was diagnosed in all of the children when left ventricular ejection fraction was <40% (Teicholz) via echocardiography, and they met at least three of the following criteria: (a) systolic blood pressuremL/kg/h without diuretics, (c) lactate ≥3.5mmol/L or HCO3 <18mEq/L, and (d) oxygen extraction ratio >35%. Furthermore, an arterial thermodilution catheter (PiCCO) was inserted for cardiac output monitorisation whenever it was possible, and LCOS was established when cardiac index was <2.5L/min/m2 and clinical criteria were presented.
In the children weighing <5kg, a PICCO catheter insertion was deemed unsafe, and LCOS was considered when they exhibited ventricular ejection fraction <40% (left ventricle, with the exception of hypoplastic left heart syndrome, in which the right ventricle functions as the systemic ventricle), and they exhibited at least three of the clinical criteria described above. Cardiac output was assessed in all of the patients to include them into the LCOS or the non-LCOS group at 2, 12, 24 and 48h after the immediate post-operative period.
Blood samplingBaseline blood samples were collected from a central venous catheter prior to corrective surgery and at 2, 12, 24, and 48h post-CPB. Assessment of the patient general biochemical parameters and quantification cTn-I levels were carried out in our department of clinical analysis. Blood samples were collected and centrifuged at 3500g for 10min. Serum was separated into aliquots and frozen at −82°C until the day of analysis. cTn-I was measured using an automated electrochemiluminescence immune assay (Architect system, Abbott Diagnostics Division, Illinois, United States). MR-proADM was measured using a simultaneous multianalyte detection immunoassay BRAHMS KRYPTOR compact (Hennigsdorf, Germany), as well as assay kits and a Multiplex system using LuminexxMAP technology. The analytical detection limits were 0.05nmol/L for MR-proADM, 4.8pmol/L.
Patient medical reportsThe data were obtained from the case history of each child. Informed written consent was obtained from the patients’ parents or legal guardians. Our study was approved by the Hospital Biomedical Ethics Committee and conformed to the ethical standards laid down in the 1964 Declaration of Helsinki.
Statistical analysisThe data were expressed either as mean±standard deviation or as median (interquartile ranges). The study of IS and VIS, as well as the comparison between the LCOS group and the non-LCOS group were undertaken using the Student t test for continuous variables when the distribution was normal (Kolmogorov–Smirnov test), and the Mann–Whitney U test when the distribution was not normal. The relationships between the categorical variables were assessed using either the chi-square test or the Fisher exact test. Comparisons among the monitored time points were tested via ANOVA test and a Sidak correction as a post hoc test.
The correlations between VIS, IS and the clinical variables (length of mechanical ventilation and PICU stay) were assessed via the Spearman correlation test. Univariate logistic regression was undertaken at 48h following surgery to identify independent predictors of LCOS. The Youden's index (J) was used to determine the optimal cutoff point to dichotomize a continuous variables (VIS, age, CPB) on the ROC plots.
For the multivariate analysis, independent predictors of LCOS were considered risk factors according to the univariate analysis (p<0.05). The Hosmer–Lemeshow test and the receiver operating characteristic curve (ROC) were applied to assess the goodness-of-fit of the model. Considering a strong correlation between VIS and IS, separate models were constructed to avoid multicollinearity. In the current study we carried out a logistic regression analysis using both only clinical variables from patients and clinical variables plus biomarkers (cTn-I >14ng/ml 2h and MR-proADM >1.5nmol/L 24h) as the latter were proven useful to predict LCOS in a previous study carried out by our research group.11 ROC curves were obtained and the area under the curve (AUC) calculated with a 95% confidence interval to determine the performance of each fitted model. The predictive power of each method was compared by the ROC curves through Hanley and Mc Neil's technique. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy (Acc) for each model were calculated from true positive, false negative, true negative and false positive outcomes.
All of the tests were 2-tailed and the results were considered statistically significant when p<0.05. The data were analysed using SPSS 18.0.0 2010 (SPSS Inc., Chicago, Illinois, United States).
ResultsThe study enrolled 117 consecutive paediatric patients (aged 10 days to 15 years) who were admitted to a PICU after corrective surgery for congenital heart disease with CPB. Two patients were excluded from the study because they perished within the first 2h following PICU admission. Six patients developed cardiogenic shock and required extracorporeal membrane oxygenation within the first 48h post-CPB. Table 1 displays the type of congenital heart disease and the patient characteristics of the entire cohort of our study.
Characteristics and description for paediatric patient with congenital heart.
| Mean±SD | Median (IQR) | |
|---|---|---|
| Age (months) | 39.84±52.43 | 14 (209.86) |
| Weight (kg) | 12.71±13.14 | 8.3 (71.8) |
| PIM-2 | 5.32±3.27 | 4.83 (18.27) |
| ACC (min) | 58.77±45.5 | 60 (180) |
| CPB time (min) | 116.9±47.46 | 110 (225) |
| Duration of MV (h) | 110.55±2.1 | 24 (1248) |
| Stay at PICU (days) | 12.8±11.93 | 9 (82) |
| Vasoactive drugs* | |
|---|---|
| I. Only with Milrinone:16 (14%) | |
| II-Sequence: 88 (75%):II.1. Milrinone+DA 35 (29.9%)II.2 Milrinone+DA+A: 29 (24.7%),II.3 Milrinone+DA+A+NA 24 (20.5%)III Other sequences: 13 (11%) | |
| Type of CHD (No. patients (%) | |
|---|---|
| T Fallot: 18 (15.4%) | CAT: 8 (6.8%) |
| AV canal defect: 15 (12.8%) | HLHS: 6 (5.1%) |
| CoA/IAo: 13 (11.1%) | MR/AoR: 3 (2.6%) |
| PA: 12 (10.3%) | TAPVC: 3 (2.6%) |
| TGA: 14 (12%) | Others: 3 (2.6%) |
| VSD/ASD: 22 (18.8%) | |
PIM: paediatric index of mortality; ACC: aortic cross-clamping; CPB: cardiopulmonary bypass; MV: mechanical ventilation; PICU: paediatric intensive care unit; IQR: interquartile range; ASD: atrial septal defect; CAT: common arterial trunk; CHD: congenital heart disease; CoA: coarctation of the aorta; HLHS: hypoplastic left heart syndrome; IAo: interrupted aortic arch; MR/AoR: mitral/aortic regurgitation; PA: pulmonary atresia; T Fallot: tetralogy of Fallot; TAPVC: total anomalous pulmonary venous connection; TGA: transposition of the great arteries; VSD: ventricular septal.
Defect. DA: Dopamine; A: Adrenaline; NA: Noradrenaline.
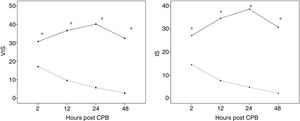
Thirty-three patients (29%) exhibited LCOS following corrective cardiac surgery with CPB, while the other patients were included in the non-LCOS group. IS and VIS levels were significantly more elevated in LCOS patients in all the CPB time points (p<0.001) (Fig. 1). cTn-I and MR-proADM levels, after attaining a peak value at 2h post-operatively, decreased in the group without LCOS, whereas they increased in the LCOS group (these data have been published in Rev Esp Cardiol (Engl Ed). 2017; 70: 267–274).
(A, B) Differences in inotrope score (IS) and vasoactive–inotropic score (VIS) among patients according to presence or absence of low cardiac output syndrome (LCOS) in the immediate postoperative period of cardiopulmonary bypass (CPB) at a Paediatric Intensive Care Unit (PICU). —— LCOS; - - - - Absence LCOS,. Represent the mean, * Significant differences (p=0.01) between LCOS and Absence of LCOS.
The results by the univariate and multivariate logistic regression analyses to predict LCOS after CHD surgery with CPB are shown in Table 2. There was no significant association between the gender of the patients, PIM-2 and the Aristotle score with the development of LCOS at 48h post-CPB. Although the univariate logistic regression analysis indicated that there was an association between IS levels and LCOS, in the multivariate logistic regression we did not observe such association. For this reason, we constructed models to predict LCOS using the VIS score, the significant variables of the univariate model, and cTn-I and ADM levels. The cut-off points for these two cardiac biomarkers were the same as established as predictors of LCOS in a previous study11 (cTn-l>14ng/ml and ADM>1.5nMol/L).
Univariate and multivariate logistic regression for the prediction of low cardiac output syndrome after cardiac surgery with cardiopulmonary bypass.
| Prognostic factor of LCOS | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age <12 months | 5.45(2.16–13.7) | 0.001 | 4.89 (1.6–14.7) | 0.004 | 1.7 (0.4–6.7) | 0.427 |
| Sex (hombre) | 0.86 (0.31–2.33) | 0.76 | ||||
| CPB >120min | 4.1 (1.7–10.2) | 0.002 | 2.28 (1.08–7.38) | 0.034 | 2.1 (0.6–7.5) | 0.237 |
| PIM-2 | 0.99 (0.86–1.13) | 0.88 | ||||
| Aristotle score | 1.21 (0.89–1.64) | 0.21 | ||||
| IS 2h PO | 1.029 (1.01–1.05) | 0.009 | ||||
| VIS 2h>15.5 PO | 6.04 (2.38–17.1) | 0.007 | 4.89 (1.6–14.4) | 0.003 | 4.43 (1.4–13.9) | 0.019 |
| cTn-I>14ng/ml 2h PO | 7.2 (2.87–18.03)* | <0.001 | 5.48 (1.7–15.2) | 0.003 | ||
| MR-proADM>1.5nmol/l 24h PO | 13.75 (4.4–42.99)* | <0.001 | 7.28 (2.4–25.4) | 0.002 | ||
| Model performance | ||||||
| AUC | 0.81 (0.73–0.89) | 0.001 | 0.85 (0.78–0.93) | 0.03 | ||
| Hosmer–Lemeshow goodness-of-fit test | X2: 3.12; P: 0.68 | χ2:3.6; P=0.73 | ||||
CPB: cardiopulmonary bypass; PIM-2: paediatric index of mortality; IS: inotrope score; VIS: vasoactive-inotropic score; CI: confidence interval; OR: odds ratio; PO, postoperative; mid-regional pro-adrenomedullin (MR-proADM) and cardiac troponin I (cTn-I). AUC: area under the curve.
The model 1 exclusively comprised, aside from VIS, variables such as age and CPB time points. Age and CPB were excluded from the model 2 when cardiac biomarker were included in it. Comparisons between AUCs in both predictive models did not show statistical differences by Hanley and Mc Nail methods (p>0.05). Table 3 shows the sensitivity, specificity, predictive values and the likelihood ratio of the curves generated by the two models. The VIS score correlated positively with the duration of MV (rho: 0.385; p: 0.001) and with the stay at a PICU (rho: 0.465; p: 0.001).
Sensibility, specificity and predictive values of Logistic Regression Model 1 and Model 2 to predict low cardiac output syndrome.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Value (%) | CI (95%) | Value (%) | CI (95%) | |
| Sensibility | 55.48 | 42.13–75.45 | 61.29 | 42.53–80.05 |
| Specificity | 92.87 | 86.75–98.96 | 85.71 | 77.64–93.79 |
| PPV | 64.71 | 49.05–90.36 | 61.29 | 42.5–80.05 |
| NPV | 79.59 | 71.10–88.08 | 85.71 | 77.64–93.79 |
| Likelihood ratio+ | 4.97 | 2.01–12.29 | 4.29 | 2.37–7.77 |
| Likelihood ratio− | 0.69 | 0.53–0.91 | 0.45 | 0.29–0.71 |
| Accuracy | 77.39 | 69.31–85.47 | 79.13 | 71.27–86.99 |
CI: confidence interval; VIS: vasoactive-inotropic score; NPV: negative predictive value; PO: post-operative; PPV: positive predictive value.
Variables of model 1: VIS 2h>15.5, age<12 months and CPB>120min.
Variables of model 2: VIS 2h>15.5, cTn-I>14ng/ml 2h and MR-proADM>1.5nmol/l 24h.
LCOS after CPB is frequently observed after corrective surgery for CHD in children. Normally, ventricular dysfunction following cardiac surgery with CPB peaks within 8–12h post-CPB, and it gradually recovers within 24–48h post-operatively.17 However, there are patients in whom LCOS is prolonged, as demonstrated in our study, in which LCOS occurrence at 48h post-CPB was in 29% of the patients.
To diagnose LCOS is hard to achieve, especially in children, in whom the validation of estimation methods for LCOS is complex, and invasive procedures present great many limitations, particularly in younger children. In consequence, the current study includes cardiac function measurement by echocardiography, clinical variables that have been traditionally utilised as shock markers and quantitative data, such as the data obtained by arterial thermodilution.
The development of LCOS suggests a higher rate of complications, hence predicting it could positively influence in the post-operative course of the patient. Assessing the need for post-CPB vasoactive inotropic support using the IS and VIS scales to predict early LCOS can contribute to decision making in clinical practice. Accordingly, it has been observed that the prediction of LCOS sustained after 48h post-CPB was strongly associated with VIS at 2h of PICU admission, adjusted forage and duration of the CPB. These results support those indicated by Wernosky,1 who has determined the value of these scales in a group of neonate sunder going CPB.
Following PICU admission, according to results, the use of inotropic support varies through to the period after CPB, which increases in LCOS patients. This relatively facilitates LCOS prediction as the time span fore estimating LCOS expands but its practical utility diminishes. Consequently, it is pivotal to find a nearly indicator of LCOS by assessing the efficacy of IS and VIS at different time points. In this study, at 2h after PICU admission, VIS displayed a high LCOS predictive power post-CPB, with a cut-off point of 15.5.IS, at 2h post-CPB, also predicted LCOS in the univariate analysis but opposing VIS, it did not obtain significance in the multivariate analysis. Therefore, VIS appeared to be more efficient than IS, probably because the use of IS has some important limitations as this mainly excludes vasoactive medication, such as nitroprusside, nitro-glycerine, phosphodiesterase inhibitors and vasopressin. For this reason, Davidson et al.,3 who have undertaken a prospective, observational study of 70 children submitted to cardiac surgery, proposes a modified version of IS that includes, like VIS, vasoactive drugs.
The ability of the different variables assessed in our study to predict LCOS was analysed by multivariate logistic regression, obtaining important significance in age, CPB duration and VIS at 2h post-CPB, with a cut-off point >15.5 for VIS (Table 3). This cut-off point showed high specificity and high negative predictive value to predict LCOS at 48h following CPB.
cTn-I levels, which increase rapidly after cardiac injury, could function as a predictive cardiac biomarker during the post-operative period in patients with CHD submitted to cardiac surgery.18,19 Preceding studies have demonstrated that cTn-I levels at 4h post-CPB enable to predict the post-operative course of paediatric patients undergoing cardiac surgery.20 Bojan et al.19 have described that an early increase in cTn-I levels may be useful in predicting the post-operative course of neonates and infants subjected to cardiac surgery. Froese et al.,20 using a cut-off point 13ng/ml for cTn-I at 4h post-CPB, have proven that the early measurement of cTn-I may allow the early diagnosis of LCOS in CHD children undergoing surgery with CPB.
Adrenomedullin (ADM) was discovered in 1993 in human pheochromocytoma tissue and subsequently found to be a circulating hormone. Several studies have demonstrated that MR-proADM alone or combined with other parameters may be a predictor of LCOS in children undergoing CPB.21,22 Moreover, our research group has shown that a cut-off point >14ng/mL for cTn-I and a cut-off point 1.5nMol/L for ADM at 2 and 24h post-CPB, respectively, has high sensibility and specificity for determining LCOS.11
Based on previous findings, our study includes cTn-I and ADM levels as predictive variables with the object of improving the goodness-of-fit. However, the predictive power for LCOS did not rise when these cardiac biomarkers were utilised. We consider these results to be of great relevance as they enable an early estimation of the post operatory course of the patients with CHD. In addition, VIS scores were related to indirect indicators of worse evolution, such as MV duration and the length of PICU stay. The results of this work indicate that the monitoring of cardiac biomarkers in the immediate postoperative period following CHD does not provide more any further clinical information, as the ability to predict LCOS with VIS does not increase with the measurement of cardiac biomarker levels.
The present study has some limitations to consider. The wide range of CHDs required different types of surgical corrections. A solution to this drawback was to study a large sample size in a single hospital, resulting in minimal variability for patient management. LCOS was considered based on restrictive inclusion criteria. All of the data were adjusted for the age and the body-weight of the patients to prevent these variables from influencing our results.
In conclusion, our findings have shown that the value of VIS at 2h post-CPB was itself an early independent predictor of LCOS. This predictive value did not increase when associated with LCOS cardiac biomarkers. VIS was more useful than IS in making early therapeutic decisions in clinical practice after corrective surgery of CHDs by CPB. Therefore, these results could be useful to improve the prognosis of CHDs patients after corrective surgery.
Financial support receivedBRAHMS GmbH Biotechnology Centre, Hennugsdorf, Berlin, Germany, in part supported this study. The partial sponsor of the study had no role in the study design or in analysis, and did not participate in writing the report.
Authors’ contributionsJuan L. Pérez-Navero, Ignacio Ibarra de la Rosa and Maria José de la Torre-Aguilar conceived, planned the project and drafted the manuscript experiments. Carlos Merino-Cejas, Susana Jaraba-Caballero and Manuel Frias-Perez carried out the experiments and contributed to sample preparation. Mercedes Gil-Campos and Elena Gómez-Guzmán contributed to the interpretation of the results. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Conflict of interestThe authors report no conflicts of interest. The authors are responsible for the contents and writing of the paper.
The authors wish to thank the staff of the Maimónides Biomedical Research Institute of Córdoba (IMIBIC) at the University of Córdoba (Córdoba, Spain).